Effects of Particle Size on the Morphology and Water- and Thermo-Resistance of Washed Cottonseed Meal-Based Wood Adhesives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bonded Wood Specimens
2.3. Adhesive Strength Testing
2.4. Optical Microscopy
2.5. Scanning Electron Microscopy (SEM) and Scanning Electron Microscopy-Energy Dispersive Spectroscopy (SEM-EDS)
3. Results and Discussion
3.1. Morphology of WCSM Prodcuts
3.2. Surface Compostion of WCSM Products
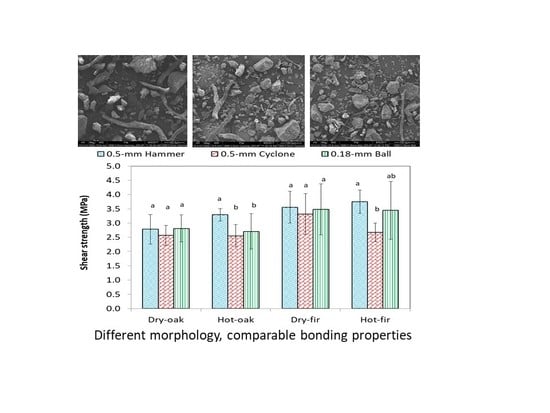
3.3. Effects of Particle Size of WCSM on the Shear Strength of White Oak and Douglas Fir Bonding Joints
3.4. Effects of Particle Size of WCSM on the Water Resistance of White Oak and Douglas Fir Bonding Joints
3.5. Microscopic Examination of the Bondline Features
3.6. Speculation for Practical Applications
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pizzi, A.; Mittal, K.L. (Eds.) Wood Adhesives; CRC Press: Boca Raton, FL, USA, 2011; p. 451. [Google Scholar]
- He, Z. (Ed.) Bio-Based Wood Adhesives: Preparation, Characterization, and Testing; CRC Press: Boca Raton, FL, USA, 2017; p. 356. [Google Scholar]
- Santoni, I.; Pizzo, B. Evaluation of alternative vegetable proteins as wood adhesives. Ind. Crops Prod. 2013, 45, 148–154. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Yuan, C.; Zhang, W.; Li, J.; Gao, Q.; Chen, H. An eco-friendly wood adhesive from soy protein and lignin: Performance properties. RSC Adv. 2015, 5, 100849–100855. [Google Scholar] [CrossRef]
- Qi, G.; Li, N.; Sun, X.S.; Wang, D. Adhesion properties of soy protein subunits and protein adhesive modification. In Bio-Based Wood Adhesives: Preparation, Characterization, and Testing; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 59–85. [Google Scholar]
- Li, J.; Li, X.; Li, J.; Gao, Q. Investigating the use of peanut meal: A potential new resource for wood adhesives. RSC Adv. 2015, 5, 80136–80141. [Google Scholar] [CrossRef]
- Li, N.; Qi, G.; Sun, X.S.; Wang, D. Canola protein and oil-based wood adhesives. In Bio-Based Wood Adhesives: Preparation, Characterization, and Testing; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 111–139. [Google Scholar]
- Cheng, H.N.; He, Z. Wood adhesives containing proteins and carbohydrates. In Bio-Based Wood Adhesives: Preparation, Characterization, and Testing; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 140–155. [Google Scholar]
- Ghahri, S.; Mohebby, B.; Pizzi, A.; Mirshokraie, A.; Mansouri, H.R. Improving water resistance of soy-based adhesive by vegetable tannin. J. Polym. Environ. 2017. [Google Scholar] [CrossRef]
- Zheng, P.; Li, Y.; Li, F.; Ou, Y.; Lin, Q.; Chen, N. Development of defatted soy flour-based adhesives by acid hydrolysis of carbohydrates. Polymers 2017, 9, 153. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; Zhang, J.; Gao, Q.; Zhang, S.; Li, J. Physico-chemical properties of soybean meal-based adhesives reinforced by ethylene glycol diglycidyl ether and modified nanocrystalline cellulose. Polymers 2017, 9, 463. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N. Preparation and utilization of water washed cottonseed meal as wood adhesives. In Bio-Based Wood Adhesives: Preparation, Characterization, and Testing; He, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 156–178. [Google Scholar]
- Cheng, H.N.; Dowd, M.K.; He, Z. Investigation of modified cottonseed protein adhesives for wood composites. Ind. Crops Prod. 2013, 46, 399–403. [Google Scholar] [CrossRef]
- Cheng, H.N.; Ford, C.; Dowd, M.K.; He, Z. Effects of phosphorus-containing additives on soy and cottonseed protein as wood adhesives. Int. J. Adhes. Adhes. 2017, 77, 51–57. [Google Scholar] [CrossRef]
- Cheng, H.N.; Ford, C.V.; Dowd, M.K.; He, Z. Soy and cottonseed protein blends as wood adhesives. Ind. Crops Prod. 2016, 85, 324–330. [Google Scholar] [CrossRef]
- He, Z.; Chapital, D.C.; Cheng, H.N.; Dowd, M.K. Comparison of adhesive properties of water- and phosphate buffer-washed cottonseed meals with cottonseed protein isolate on maple and poplar veneers. Int. J. Adhes. Adhes. 2014, 50, 102–106. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N.; Chapital, D.C.; Dowd, M.K. Sequential fractionation of cottonseed meal to improve its wood adhesive properties. J. Am. Oil Chem. Soc. 2014, 91, 151–158. [Google Scholar] [CrossRef]
- He, Z.; Klasson, K.T.; Wang, D.; Li, N.; Zhang, H.; Zhang, D.; Wedegaertner, T.C. Pilot-scale production of washed cottonseed meal and co-products. Modern Appl. Sci. 2016, 10, 25–33. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N. Evaluation of wood bonding performance of water-washed cottonseed meal-based adhesives with high solid contents and low press temperatures. J. Adhes. Sci. Technol. 2017, 31, 2620–2629. [Google Scholar] [CrossRef]
- Li, N.; Prodyawong, S.; He, Z.; Sun, X.S.; Wang, D. Effect of drying methods on the physicochemical properties and adhesion performance of water-washed cottonseed meal. Ind. Crops Prod. 2017, 109, 281–287. [Google Scholar] [CrossRef]
- He, Z.; Chiozza, F. Adhesive strength of pilot-scale-produced water-washed cottonseed meal in comparison with a synthetic glue for non-structural interior application. J. Mater. Sci. Res. 2017, 6, 20–26. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Olk, D.C. Chemical composition of defatted cottonseed and soy meal products. PLoS ONE 2015, 10, e0129933. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Uchimiya, S.M.; Guo, M. Production and characterization of biochar from agricultural by-products: Overview and use of cotton biomass residues. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; pp. 63–86. [Google Scholar]
- American National Standards Institute (ANSI); Hardwood Plywood and Veneer Association (HPVA). American National Standard for Hardwood and Decorative Plywood; The Hardwood Plywood & Veneer Association: Reston, VA, USA, 2016. [Google Scholar]
- He, Z.; Chapital, D.C.; Cheng, H.N.; Olanya, O.M. Adhesive properties of water-washed cottonseed meal on four types of wood. J. Adhes. Sci. Technol. 2016, 30, 2109–2119. [Google Scholar] [CrossRef]
- Modzel, G.; Kamke, F.; De Carlo, F. Comparative analysis of a wood: Adhesive bondline. Wood Sci. Technol. 2011, 45, 147–158. [Google Scholar] [CrossRef]
- He, Z.; Chapital, D.C. Preparation and testing of plant seed meal-based wood adhesives. J. Vis. Exp. 2015, 97, e52557. [Google Scholar] [CrossRef] [PubMed]
- British Standards. EN204 Classification of Thermoplastic Wood Adhesives for Non-Structural Applications; European Committee for Standardization (CEN): Brussels, Belgium, 2016. [Google Scholar]
- British Standards. EN14257 Adhesives—Wood Adhesives—Determination of Tensile Strength of Lap Joints at Elevated Temperature (WATT’91); European Committee for Standardization (CEN): Brussels, Belgium, 2006. [Google Scholar]
- Gazulla, M.; Rodrigo, M.; Blasco, E.; Orduna, M. Nitrogen determination by SEM-EDS and elemental analysis. X-ray Spectrom. 2013, 42, 394–401. [Google Scholar] [CrossRef]
- Hou, D.; Lin, D.; Zhao, C.; Wang, J.; Fu, C. Control of protein (BSA) fouling by ultrasonic irradiation during membrane distillation process. Sep. Purif. Technol. 2017, 175, 287–297. [Google Scholar] [CrossRef]
- Muruganantham, S.; Anbalagan, G.; Ramamurthy, N. FT-IR and SEM-EDS comparative analysis of medicinal plants, Eclipta alba Hassk and Eclipta prostrata Linn. Rom. J. Biophys. 2009, 19, 285–294. [Google Scholar]
- Luna-Valdez, J.G.; Balandran-Quintana, R.R.; Azamar-Barrios, J.A.; Ramos Clamont-Montfort, G.; Mendoza-Wilson, A.M.; Mercado-Ruiz, J.N.; Madera-Santana, T.J.; Rascon-Chu, A.; Chaquilla-Quilca, G. Structural and physicochemical characterization of nanoparticles synthesized from an aqueous extract of wheat bran by a cold-set gelation/desolvation approach. Food Hydrocoll. 2017, 62, 165–173. [Google Scholar] [CrossRef]
- Miculescu, F.; Jepu, I.; Porosnicu, C.; Lungu, C.; Miculescu, M.; Burhala, B. A study on the influence of the primary electronbeam on nanodimensional layers analysis. Dig. J. Nanomater. Biostruct. 2011, 6, 307–317. [Google Scholar]
- He, Z.; Chapital, D.C.; Cheng, H.N. Effects of pH and storage time on the adhesive and rheological properties of cottonseed meal-based products. J. Appl. Polym. Sci. 2016, 133, 43637. [Google Scholar] [CrossRef]
- Xiao, Z.; Li, Y.; Wu, X.; Qi, G.; Li, N.; Zhang, K.; Wang, D.; Sun, X.S. Utilization of sorghum lignin to improve adhesion strength of soy protein adhesives on wood veneer. Ind. Crops Prod. 2013, 50, 501–509. [Google Scholar] [CrossRef]
- Pradyawong, S.; Qi, G.; Li, N.; Sun, X.S.; Wang, D. Adhesion properties of soy protein adhesives enhanced by biomass lignin. Int. J. Adhes. Adhes. 2017, 75, 66–73. [Google Scholar] [CrossRef]
- Premjai, P.; Khammuang, P.; Somord, K.; Tawichai, N.; Intatha, U.; Soykeabkaew, N. Bio-based composites of sugarcane bagasse: Effect of bagasse particle size. In Proceedings of the 26th Annual Meeting of the Thai Society for Biotechnology and International Conference, Thai Society for Biotechnology (TSB) and Mae Fah Lunag University (School of Science), Chiang Rai, Thailand, 26–29 November 2014. [Google Scholar]
- Nordqvist, P.; Thedjil, D.; Khosravi, S.; Lawther, M.; Malmstrom, E.; Khabbaz, F. Wheat gluten fractions as wood adhesives-glutenins versus gliadins. J. Appl. Polym. Sci. 2012, 123, 1530–1538. [Google Scholar] [CrossRef]
- He, Z.; Chapital, D.C.; Cheng, H.N.; Klasson, K.T.; Olanya, M.O.; Uknalis, J. Application of tung oil to improve adhesion strength and water resistance of cottonseed meal and protein adhesives on maple veneer. Ind. Crops Prod. 2014, 61, 398–402. [Google Scholar] [CrossRef]
- Ugovsek, A.; Skapin, A.; Humar, M.; Sernek, M. Microscopic analysis of the wood bond line using liquefied wood as adhesive. J. Adhes. Sci. Technol. 2013, 27, 1247–1258. [Google Scholar] [CrossRef]
- Chen, N.; Zheng, P.; Zeng, Q.; Lin, Q.; Rao, J. Characterization and performance of soy-based adhesives cured with epoxy resin. Polymers 2017, 9, 514. [Google Scholar] [CrossRef]
- He, Z.; Chapital, D.C.; Cheng, H.N. Comparison of the adhesive performances of soy meal, water washed meal fractions, and protein isolates. Modern Appl. Sci. 2016, 10, 112–120. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Zhang, W.; He, C. Performance comparison of different plant fiber/soybean protein adhesive composites. BioResources 2017, 12, 8813–8826. [Google Scholar]
- Nordqvist, P.; Khabbaz, F.; Malmstrom, E. Comparing bond strength and water resistance of alkali-modified soy protein isolate and wheat gluten adhesives. Int. J. Adhes. Adhes. 2010, 30, 72–79. [Google Scholar] [CrossRef]
- He, Z.; Cheng, H.N.; Klasson, K.T.; Barroso, V.A.B. Cottonseed meal-based wood adhesives for non-structural interior applications. In Proceedings of the 2017 International Conference on Wood Adhesives, Atlanta, GA, USA, 25–27 October 2017; Hunt, C.G., Smith, G.D., Yan, N., Eds.; Forest Products Society: Gwinnett County, GA, USA, 2017. [Google Scholar]






| 0.5-mm Hammer | 0.5-mm Cyclone | 0.18-mm Ball | ||||
|---|---|---|---|---|---|---|
| Element | Chunk | Filament | Chunk | Filament | Chunk | Filament |
| C | 65.4 ± 0.4 | 70.5 ± 0.2 | 63.2 ± 0.3 | 65.0 ± 0.3 | 65.0 ± 0.2 | 68.1 ± 0.2 |
| O | 32.5 ± 0.4 | 28.5 ± 0.2 | 33.9 ± 0.3 | 34.6 ± 0.3 | 30.5 ± 0.2 | 31.4 ± 0.2 |
| K | 1.5 ± 0.0 | 0.3 ± 0.0 | 1.5 ± 0.0 | 0.2 ± 0.0 | 1.2 ± 0.0 | 0.2 ± 0.0 |
| Mg | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.1 ± 0.0 | 1.0 ± 0.0 | 0.1 ± 0.0 |
| Ca | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.0 ± 0.0 |
| S | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.0 | 0.0 ± 0.0 |
| P | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.0 ± 0.0 | 1.5 ± 0.0 | 0.0 ± 0.0 |
| Cu | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Cheng, H.N.; Klasson, K.T.; Olanya, O.M.; Uknalis, J. Effects of Particle Size on the Morphology and Water- and Thermo-Resistance of Washed Cottonseed Meal-Based Wood Adhesives. Polymers 2017, 9, 675. https://doi.org/10.3390/polym9120675
He Z, Cheng HN, Klasson KT, Olanya OM, Uknalis J. Effects of Particle Size on the Morphology and Water- and Thermo-Resistance of Washed Cottonseed Meal-Based Wood Adhesives. Polymers. 2017; 9(12):675. https://doi.org/10.3390/polym9120675
Chicago/Turabian StyleHe, Zhongqi, Huai N. Cheng, K. Thomas Klasson, O. Modesto Olanya, and Joseph Uknalis. 2017. "Effects of Particle Size on the Morphology and Water- and Thermo-Resistance of Washed Cottonseed Meal-Based Wood Adhesives" Polymers 9, no. 12: 675. https://doi.org/10.3390/polym9120675
APA StyleHe, Z., Cheng, H. N., Klasson, K. T., Olanya, O. M., & Uknalis, J. (2017). Effects of Particle Size on the Morphology and Water- and Thermo-Resistance of Washed Cottonseed Meal-Based Wood Adhesives. Polymers, 9(12), 675. https://doi.org/10.3390/polym9120675