Effect of Glycerol Pretreatment on Levoglucosan Production from Corncobs by Fast Pyrolysis
Abstract
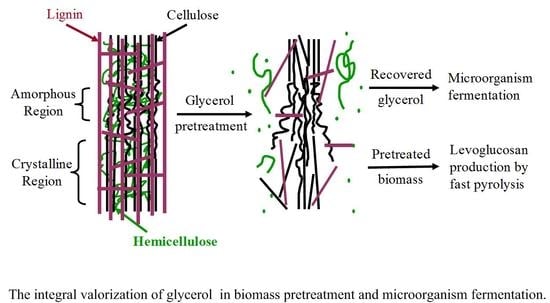
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Glycerol Pretreatment of Corncobs
2.3. Fermentation Experiments of the Recovered Glycerol
2.4. Elemental Analysis of Un-Treated and Glycerol Pretreated Corncobs
2.5. Compositional Analysis of Un-Pretreated and Glycerol Pretreated Corncobs
2.6. Thermogravimetric Analysis
2.7. Structural Characterization of Un-Pretreated and Glycerol Pretreated Corncobs
2.8. Fast Pyrolysis of Un-Pretreated and Glycerol Pretreated Corncobs
3. Results
3.1. The Fermentability of the Recovered Glycerol
3.2. Main Composition and Elemental Analysis of Glycerol Pretreated Corncobs
3.3. Mapping the Structural Changes of Corncobs after Glycerol Pretreatment
3.4. Thermogravimetric Analysis of Corncobs after Glycerol Pretreatment
3.5. Levoglucosan Production from Glycerol Pretreated Corncobs
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Y.; Via, B.K.; Pan, Y.F.; Cheng, Q.Z.; Guo, H.W.; Auad, M.L.; Taylor, S. Preparation and characterization of epoxy resin cross-linked with high wood pyrolysis bio-oil substitution by acetone pretreatment. Polymers 2017, 9, 106. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Fermentable sugars by chemical hydrolysis of biomass. Proc. Natl. Acad. Sci. USA 2010, 107, 4516–4521. [Google Scholar] [CrossRef] [PubMed]
- Rover, M.R.; Johnston, P.A.; Jin, T.; Smith, R.G.; Brown, R.C.; Jarboe, L. Production of clean pyrolytic sugars for fermentation. ChemSusChem 2014, 7, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Bailliez, V.; Olesker, A.; Cleophax, J. Synthesis of polynitrogenated analogues of glucopyranoses from levoglucosan. Tetrahedron 2004, 60, 1079–1085. [Google Scholar] [CrossRef]
- Cao, F.; Schwartz, T.J.; McClelland, D.J.; Krishna, S.H.; Dumesic, J.A.; Huber, G.W. Dehydration of cellulose to levoglucosenone using polar aprotic solvents. Energy Environ. Sci. 2015, 6, 1808–1815. [Google Scholar] [CrossRef]
- Kitamura, Y.; ABE, Y.; Yasui, T. Metabolism of levoglucosan (1,2-anhydro-β-d-glucopyranose) in Microorganisms. Agric. Biol. Chem. 1991, 55, 515–521. [Google Scholar]
- Nakagawa, M.; Sakai, Y.; Yasui, T. Itaconic acid fermentation of levoglucosan. J. Ferment. Technol. 1984, 62, 201–203. [Google Scholar]
- Zhuang, X.L.; Zhang, H.X. Identification, characterization of levoglucosan kinase, and cloning and expression of levoglucosan kinase cDNA from Aspergillus. niger CBX-209 in Escherichia coli. Protein Expr. Purif. 2002, 26, 71–81. [Google Scholar] [CrossRef]
- Lian, J.N.; Garcia-Perez, M.; Chen, S.L. Fermentation of levoglucosan with oleaginous yeasts for lipid production. Bioresour. Technol. 2013, 133, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Layton, D.S.; Ajjarapu, A.; Choi, D.W.; Jarboe, L.R. Engineering ethanologenic Escherichia coli for levoglucosan utilization. Bioresour. Technol. 2011, 102, 8318–8322. [Google Scholar] [CrossRef] [PubMed]
- Prosen, E.M.; Radlein, D.; Piskorz, J.; Scott, D.S.; Legge, R.L. Microbial utilization of levoglucosan in wood pyrolysate as a carbon and energy source. Biotechnol. Bioeng. 1993, 42, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.M.; Helle, S.S.; Juff, S.J.B. Extraction and hydrolysis of levoglucosan from pyrolysis oil. Bioresour. Technol. 2009, 100, 6059–6063. [Google Scholar] [CrossRef] [PubMed]
- Luque, L.; Oudenhoven, S.; Westerhof, R.; Rossum, G.V.; Berruti, F.; Kersten, S.; Rehmann, L. Comparison of ethanol production from corn cobs and switchgrass following a pyrolysis-based biorefinery approach. Biotechnol. Biofuels 2016, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Kawamoto, H.; Saka, S. Cellulose-hemicellulose and cellulose-lignin interactions in wood pyrolysis at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 80, 118–125. [Google Scholar] [CrossRef]
- Zheng, A.Q.; Zhao, Z.L.; Huang, Z.; Zhao, K.; Wei, G.Q.; Jiang, L.Q.; Wang, X.B.; He, F.; Li, H.B. Overcoming biomass recalcitrance for enhancing sugar production from fast pyrolysis of biomass by microwave pretreatment in glycerol. Green Chem. 2015, 17, 1167–1175. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Wu, N.N.; Zheng, A.Q.; Zhao, Z.L.; He, F.; Li, H.B. The integration of dilute acid hydrolysis of xylan and fast pyrolysis of glucan to obtain fermentable sugars. Biotechnol. Biofuels 2016, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Q.; Wu, N.N.; Zheng, A.Q.; Liu, A.Q.; Zhao, Z.L.; Zhang, F.; He, F.; Li, H.B. Comprehensive utilization of hemicellulose and cellulose to release fermentable sugars from corncobs via acid hydrolysis and fast pyrolysis. ACS Susbtain. Chem. Eng. 2017, 5, 5208–5213. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, B.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimation the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Zambanini, T.; Kleineberg, W.; Sarikaya, E.; Buescher, J.M.; Meurer, G.; Wierckx, N.; Blank, L.M. Enhanced malic acid production from glycerol with high-cell density Ustilago. trichophora TZ1 cultivations. Biotechnol. Biofuels 2016, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Zambanini, T.; Tehrani, H.H.; Geiser, E.; Merker, D.; Krabbe, J.; Buescher, J.M.; Meurer, G.; Wierckx, N.; Blank, L.M. Efficient itaconic acid production from glycerol with Ustilago. vetiveriae TZ1. Biotechnol. Biofuels 2017, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Le Brech, Y.; Ghislain, T.; Leclerc, S.; Bouroukba, M.; Delmotte, L.; Brosse, N.; Snape, C.; Chaimbault, P.; Dufour, A. Effect of potassium on the mechanisms of biomass pyrolysis studied using complementary analytical techniques. ChemSusChem 2016, 9, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; McDonald, A.G.; Westerhof, R.J.M.; Kersten, S.R.A.; Cuba-Torres, C.M.; Ha, S.; Pecha, B.; Garcia-Perez, M. Effect of cellulose crystallinity on the formation of a liquid intermediate product distribution during pyrolysis. J. Anal. Appl. Pyrolysis 2013, 100, 56–66. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Zheng, A.Q.; Zhao, Z.L.; He, F.; Li, H.B.; Wu, N.N. The comparison of obtaining fermentable sugars from cellulose by enzymatic hydrolysis and fast pyrolysis. Bioresour. Technol. 2016b, 200, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Hosoya, T.; Sakaki, S. Levoglucosan formation from crystalline cellulose: Importance of a hydrogen bonding network in the reaction. ChemSusChem 2013, 6, 2356–2368. [Google Scholar] [CrossRef] [PubMed]
- Mukarakate, C.; Mittal, A.; Ciesielski, P.N.; Budhi, S.; Thompson, L.; Iisa, K.; Nimlos, M.R.; Donohoe, B.S. Influence of crystal allomorph and crystallinity on products and behavior of cellulose during fast pyrolysis. ACS Susbtain. Chem. Eng. 2016, 4, 4662–4674. [Google Scholar] [CrossRef]
- Piskorz, J.; Radlein, D.S.; Scott, D.S.; Czernik, S. Pretreatment of wood and cellulose for production of sugars by fast pyrolysis. J. Anal. Appl. Pyrolysis 1989, 16, 127–142. [Google Scholar] [CrossRef]
- Fuentes, M.E.; Nowakowski, D.J.; Kubacki, M.L.; Cove, J.M.; Bridgeman, T.G.; Jones, J.M. Survey of influence of biomass mineral matter in thermochemical conversion of short rotation willow coppice. J. Energy Inst. 2008, 81, 234–241. [Google Scholar] [CrossRef]
- Wang, K.G.; Zhang, J.; Shanks, B.H.; Brown, R.C. The deleterious effect of inorganic salts on hydrocarbon yields from catalytic pyrolysis of lignocellulosic biomass and its mitigation. Appl. Energy 2015, 148, 115–120. [Google Scholar] [CrossRef]
- Patwardhan, P.R.; Satrio, J.A.; Brown, R.C.; Shanks, B.H. Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 2010, 101, 4646–4655. [Google Scholar] [CrossRef] [PubMed]
- Jarboe, L.R.; Liu, P.; Royce, L.A. Engineering inhibitor tolerance for the production of biorenewable fuels and chemicals. Curr. Opin. Chem. Eng. 2011, 1, 38–42. [Google Scholar] [CrossRef]



| Substrates | Cell mass (g/L) | Glycerol consumption (g/L) | d-Lactate (g/L) | Productivity (g/L DCW) |
|---|---|---|---|---|
| Pure glycerol | 3.7 | 19.6 | 6.1 | 1.7 |
| 220-0.5 | 3.5 | 18.9 | 6.0 | 1.7 |
| 220-1 | 3.5 | 18.9 | 6.0 | 1.7 |
| 220-2 | 3.6 | 18.7 | 6.0 | 1.7 |
| 220-3 | 3.7 | 19.2 | 5.6 | 1.5 |
| 240-0.5 | 3.5 | 19.1 | 6.1 | 1.7 |
| 240-1 | 3.4 | 19.6 | 5.8 | 1.7 |
| 240-2 | 3.7 | 19.3 | 5.3 | 1.4 |
| 240-3 | 3.5 | 19.2 | 5.7 | 1.6 |
| 240-3-10 | 3.6 | 18.6 | 6.0 | 1.7 |
| 240-3-15 | 3.6 | 19.1 | 5.8 | 1.6 |
| 240-3-20 | 3.6 | 19.1 | 5.2 | 1.4 |
| Samples | Recovery rate (%) | Cellulose (wt %) | Hemicellulose (wt %) | Lignin (wt %) |
|---|---|---|---|---|
| Un-treated | — | 32.3 | 28.0 | 24.2 |
| 220-0.5 | 78.9 | 38.2 | 26.8 | 20.6 |
| 220-1 | 64.7 | 50.8 | 20.8 | 16.3 |
| 220-2 | 53.4 | 60.4 | 15.6 | 12.5 |
| 220-3 | 49.6 | 64.4 | 13.1 | 10.9 |
| 240-0.5 | 70.5 | 45.1 | 23.4 | 18.4 |
| 240-1 | 57.2 | 56.3 | 16.9 | 14.7 |
| 240-2 | 50.6 | 63.8 | 13.4 | 11.5 |
| 240-3 | 47.5 | 69.1 | 10.3 | 9.2 |
| 240-3-10 | 49.1 | 65.6 | 11.7 | 10.6 |
| 240-3-15 | 52.3 | 60.5 | 13.4 | 12.5 |
| 240-3-20 | 54.0 | 56.9 | 16.1 | 14.1 |
| Samples | C (wt %) | H (wt %) | N (wt %) | O (wt %) |
|---|---|---|---|---|
| Un-treated | 44.6 | 5.9 | 0.3 | 38.7 |
| 220-0.5 | 44.2 | 6.3 | 0.1 | 41.3 |
| 220-1 | 43.7 | 6.3 | 0.1 | 42.5 |
| 220-2 | 41.8 | 6.0 | 0.1 | 44.9 |
| 220-3 | 43.1 | 6.2 | 0.1 | 44.6 |
| 240-0.5 | 42.8 | 6.3 | 0.1 | 43.8 |
| 240-1 | 42.1 | 6.1 | 0.1 | 45.2 |
| 240-2 | 42.7 | 6.0 | 0.1 | 44.9 |
| 240-3 | 43.1 | 6.0 | 0.1 | 45.0 |
| 240-3-10 | 43.3 | 6.1 | 0.1 | 44.0 |
| 240-3-15 | 43.6 | 6.0 | 0.1 | 42.5 |
| 240-3-20 | 43.8 | 6.1 | 0.1 | 42.1 |
| Samples | AAEMs (mg/kg) | Normalized total AAEMs valencies | ||||
|---|---|---|---|---|---|---|
| Mg | Ca | Na | K | Total | ||
| Un-treated | 566.0 | 1185.2 | 82.4 | 4809.9 | 6643.5 | — |
| 220-0.5 | 294.8 | 993.3 | 40.5 | 190.1 | 1518.7 | 0.3 |
| 220-1 | 275.1 | 740.0 | 30.4 | 145.4 | 1190.9 | 0.3 |
| 220-2 | 274.7 | 482.0 | 17.7 | 120.2 | 894.6 | 0.2 |
| 220-3 | 274.9 | 325.6 | 17.7 | 82.9 | 701.1 | 0.2 |
| 240-0.5 | 306.5 | 802.3 | 39.8 | 182.8 | 1331.4 | 0.3 |
| 240-1 | 282.6 | 527.1 | 29.2 | 139.4 | 978.3 | 0.2 |
| 240-2 | 241.8 | 487.0 | 15.9 | 117.5 | 862.2 | 0.2 |
| 240-3 | 205.2 | 395.3 | 18.7 | 67.7 | 686.9 | 0.1 |
| 240-3-10 | 261.5 | 513.2 | 16.5 | 126.3 | 917.5 | 0.2 |
| 240-3-15 | 255.4 | 669.0 | 28.1 | 152.5 | 1105.0 | 0.2 |
| 240-3-20 | 282.0 | 823.2 | 24.2 | 138.8 | 1268.2 | 0.3 |
| Samples | CrI (%) | Ti (°C) | Tmax (°C) | DTGmax (%/°C) | Residue (%) |
|---|---|---|---|---|---|
| Un-treated | 40.7 | 211.2 | 307.6 | 0.9 | 19.8 |
| 220-0.5 | 45.1 | 246.6 | 330.2 | 1.3 | 16.7 |
| 220-1 | 56.3 | 266.6 | 330.9 | 1.6 | 14.1 |
| 220-2 | 66.9 | 260.4 | 328.6 | 1.6 | 12.1 |
| 220-3 | 69.9 | 283.2 | 327.4 | 1.7 | 11.3 |
| 240-0.5 | 50.7 | 268.7 | 328.0 | 1.6 | 14.8 |
| 240-1 | 62.9 | 266.3 | 326.9 | 1.8 | 14.4 |
| 240-2 | 70.6 | 284.4 | 326.5 | 1.9 | 11.5 |
| 240-3 | 72.9 | 282.7 | 325.0 | 1.7 | 10.3 |
| 240-3-10 | 71.2 | 281.5 | 327.3 | 1.7 | 10.8 |
| 240-3-15 | 67.1 | 277.2 | 330.6 | 1.5 | 12.6 |
| 240-3-20 | 64.8 | 281.5 | 330.6 | 1.5 | 12.6 |
| Samples | Acetic Acid | Furfural | 5-HMF | Levoglucosan |
|---|---|---|---|---|
| Un-treated | 7.8 | 0.8 | 0.4 | 2.2 |
| 220-0.5 | 6.6 | 0.6 | 0.4 | 10.4 |
| 220-1 | 5.8 | 0.6 | 0.4 | 20.0 |
| 220-2 | 4.6 | 0.5 | 0.5 | 27.5 |
| 220-3 | 3.9 | 0.4 | 0.6 | 30.3 |
| 240-0.5 | 6.1 | 0.6 | 0.5 | 19.3 |
| 240-1 | 4.3 | 0.5 | 0.5 | 26.8 |
| 240-2 | 3.1 | 0.5 | 0.6 | 33.0 |
| 240-3 | 2.9 | 0.4 | 0.7 | 35.8 |
| 240-3-10 | 3.2 | 0.5 | 0.7 | 33.1 |
| 240-3-15 | 3.8 | 0.6 | 0.6 | 30.7 |
| 240-3-20 | 4.4 | 0.6 | 0.6 | 28.6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Wu, N.; Zheng, A.; Wang, X.; Liu, M.; Zhao, Z.; He, F.; Li, H.; Feng, X. Effect of Glycerol Pretreatment on Levoglucosan Production from Corncobs by Fast Pyrolysis. Polymers 2017, 9, 599. https://doi.org/10.3390/polym9110599
Jiang L, Wu N, Zheng A, Wang X, Liu M, Zhao Z, He F, Li H, Feng X. Effect of Glycerol Pretreatment on Levoglucosan Production from Corncobs by Fast Pyrolysis. Polymers. 2017; 9(11):599. https://doi.org/10.3390/polym9110599
Chicago/Turabian StyleJiang, Liqun, Nannan Wu, Anqing Zheng, Xiaobo Wang, Ming Liu, Zengli Zhao, Fang He, Haibin Li, and Xinjun Feng. 2017. "Effect of Glycerol Pretreatment on Levoglucosan Production from Corncobs by Fast Pyrolysis" Polymers 9, no. 11: 599. https://doi.org/10.3390/polym9110599
APA StyleJiang, L., Wu, N., Zheng, A., Wang, X., Liu, M., Zhao, Z., He, F., Li, H., & Feng, X. (2017). Effect of Glycerol Pretreatment on Levoglucosan Production from Corncobs by Fast Pyrolysis. Polymers, 9(11), 599. https://doi.org/10.3390/polym9110599