Ionic Conductivity and Assembled Structures of Imidazolium Salt-Based Block Copolymers with Thermoresponsive Segments
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Block Copolymers
2.3. Anion-Exchange Reactions of the Block Copolymers
2.4. Instrumentation
3. Results and Discussion
3.1. Synthesis and Anion-Exchange Reaction of Imidazolium Salt-Based Block Copolymers
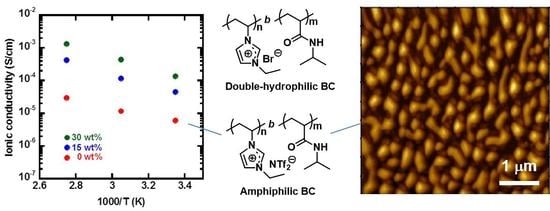
3.2. Self-Assembly and Ionic Conductivity of Amphiphilic Block Copolymers
3.3. Self-Assembly and Ionic Conductivity of Double-Hydrophilic Block Copolymers
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Green, O.; Grubjesic, S.; Lee, S.; Firestone, M.A. The Design of Polymeric Ionic Liquids for the Preparation of Functional Materials. Polym. Rev. 2009, 49, 339–360. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Marcilla, R.; Mecerreyes, D. Recent Advances in Innovative Polymer Electrolytes based on Poly(ionic liquid)s. Electrochem. Acta 2015, 175, 18–34. [Google Scholar] [CrossRef]
- Yuan, J.; Mecerreyes, D.; Antonietti, M. Poly(ionic liquid)s: An update. Prog. Polym. Sci. 2013, 38, 1009–1036. [Google Scholar] [CrossRef]
- Choi, J.-H.; Ye, Y.; Elabd, Y.A.; Winey, K.I. Network Structure and Strong Microphase Separation for High Ion Conductivity in Polymerized Ionic Liquid Block Copolymers. Macromolecules 2013, 46, 5290–5300. [Google Scholar] [CrossRef]
- Schneider, Y.; Modestino, M.A.; McCulloch, B.L.; Hoarfrost, M.L.; Hess, R.W.; Segalman, R.A. Ionic Conduction in Nanostructured Membranes Based on Polymerized Protic Ionic Liquids. Macromolecules 2013, 46, 1543–1548. [Google Scholar] [CrossRef]
- Ye, Y.; Choi, J.-H.; Winey, K.I.; Elabd, Y.A. Polymerized Ionic Liquid Block and Random Copolymers: Effect of Weak Microphase Separation on Ion Transport. Macromolecules 2012, 45, 7027–7035. [Google Scholar] [CrossRef]
- Green, M.D.; Choi, J.-H.; Winey, K.I.; Long, T.E. Synthesis of Imidazolium-Containing ABA Triblock Copolymers: Role of Charge Placement, Charge Density, and Ionic Liquid Incorporation. Macromolecules 2012, 45, 4749–4757. [Google Scholar] [CrossRef]
- Evans, C.M.; Sanoja, G.E.; Popere, B.C.; Segalrnan, R.A. Anhydrous Proton Transport in Polymerized Ionic Liquid Block Copolymers: Roles of Block Length, Ionic Content, and Confinement. Macromolecules 2016, 49, 395–404. [Google Scholar] [CrossRef]
- Margaretta, E.; Fahs, G.B.; Inglefield, J.; David, L.; Jangu, C.; Wang, D.; Heflin, J.R.; Moore, R.B.; Long, T.E. Imidazolium-Containing ABA Triblock Copolymers as Electroactive Devices. ACS Appl. Mater. Interfaces 2016, 8, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Pont, A.-L.; Marcilla, R.; De Meatza, I.; Grande, H.; Mecerreyes, D. Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes. J. Power Sources 2009, 188, 558–563. [Google Scholar] [CrossRef]
- Meek, K.M.; Elabd, Y.A. Sulfonated Polymerized Ionic Liquid Block Copolymers. Macromol. Rapid Commun. 2016, 37, 1200–1206. [Google Scholar] [CrossRef] [PubMed]
- Green, M.D.; Allen, M.H., Jr.; Dennis, J.M.; Salas-de la Cruz, D.; Gao, R.; Winey, K.I.; Long, T.E. Tailoring macromolecular architecture with imidazole functionality: A perspective for controlled polymerization processes. Eur. Polym. J. 2011, 47, 486–496. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Mori, H. Recent progress in controlled radical polymerization of N-vinyl monomers. Eur. Polym. J. 2013, 49, 2808–2838. [Google Scholar] [CrossRef]
- Texter, J.; Vasantha, V.A.; Crombez, R.; Maniglia, R.; Slater, L.; Mourey, T. Triblock Copolymer Based on Poly(propylene oxide) and Poly(1-[11-acryloylundecyl]-3-methyl-imidazolium bromide). Macromol. Rapid Commun. 2012, 33, 69–74. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Luebke, D.; Nuwala, H.; Matyjaszewski, K. Synthesis of Poly(ionic liquid)s by Atom Transfer Radical Polymerization with ppm of Cu Catalyst. Macromolecules 2014, 47, 6601–6609. [Google Scholar] [CrossRef]
- Mori, H.; Yahagi, M.; Endo, T. RAFT Polymerization of N-vinylimidazolium salts and synthesis of thermoresponsive ionic liquid block copolymers. Macromolecules 2009, 42, 8082–8092. [Google Scholar] [CrossRef]
- Yang, J.; Sun, W.; Lin, W.; Shen, Z. Synthesis and magnetic properties of comb-like copolymeric complexes based on thiazole ring and ionic liquid. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 5123–5132. [Google Scholar] [CrossRef]
- Yuan, J.; Schlaad, H.; Giordano, C.; Antonietti, M. Double hydrophilic diblock copolymers containing a poly(ionic liquid) segment: Controlled synthesis, solution property, and application as carbon precursor. Eur. Polym. J. 2011, 47, 772–781. [Google Scholar] [CrossRef]
- Vijayakrishna, K.; Jewrajka, S.K.; Ruiz, A.; Marcilla, R.; Pomposo, J.A.; Mecerreyes, D.; Taton, D.; Gnanou, Y. Synthesis by RAFT and Ionic Responsiveness of Double Hydrophilic Block Copolymers Based on Ionic Liquid Monomer Units. Macromolecules 2008, 41, 6299–6308. [Google Scholar] [CrossRef]
- Mori, H.; Ebina, Y.; Kambara, R.; Nakabayashi, K. Temperature-responsive self-assembly of star block copolymers with poly(ionic liquid) segments. Polym. J. 2012, 44, 550–560. [Google Scholar] [CrossRef]
- Detrembleur, C.; Debuigne, A.; Hurtgen, M.; Jerome, C.; Pinaud, J.; Fevre, M.; Coupillaud, P.; Vignolle, J.; Taton, D. Synthesis of 1-Vinyl-3-ethylimidazolium-Based Ionic Liquid (Co)polymers by Cobalt-Mediated Radical Polymerization. Macromolecules 2011, 44, 6397–6404. [Google Scholar] [CrossRef]
- Cordella, D.; Kermagoret, A.; Debuigne, A.; Jerome, C.; Mecerreyes, D.; Isik, M.; Taton, D.; Detrembleur, C. All Poly(ionic liquid)-Based Block Copolymers by Sequential Controlled Radical Copolymerization of Vinylimidazolium Monomers. Macromolecules 2015, 48, 5230–5243. [Google Scholar] [CrossRef]
- Cordella, D.; Ouhib, F.; Aqil, A.; Defize, T.; Jerome, C.; Serghei, A.; Drockenmuller, E.; Aissou, K.; Taton, D.; Detrembleur, C. Fluorinated Poly(ionic liquid) Diblock Copolymers Obtained by Cobalt-Mediated Radical Polymerization-Induced Self-Assembly. ACS Macro Lett. 2017, 6, 121–126. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Umeda, A.; Sato, Y.; Mori, H. Synthesis of 1,2,4-triazolium salt-based polymers and block copolymers by RAFT polymerization: Ion conductivity and assembled structures. Polymer 2016, 96, 81–93. [Google Scholar] [CrossRef]
- Hall, C.C.; Zhou, C.; Danielsen, S.P.O.; Lodge, T.P. Formation of Multicompartment Ion Gels by Stepwise Self-Assembly of a Thermoresponsive ABC Triblock Terpolymer in an Ionic Liquid. Macromolecules 2016, 49, 2298–2306. [Google Scholar] [CrossRef]
- Ribot, J.C.; Guerrero-Sanchez, C.; Greaves, T.L.; Kennedy, D.F.; Hoogenboom, R.; Schubert, U.S. Amphiphilic oligoether-based ionic liquids as functional materials for thermoresponsive ion gels with tunable properties via aqueous gelation. Soft Matter 2012, 8, 1025–1032. [Google Scholar] [CrossRef]
- Soni, S.S.; Fadadu, K.B.; Gibaud, A. Ionic Conductivity through Thermoresponsive Polymer Gel: Ordering Matters. Langmuir 2012, 28, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Ribot, J.C.; Guerrero-Sanchez, C.; Hoogenboom, R.; Schubert, U.S. Aqueous gelation of ionic liquids: Reverse thermoresponsive ion gels. Chem. Commun. 2010, 43, 6971–6973. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Thermo-sensitive polymeric micelles based on poly(N-isopropylacrylamide) as drug carriers. Prog. Polym. Sci. 2009, 34, 893–910. [Google Scholar] [CrossRef]
- Rzaev, Z.M.O.; Dincer, S.; Piskin, E. Functional copolymers of N-isopropylacrylamide for bioengineering applications. Prog. Polym. Sci. 2007, 32, 534–595. [Google Scholar] [CrossRef]
- Marcilla, R.; Blazquez, J.A.; Rodriguez, J.; Pomposo, J.A.; Mecerreyes, D. Tuning the solubility of polymerized ionic liquids by simple anion-exchange reactions. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 208–212. [Google Scholar] [CrossRef]
- Green, M.D.; Salas-de la Cruz, D.; Ye, Y.; Layman, J.M.; Elabd, Y.A.; Winey, K.I.; Long, T.E. Alkyl-Substituted N-Vinylimidazolium Polymerized Ionic Liquids: Thermal Properties and Ionic Conductivities. Macromol. Chem. Phys. 2011, 212, 2522–2528. [Google Scholar] [CrossRef]
- Ogihara, W.; Suzuki, N.; Nakamura, N.; Ohno, H. Electrochemical and spectroscopic analyses of lithium ion-conductive polymers prepared by the copolymerization of ionic liquid monomer with lithium salt monomer. Polym. J. 2006, 38, 117–121. [Google Scholar]
- Dimitrov-Raytchev, P.; Beghdadi, S.; Serghei, A.; Drockenmuller, E. Main-chain 1,2,3-triazolium-based poly(ionic liquid)s issued from AB plus AB click chemistry polyaddition. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 34–38. [Google Scholar]
- Sood, R.; Obadia, M.M.; Mudraboyina, B.P.; Zhang, B.; Serghei, A.; Bernard, J.; Drockenmuller, E. 1,2,3-Triazolium-based poly(acrylate ionic liquid)s. Polymer 2014, 55, 3314–3319. [Google Scholar]
- Obadia, M.M.; Mudraboyina, B.P.; Serghei, A.; Phan, T.N.T.; Gigmes, D.; Drockenmuller, E. Enhancing Properties of Anionic Poly(ionic liquid)s with 1,2,3-Triazolium Counter Cations. ACS Macro Lett. 2014, 3, 658–662. [Google Scholar]
- Nakabayashi, K.; Higashihara, T.; Ueda, M. Highly Sulfonated Multiblock Copoly(ether sulfone)s for Fuel Cell Membranes. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2757–2764. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Z.; Holdcroft, S. Synthesis of sulfonated polysulfone-block-PVDF copolymers: Enhancement of proton conductivity in low ion exchange capacity membranes. Macromolecules 2004, 37, 1678–1681. [Google Scholar] [CrossRef]
- Ding, J.; Chuy, C.; Holdcroft, S. Solid polymer electrolytes based on ionic graft polymers: Effect of graft chain length on nano-structured, ionic networks. Adv. Funct. Mater. 2002, 12, 389–394. [Google Scholar] [CrossRef]
- Biswas, C.S.; Patel, V.K.; Vishwakarma, N.K.; Tiwari, V.K.; Maiti, B.; Maiti, P.; Kamigaito, M.; Okamoto, Y.; Ray, B. Effects of Tacticity and Molecular Weight of Poly(N-isopropylacrylamide) on Its Glass Transition Temperature. Macromolecules 2011, 44, 5822–5824. [Google Scholar] [CrossRef]








| Composition b (NVI/NIPAM) | Mw/Mn c | Double-Hydrophilic BC Mn, d Poly(NVI-Br)/Poly(NIPAM) | Amphiphilic BC Mn, d Poly(NVI-NTf2)/Poly(NIPAM) |
|---|---|---|---|
| 68/32 | 1.39 | 13,100/6300 | 26,000/6300 |
| 48/52 | 1.26 | 6100/6600 | 12,100/6600 |
| 21/79 | 1.38 | 9800/36,900 | 19,500/36,900 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakabayashi, K.; Sato, Y.; Isawa, Y.; Lo, C.-T.; Mori, H. Ionic Conductivity and Assembled Structures of Imidazolium Salt-Based Block Copolymers with Thermoresponsive Segments. Polymers 2017, 9, 616. https://doi.org/10.3390/polym9110616
Nakabayashi K, Sato Y, Isawa Y, Lo C-T, Mori H. Ionic Conductivity and Assembled Structures of Imidazolium Salt-Based Block Copolymers with Thermoresponsive Segments. Polymers. 2017; 9(11):616. https://doi.org/10.3390/polym9110616
Chicago/Turabian StyleNakabayashi, Kazuhiro, Yu Sato, Yuta Isawa, Chen-Tsyr Lo, and Hideharu Mori. 2017. "Ionic Conductivity and Assembled Structures of Imidazolium Salt-Based Block Copolymers with Thermoresponsive Segments" Polymers 9, no. 11: 616. https://doi.org/10.3390/polym9110616
APA StyleNakabayashi, K., Sato, Y., Isawa, Y., Lo, C. -T., & Mori, H. (2017). Ionic Conductivity and Assembled Structures of Imidazolium Salt-Based Block Copolymers with Thermoresponsive Segments. Polymers, 9(11), 616. https://doi.org/10.3390/polym9110616