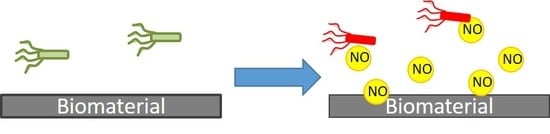
Nitric Oxide Releasing Polymeric Coatings for the Prevention of Biofilm Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Polymer Substrates for Analysis
2.2. Preparation of Aminosilanised Substrates
2.3. Preparation of Diazeniumdiolate-Tethered Substrates
2.4. Contact Angle Analysis
2.5. XPS Analysis
2.6. Atomic Force Microscopy
2.7. Electrochemical NO Detection
2.8. Biofilm CFU Assay
2.9. Statistical Analysis
3. Results
3.1. Surface Wettability: Contact Angle
3.2. XPS Analysis
3.2.1. PET
3.2.2. SE
3.3. Atomic Force Microscopy
3.3.1. PET
3.3.2. SE
3.4. NO Release: Electrochemical Detection
3.4.1. PET
3.4.2. SE
3.5. Bacterial Response
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wells, R.K.; Badyal, J.P.S.; Drummond, I.W.; Robinson, K.S.; Street, F.J. A comparison of plasma-oxidized and photooxidized polystyrene surfaces. Polymer 1993, 34, 3611–3613. [Google Scholar] [CrossRef]
- National Audit Office (NAO). The Management and Control. of Hospital Acquired Infection in Acute nhs Trusts in England; The Stationery Office: London, UK, 2000. [Google Scholar]
- Donlan, R. Biofilms on central venous catheters: Is eradication possible? In Bacterial Biofilms; Springer: Berlin, Germany, 2008; pp. 133–161. [Google Scholar]
- Public Health England (HPA). Healthcare-Associated Infection and Antimicrobial Resistance: 2010–2011; Public Health England: London, UK, 2012.
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Reid, G. Biofilms in infectious disease and on medical devices. Int. J. Antimicrob. Agents 1999, 11, 223–226. [Google Scholar] [CrossRef]
- Fedtke, I.; Götz, F.; Peschel, A. Bacterial evasion of innate host defenses—The staphylococcus aureus lesson. Int. J. Med. Microbiol. 2004, 294, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.; Pereira, M.O. Pseudomonas aeruginosa diversification during infection development in cystic fibrosis lungs—A review. Pathogens 2014, 3, 680–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, V.E.; Filiatrault, M.J.; Picardo, K.F.; Iglewski, B.H. Pseudomonas aeruginosa virulence and pathogenesis. In Pseudomonas: Genomics and Molecular Biology; Caister Academic Press: Norfolk, UK, 2008. [Google Scholar]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-resistant pseudomonas aeruginosa: Clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Donadio, S.; Maffioli, S.; Monciardini, P.; Sosio, M.; Jabes, D. Antibiotic discovery in the twenty-first century: Current trends and future perspectives. J. Antibiot. 2010, 63, 423. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, H.; Solgi, H.; Havaei, S.A.; Shokri, D.; Norouzi Barogh, M.; Zamani, F.Z. Carbapenem and fluoroquinolone resistance in multidrug resistant pseudomonas aeruginosa isolates from al-zahra hospital, isfahan, iran. J. Med. Microbiol. Infect. Dis. 2014, 2, 147–152. [Google Scholar]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- De Nys, R.; Givskov, M.; Kumar, N.; Kjelleberg, S.; Steinberg, P. Furanones. In Antifouling Compounds; Springer: Berlin/Heidelberg, Germany, 2006; pp. 55–86. [Google Scholar]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Higgs, A. The l-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [PubMed]
- Seabra, A.B.; Martins, D.; Simões, M.M.S.G.; Da Silva, R.; Brocchi, M.; De Oliveira, M.G. Antibacterial nitric oxide-releasing polyester for the coating of blood-contacting artificial materials. Artif. Organs 2010, 34, E204–E214. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part ii. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Backlund, C.J.; Worley, B.V.; Schoenfisch, M.H. Anti-biofilm action of nitric oxide-releasing alkyl-modified poly(amidoamine) dendrimers against streptococcus mutans. Acta Biomater. 2016, 29, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Barraud, N.; Kelso, J.M.; Rice, S.A.; Kjelleberg, S. Nitric oxide: A key mediator of biofilm dispersal with applications in infectious diseases. Curr. Pharm. Des. 2015, 21, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Barraud, N.; Schleheck, D.; Klebensberger, J.; Webb, J.S.; Hassett, D.J.; Rice, S.A.; Kjelleberg, S. Nitric oxide signaling in pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-gmp levels, and enhanced dispersal. J. Bacteriol. 2009, 191, 7333–7342. [Google Scholar] [CrossRef] [PubMed]
- Weller, R.B. Nitric oxide-containing nanoparticles as an antimicrobial agent and enhancer of wound healing. J. Investig. Dermatol. 2009, 129, 2335–2337. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Friedman, J. New biomaterials for the sustained release of nitric oxide: Past, present and future. Expert Opin. Drug Deliv. 2009, 6, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Riccio, D.A.; Schoenfisch, M.H. Nitric oxide release: Part i. Macromolecular scaffolds. Chem. Soc. Rev. 2012, 41, 3731–3741. [Google Scholar] [CrossRef] [PubMed]
- Coneski, P.N.; Schoenfisch, M.H. Nitric oxide release: Part iii. Measurement and reporting. Chem. Soc. Rev. 2012, 41, 3753–3758. [Google Scholar] [CrossRef] [PubMed]
- Keefer, L.K.; Nims, R.W.; Davies, K.M.; Wink, D.A. “Nonoates”(1-substituted diazen-1-ium-1, 2-diolates) as nitric oxide donors: Convenient nitric oxide dosage forms. Methods Enzymol. 1996, 268, 281–293. [Google Scholar] [PubMed]
- Fitzhugh, A.L.; Keefer, L.K. Diazeniumdiolates: Pro- and antioxidant applications of the “nonoates”. Free Radic. Biol. Med. 2000, 28, 1463–1469. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Wink, D.A.; Saavedra, J.E.; Keefer, L.K. Chemistry of the diazeniumdiolates. 2. Kinetics and mechanism of dissociation to nitric oxide in aqueous solution. J. Am. Chem. Soc. 2001, 123, 5473–5481. [Google Scholar] [CrossRef] [PubMed]
- Drago, R.S.; Paulik, F.E. The reaction of nitrogen(ii) oxide with diethylamine. J. Am. Chem. Soc. 1960, 82, 96–98. [Google Scholar] [CrossRef]
- Drago, R.S.; Karstetter, B.R. The reaction of nitrogen(ii) oxide with various primary and secondary amines. J. Am. Chem. Soc. 1961, 83, 1819–1822. [Google Scholar] [CrossRef]
- Hrabie, J.A.; Keefer, L.K. Chemistry of the nitric oxide-releasing diazeniumdiolate (“nitrosohydroxylamine”) functional group and its oxygen-substituted derivatives. Chem. Rev. 2002, 102, 1135–1154. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.A.; Carrington, A.L.; Ashe, H.; Bath, S.; Every, L.C.; Griffiths, J.; Hann, A.W.; Hussein, A.; Jackson, N.; Johnson, K.E.; et al. The north-west diabetes foot care study: Incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet. Med. 2002, 19, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Keefer, L.K. Progress toward clinical application of the nitric oxide-releasing diazeniumdiolates. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Goudie, M.J.; Brisbois, E.J.; Pant, J.; Thompson, A.; Potkay, J.A.; Handa, H. Characterization of an s-nitroso-n-acetylpenicillamine—Based nitric oxide releasing polymer from a translational perspective. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, M.M.; Reoma, S.L.; Fleser, P.S.; Nuthakki, V.K.; Callahan, R.E.; Shanley, C.J.; Politis, J.K.; Elmore, J.; Merz, S.I.; Meyerhoff, M.E. More lipophilic dialkyldiamine-based diazeniumdiolates: Synthesis, characterization, and application in preparing thromboresistant nitric oxide release polymeric coatings. J. Med. Chem. 2003, 46, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Nablo, B.J.; Prichard, H.L.; Butler, R.D.; Klitzman, B.; Schoenfisch, M.H. Inhibition of implant-associated infections via nitric oxide release. Biomaterials 2005, 26, 6984–6990. [Google Scholar] [CrossRef] [PubMed]
- Nablo, B.J.; Schoenfisch, M.H. Poly(vinyl chloride)-coated sol−gels for studying the effects of nitric oxide release on bacterial adhesion. Biomacromolecules 2004, 5, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Marxer, S.M.; Rothrock, A.R.; Nablo, B.J.; Robbins, M.E.; Schoenfisch, M.H. Preparation of nitric oxide (no)-releasing sol−gels for biomaterial applications. Chem. Mater. 2003, 15, 4193–4199. [Google Scholar] [CrossRef]
- Shin, J.H.; Marxer, S.M.; Schoenfisch, M.H. Nitric oxide-releasing sol−gel particle/polyurethane glucose biosensors. Anal. Chem. 2004, 76, 4543–4549. [Google Scholar] [CrossRef] [PubMed]
- Stasko, N.A.; Schoenfisch, M.H. Dendrimers as a scaffold for nitric oxide release. J. Am. Chem. Soc. 2006, 128, 8265–8271. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Davies, I.R.; Zhang, X. Nitric oxide selective electrodes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 436, pp. 63–95. [Google Scholar]
- Wadsworth, R.; Stankevicius, E.; Simonsen, U. Physiologically relevant measurements of nitric oxide in cardiovascular research using electrochemical microsensors. J. Vasc. Res. 2006, 43, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, U.; Wadsworth, R.M.; Buus, N.H.; Mulvany, M.J. In vitro simultaneous measurements of relaxation and nitric oxide concentration in rat superior mesenteric artery. J. Physiol. 1999, 516, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.A.; Steinert, J.R. Characterisation and comparison of temporal release profiles of nitric oxide generating donors. J. Neurosci. Methods 2015, 245, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Urbach, J.M.; Wu, G.; Liberati, N.T.; Feinbaum, R.L.; Miyata, S.; Diggins, L.T.; He, J.; Saucier, M.; Deziel, E.; et al. Genomic analysis reveals that pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006, 7, R90. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chaudhury, M.K.; Owen, M.J. Hydrophobic recovery of polydimethylsiloxane elastomer exposed to partial electrical discharge. J. Colloid Interface Sci. 2000, 226, 231–236. [Google Scholar] [CrossRef]
- Zhu, M.; Lerum, M.Z.; Chen, W. How to prepare reproducible, homogeneous, and hydrolytically stable aminosilane-derived layers on silica. Langmuir 2011, 28, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, V.; Suvanto, P.; Franssila, S. Oxygen and nitrogen plasma hydrophilization and hydrophobic recovery of polymers. Biomicrofluidics 2012, 6, 16501–1650110. [Google Scholar] [CrossRef] [PubMed]
- Morra, M.; Occhiello, E.; Marola, R.; Garbassi, F.; Humphrey, P.; Johnson, D. On the aging of oxygen plasma-treated polydimethylsiloxane surfaces. J. Colloid Interface Sci. 1990, 137, 11–24. [Google Scholar] [CrossRef]
- Fritz, J.L.; Owen, M.J. Hydrophobic recovery of plasma-treated polydimethylsiloxane. J. Adhes. 1995, 54, 33–45. [Google Scholar] [CrossRef]
- Owen, M.J.; Smith, P.J. Plasma treatment of polydimethylsiloxane. J. Adhes. Sci. Technol. 1994, 8, 1063–1075. [Google Scholar] [CrossRef]
- Rubbo, H.; Radi, R.; Trujillo, M.; Telleri, R.; Kalyanaraman, B.; Barnes, S.; Kirk, M.; Freeman, B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994, 269, 26066–26075. [Google Scholar] [PubMed]
- Miranda, K.; Espey, M.; Jourd’heuil, D.; Grisham, M.; Fukuto, J.; Freelisch, M.; Wink, D. Nitric Oxide: Biology and Pathobiology; Ignarro, L.J., Ed.; Academic Press: New York, NY, USA, 2000; pp. 41–55. [Google Scholar]
- Raulli, R.; McElhaney-Feser, G.; Hrabie, J.; Cihlar, R. Antimicrobial properties of nitric oxide using diazeniumdiolates as the nitric oxide donor. Recent Res. Dev. Microbiol. 2002, 6, 177–183. [Google Scholar]
- Schoenfisch, M.H.; Mowery, K.A.; Rader, M.V.; Baliga, N.; Wahr, J.A.; Meyerhoff, M.E. Improving the thromboresistivity of chemical sensors via nitric oxide release: Fabrication and in vivo evaluation of no-releasing oxygen-sensing catheters. Anal. Chem. 2000, 72, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Mowery, K.A.; Schoenfisch, H.M.; Saavedra, J.E.; Keefer, L.K.; Meyerhoff, M.E. Preparation and characterization of hydrophobic polymeric films that are thromboresistant via nitric oxide release. Biomaterials 2000, 21, 9–21. [Google Scholar] [CrossRef]
- Nablo, B.J.; Schoenfisch, M.H. In vitro cytotoxicity of nitric oxide-releasing sol–gel derived materials. Biomaterials 2005, 26, 4405–4415. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Schoenfisch, M.H. Antibacterial nitric oxide-releasing xerogels: Cell viability and parallel plate flow cell adhesion studies. Biomaterials 2007, 28, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C.M.; Morley, D.; Wink, D.A.; Dunams, T.M.; Saavedra, J.E.; Hoffman, A.; Bove, A.A.; Isaac, L.; Hrabie, J.A.; Keefer, L.K. Complexes of ·NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 1991, 34, 3242–3247. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, R.O.; Karstetter, B.R.; Drago, R.S. Decomposition of the adducts of diethylamine and isopropylamine with nitrogen(ii) oxide. Inorg. Chem. 1965, 4, 420–422. [Google Scholar] [CrossRef]









| Surface | Contact Angle (°) | |
|---|---|---|
| PET | SE | |
| Pristine | 88.1 ± 1.0 | 113.0 ± 2.3 |
| Plasma treated | 19.7 ± 1.8 | 11.0 ± 1.2 |
| DET3 | 90.2 ± 0.9 | 116.6 ± 1.8 |
| DET3/NO | 79.0 ± 0.4 | 108.8 ± 2.0 |
| PTMSPA | 88.5 ± 0.3 | 119.2 ± 1.5 |
| PTMSPA/NO | 81.7 ± 1.9 | 108.6 ± 2.8 |
| Sample | at % | |||
|---|---|---|---|---|
| C 1s | O 1s | N 1s | Si 2p | |
| PET | 73.0 ± 0.4 | 27.0 ± 0.4 | - | - |
| PETox | 66.0 ± 0.2 | 34.0 ± 0.2 | - | - |
| PET-DET3 | 56.0 ± 1.1 | 22.5 ± 0.2 | 4.7 ± 0.3 | 16.8 ± 1.2 |
| PET-DET3/NO | 57.2 ± 0.3 | 26.9 ± 0.1 | 5.7 ± 0.7 | 10.2 ± 0.4 |
| PET-PTMSPA | 61.7 ± 1.1 | 25.4 ± 0.2 | 3.3 ± 0.3 | 9.6 ± 0.6 |
| PET-PTMSPA/NO | 59.6 ± 0.6 | 25.5 ± 0.6 | 4.5 ± 0.9 | 10.4 ± 1.4 |
| SE | 38.2 ± 1.5 | 32.2 ± 1.2 | - | 29.6 ± 0.3 |
| SEox | 35.4 ± 1.5 | 35.1 ± 1.6 | - | 29.6 ± 0.5 |
| SE-DET3 | 41.3 ± 1.2 | 28.4 ± 1.0 | 3.7 ± 0.5 | 26.6 ± 0.7 |
| SE-DET3/NO | 33.8 ± 0.9 | 35.1 ± 0.7 | 2.4 ± 0.2 | 29.4 ± 0.7 |
| SE-PTMSPA | 40.5 ± 1.5 | 30.0 ± 1.3 | 1.8 ± 0.0 | 27.7 ± 0.3 |
| SE-PTMSPA/NO | 37.1 ± 0.4 | 33.4 ± 0.4 | 1.6 ± 0.1 | 28.0 ± 0.0 |
| Sample | at % | |||||
|---|---|---|---|---|---|---|
| C 1s | N 1s | |||||
| C–H, C–C | O–CH2CH2 | O=C–O | N–H | N+ | N–O | |
| PET | 59.6 ± 0.2 | 24.0 ± 0.2 | 16.5 ± 0.2 | - | - | - |
| PETox | 41.1 ± 0.6 | 37.4 ± 0.6 | 21.5 ± 0.1 | - | - | - |
| PET-DET3 | 54.8 ± 3.7 | 40.4 ± 3.4 | 4.8 ± 0.7 | 52.0 ± 0.9 | 48.0 ± 0.9 | - |
| PET-DET3/NO | 62.0 ± 2.7 | 26.4 ± 2.1 | 11.6 ± 0.6 | 34.7 ± 0.5 | 32.7 ± 0.2 | 32.7 ± 0.3 |
| PET-PTMSPA | 69.9 ± 0.3 | 19.4 ± 0.8 | 10.7 ± 0.7 | 68.7 ± 1.2 | 31.3 ± 1.2 | - |
| PET-PTMSPA/NO | 66.2 ± 1.7 | 25.4 ± 0.9 | 8.6 ± 0.9 | 28.1 ± 3.1 | 35.9 ± 1.5 | 35.9 ± 1.5 |
| Sample | at % | ||||
|---|---|---|---|---|---|
| C 1s | N 1s | ||||
| C–H, C–C, C–Si | C–O | N–H | N+ | N–O | |
| SE | 100.0 ± 0.0 | - | - | - | - |
| SEox | 82.2 ± 2.9 | 17.8 ± 2.9 | - | - | - |
| SE-DET3 | 62.0 ± 6.2 | 38.0 ± 6.2 | 54.8 ± 3.2 | 45.2 ± 3.2 | - |
| SE-DET3/NO | 77.3 ± 5.3 | 22.7 ± 5.3 | 39.3 ± 0.2 | 30.4 ± 0.1 | 30.4 ± 0.1 |
| SE-PTMSPA | 70.9 ± 2.6 | 29.1 ± 2.6 | 66.6 ± 0.2 | 33.4 ± 0.2 | - |
| SE-PTMSPA/NO | 79.0 ± 2.5 | 21.0 ± 2.5 | 43.8 ± 4.5 | 28.1 ± 2.2 | 28.1 ± 2.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleming, G.; Aveyard, J.; Fothergill, J.L.; McBride, F.; Raval, R.; D’Sa, R.A. Nitric Oxide Releasing Polymeric Coatings for the Prevention of Biofilm Formation. Polymers 2017, 9, 601. https://doi.org/10.3390/polym9110601
Fleming G, Aveyard J, Fothergill JL, McBride F, Raval R, D’Sa RA. Nitric Oxide Releasing Polymeric Coatings for the Prevention of Biofilm Formation. Polymers. 2017; 9(11):601. https://doi.org/10.3390/polym9110601
Chicago/Turabian StyleFleming, George, Jenny Aveyard, Joanne L. Fothergill, Fiona McBride, Rasmita Raval, and Raechelle A. D’Sa. 2017. "Nitric Oxide Releasing Polymeric Coatings for the Prevention of Biofilm Formation" Polymers 9, no. 11: 601. https://doi.org/10.3390/polym9110601
APA StyleFleming, G., Aveyard, J., Fothergill, J. L., McBride, F., Raval, R., & D’Sa, R. A. (2017). Nitric Oxide Releasing Polymeric Coatings for the Prevention of Biofilm Formation. Polymers, 9(11), 601. https://doi.org/10.3390/polym9110601