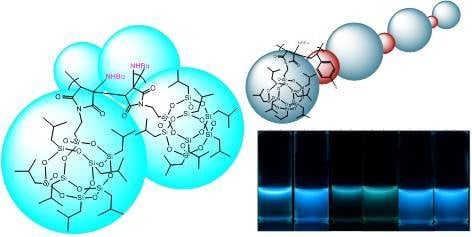
Unusual Emission of Polystyrene-Based Alternating Copolymers Incorporating Aminobutyl Maleimide Fluorophore-Containing Polyhedral Oligomeric Silsesquioxane Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
3. Results and Discussion
3.1. Synthesis of MIPOSS-2Br, MIPOSS-Br, and MIPOSS-NHBu
3.2. Synthesis of Poly(S-alt-MIPOSS-Br), Poly(S-alt-MIPOSS-NHBu), Poly(AS-alt-MIPOSS-NBu), and Poly(HS-alt-MIPOSS-NBu)
3.3. Thermal Properties of Monomers and Alternating Copolymers
3.4. Optical Properties
3.5. Fluorescence Responses of MIPOSS-NHBu and Poly(AS-alt-MIPOSS-NHBu) toward Metal Ions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hawker, C.J.; Wooley, K.L. The convergence of synthetic organic and polymer chemistries. Science 2005, 309, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Wolfbies, O.S. Optical Biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Gather, M.C.; Kohnen, A.; Meerholz, K. White organic light-emitting diodes. Adv. Mater. 2011, 23, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Guo, Z.; Zhu, W.; Yoon, J.; Tian, H. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem. Soc. Rev. 2011, 40, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Mattoussi, H.; Palui, G.; Na, H.B. Luminescent quantum dots as platforms for probing in vitro and in vivo biological processes. Adv. Drug Deliv. Rev. 2012, 64, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, E.G.; Lam, J.W.; Tang, B.Z. AIE luminogens: Emission brightened by aggregation. Mater. Today 2015, 18, 365–377. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.T.; Leung, C.W.; Lam, J.W.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.J.; Lam, J.W.Y.; Tang, B.Z. Luminogenic polymers with aggregation-induced emission characteristics. Prog. Polym. Sci. 2012, 37, 182–209. [Google Scholar] [CrossRef]
- Liu, B.; Dan, T.; Bazan, G.C. Collective response from a cationic tetrahedral fluorene for label-free DNA detection. Adv. Funct. Mater. 2007, 17, 2432–2438. [Google Scholar] [CrossRef]
- Shimizu, M.; Takeda, Y.; Higashi, M.; Hiyama, T. 1,4-Bis(alkenyl)-2,5-dipiperidinobenzenes: Minimal fluorophores exhibiting highly efficient emission in the solid state. Angew. Chem. 2009, 48, 3707–3710. [Google Scholar] [CrossRef]
- Gan, S.; Luo, W.; He, B.; Chen, L.; Nie, H.; Hu, R.; Qin, A.; Zhao, Z.; Tang, B.Z. Integration of aggregation-induced emission and delayed fluorescence into electronic donor–acceptor conjugates. J. Mater. Chem. C 2016, 4, 3705–3708. [Google Scholar] [CrossRef]
- Zhao, E.; Li, H.; Ling, J.; Wu, H.; Wang, J.; Zhang, S.; Lam, J.W.Y.; Sun, J.Z.; Qin, A.; Tang, B.Z. Structure-dependent emission of polytriazoles. Polym. Chem. 2014, 5, 2301–2308. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Zhang, D.; Zhu, D.; Tang, B.Z. Fluorescent bio/chemosensors based on silole and tetraphenylethene luminogens with aggregation-induced emission feature. J. Mater. Chem. 2010, 20, 1858–1867. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Lu, F.H.; Hong, J.L.; Kuo, S.W. Strong emission of 2, 4, 6-triphenylpyridine-functionalized polytyrosine and hydrogen-bonding interactions with poly (4-vinylpyridine). Polym. Chem. 2015, 6, 6340–6350. [Google Scholar] [CrossRef]
- Toal, J.; Jones, K.A.; Magde, D.; Trogler, W.C. Luminescent silole nanoparticles as chemoselective sensors for Cr (VI). J. Am. Chem. Soc. 2005, 127, 11661–11665. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, D.; Zhang, G.; Tang, Y.; Wang, S.; Zhu, D. Fluorescence turn-on detection of DNA and label-free fluorescence nuclease assay based on the aggregation-induced emission of silole. Anal. Chem. 2008, 80, 6443–6448. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Liu, J.; Feng, G.; Li, K.; Hu, Y.; Liu, B. Bright far-red/near-infrared conjugated polymer nanoparticles for in vivo bioimaging. Small 2013, 9, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.D.; Xie, Z.L.; Lam, J.W.Y.; Cheng, L.; Chen, H.Y.; Qiu, C.F.; Zhu, D.B.; Tang, B.Z. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, L.; Peng, Q.; Fan, D.; Yuan, W.Z.; Zhang, Y.; Tang, B.Z. Crystallization-induced dual emission from metal-and heavy atom-free aromatic acids and esters. Chem. Sci. 2015, 6, 4438–4444. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; Qin, W.; Yu, Q.; Wang, J.; Liang, G.; Tang, B.Z. Sensitive and reliable detection of glass transition of polymers by fluorescent probes based on AIE luminogens. Polym. Chem. 2015, 6, 3537–3542. [Google Scholar] [CrossRef]
- Lou, X.; Zhao, Z.; Hong, Y.; Dong, C.; Xu, X.; Jia, Y.; Xia, F.; Tang, B.Z. A new turn-on chemosensor for bio-thiols based on the nanoaggregates of a tetraphenylethene-coumarin fluorophore. Nanoscale 2014, 6, 14691–14696. [Google Scholar] [CrossRef] [PubMed]
- An, B.K.; Kwon, S.K.; Jung, S.D.; Park, S.Y. Enhanced emission and its switching in fluorescent organic nanoparticles. J. Am. Chem. Soc. 2002, 124, 14410–14415. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, L.; Guo, Z.; Nagai, A.; Jiang, D. Light-emitting conjugated polymers with microporous network architecture: Interweaving scaffold promotes electronic conjugation, facilitates exciton migration, and improves luminescence. J. Am. Chem. Soc. 2011, 133, 17622–17625. [Google Scholar] [CrossRef] [PubMed]
- Ooyama, Y.; Yoshikawa, S.; Watanabe, S.; Yoshida, K. Molecular design of novel non-planar heteropolycyclic fluorophores with bulky substituents: Convenient synthesis and solid-state fluorescence characterization. Org. Biomol. Chem. 2006, 4, 3406–3409. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Huang, P.C.; Yang, D.J.; Gao, J.Y.; Hong, J.L. Influence of the secondary structure on the AIE-related emission behavior of an amphiphilic polypeptide containing a hydrophobic fluorescent terminal and hydrophilic pendant groups. Polym. Chem. 2016, 7, 153–163. [Google Scholar] [CrossRef]
- Hsiao, T.S.; Deng, S.L.; Shih, K.Y.; Hong, J.L. Crystallization-enhanced emission through hydrogen-bond interactions in blends containing hydroxyl-functionalized azine and poly (4-vinyl pyridine). J. Mater. Chem. C 2014, 2, 4828–4834. [Google Scholar] [CrossRef]
- Shigemitsu, Y.; Mutai, T.; Houjou, H.; Araki, K. Excited-state intramolecular proton transfer (ESIPT) emission of hydroxyphenylimidazopyridine: Computational study on enhanced and polymorph-dependent luminescence in the solid state. J. Phys. Chem. A 2012, 116, 12041–12048. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Li, H.; Lam, J.W.Y.; Yuan, X.; Wei, J.; Tang, B.Z.; Zhang, H. Graphene oxide as a novel nanoplatform for enhancement of aggregation-induced emission of silole fluorophores. Adv. Mater. 2012, 24, 4191–4195. [Google Scholar]
- Fang, H.H.; Chen, Q.D.; Yang, J.; Xia, H.; Gao, B.R.; Feng, J.; Ma, Y.G.; Sun, H.B. Two-photon pumped amplified spontaneous emission from cyano-substituted oligo(p-phenylenevinylene) crystals with aggregation-induced emission enhancement. J. Phys. Chem. C 2012, 114, 11958–11961. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Hsu, K.C.; Hong, J.L.; Kuo, S.W. Unexpected fluorescence from maleimide-containing polyhedral oligomeric silsesquioxanes: Nanoparticle and sequence distribution analyses of polystyrene-based alternating copolymers. Polym. Chem. 2016, 7, 135–145. [Google Scholar] [CrossRef]
- Yu, Y.; Li, J.; Chen, S.; Hong, Y.; Ng, K.M.; Luo, K.Q.; Tang, B.Z. Thiol-reactive molecule with dual-emission-enhancement property for specific prestaining of cysteine containing proteins in SDS-PAGE. ACS. Appl. Mater. Interfaces 2013, 5, 4613–4616. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zheng, B.; Pan, D.; Yang, R.; Xu, Y.; Wang, L.; Yang, M. Unexpected fluorescence from polymers containing dithio/amino-succinimides. Polym. Chem. 2015, 6, 6133–6139. [Google Scholar] [CrossRef]
- Pucci, A.; Rause, R.; Ciardelli, F. Aggregation-induced luminescence of polyisobutene succinic anhydrides and imides. Macromol. Chem. Phys. 2008, 209, 900–906. [Google Scholar] [CrossRef]
- Zhao, E.; Lam, J.W.Y.; Meng, L.; Hong, Y.; Deng, H.; Bai, G.; Huang, X.; Hao, J.; Tang, B.Z. Poly [(maleic anhydride)-alt-(vinyl acetate)]: A pure oxygenic nonconjugated macromolecule with strong light emission and solvatochromic effect. Macromolecules 2015, 48, 64–71. [Google Scholar] [CrossRef]
- Wu, D.; Cheung, S.; Devocelle, M.; Zhang, L.J.; Chen, Z.L.; ÓShea, D.F. Synthesis and assessment of a maleimide functionalized BF 2 azadipyrromethene near-infrared fluorochrome. Chem. Commun. 2015, 51, 16667–16670. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Scott, D.W. Thermal rearrangement of branched-chain methylpolysiloxanes. J. Am. Chem. Soc. 1946, 68, 356–358. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U. Polyhedral oligomeric silsesquioxane (POSS) polymers and copolymers: A review. J. Inorg. Organomet. Polym. 2002, 11, 123–154. [Google Scholar] [CrossRef]
- Laine, R.M.; Roll, M.F. Polyhedral phenylsilsesquioxanes. Macromolecules 2011, 44, 1073–1109. [Google Scholar] [CrossRef]
- Marcolli, C.; Calzaferri, G. Monosubstituted octasilasesquioxanes. Appl. Organomet. Chem. 1999, 13, 213–226. [Google Scholar] [CrossRef]
- Zhang, W.A.; Muller, A.H.E. Architecture, self-assembly and properties of well-defined hybrid polymers based on polyhedral oligomeric silsequioxane (POSS). Prog. Polym. Sci. 2013, 38, 1121–1162. [Google Scholar] [CrossRef]
- Mark, E.J. Some interesting things about polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mather, P.T. POSS polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Li, Y.W.; Zhang, W.B.; Hsieh, I.F.; Zhang, G.L.; Cao, Y.; Li, X.P.; Wesdemiotis, C.; Lotz, B.; Xiong, H.; Cheng, S.Z.D. Breaking symmetry toward nonspherical janus particles based on polyhedral oligomeric silsesquioxanes: Molecular design, “click” synthesis, and hierarchical structure. J. Am. Chem. Soc. 2011, 133, 10712–10715. [Google Scholar] [CrossRef] [PubMed]
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Laine, R.M. Nanobuilding blocks based on the [OSiO1.5]x (x = 6, 8, 10) octasilsesquioxanes. J. Mater. Chem. 2005, 15, 3725–3744. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Wang, X.; Hu, Y.; Hu, H.; Wu, D.C.; Yu, F.J. Bioreducible POSS-cored star-shaped polycation for efficient gene delivery. ACS. Appl. Mater. Interface 2014, 6, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhong, S.; Li, X.; Tu, Y.; Yang, S.; Van Horn, R.M.; Ni, C.; Pochan, D.J.; Wesdemiotis, C.; Zhang, W.B.; et al. A giant surfactant of polystyrene–(carboxylic acid-functionalized polyhedral oligomeric silsesquioxane) amphiphile with highly stretched polystyrene tails in micellar assemblies. J. Am. Chem. Soc. 2010, 132, 16741–16744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, S.; Kong, J. Hemi-telechelic and telechelic organic/inorganic poly(ethylene oxide) hybrids based on polyhedral oligmeric silsesquioxanes (POSSs): Synthesis, morphology and self-assembly. React. Funct. Polym. 2012, 72, 580–587. [Google Scholar] [CrossRef]
- Kuo, S.W.; Lee, H.F.; Huang, W.J.; Jeong, K.U.; Chang, F.C. Solid state and solution self-assembly of helical polypeptides tethered to polyhedral oligomeric silsesquioxanes. Macromolecules 2009, 42, 1619–1626. [Google Scholar] [CrossRef]
- Ishida, Y.; Hirai, T.; Goseki, R.; Tokita, M.; Kakimoto, M.A.; Hayakawa, T. Synthesis and self-assembly of thermotropic block copolymer with long alkyl tethered cage silsesquioxane in the side chain. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2653–2664. [Google Scholar] [CrossRef]
- Hirai, T.; Leolukman, M.; Hayakawa, T.; Kakimoto, M.; Gopalam, P. Hierarchical nanostructures of organosilicate nanosheets within self-organized block copolymer films. Macromolecules 2008, 41, 4558–4560. [Google Scholar] [CrossRef]
- Zhang, Z.; Hong, L.; Gao, Y.; Zhang, W. One-pot synthesis of POSS-containing alternating copolymers by RAFT polymerization and their microphase-separated nanostructures. Polym. Chem. 2014, 5, 4534–4541. [Google Scholar] [CrossRef]
- Yan, J.J.; Wang, D.; Wu, D.C.; You, Y.Z. Synthesis of sequence-ordered polymers via sequential addition of monomers in one pot. Chem. Commun. 2013, 49, 6057–6059. [Google Scholar] [CrossRef] [PubMed]
- Chiou, C.W.; Lin, Y.C.; Hayakawa, T.; Kuo, S.W. Hydrogen bond interactions mediate hierarchical self-assembly of POSS-containing block copolymers blended with phenolic resin. Macromolecules 2014, 47, 8709–8721. [Google Scholar] [CrossRef]

















| Sample | Mn a | Mw a | PDI a | Tg b (°C) | Td c (°C) | Quantum Yield d (%) |
|---|---|---|---|---|---|---|
| S-alt-MIPOSS-Br | 23,900 | 40,000 | 1.67 | 83 | 364 | 52.4 |
| S-alt-MIPOSS-NHBu | 10,900 | 18,964 | 1.74 | 81 | 355 | 78.8 |
| AS-alt-MIPOSS-Br | 74,200 | 106,200 | 1.43 | 119 | 356 | 63.2 |
| AS-alt-MIPOSS-NHBu | 26,800 | 46,000 | 1.71 | 105 | 326 | 93.8 |
| HS-alt-MIPOSS-NHBu | 65,300 | 95,900 | 1.46 | 130 | 320 | 31.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, M.G.; Jheng, Y.-R.; Yeh, S.-L.; Chen, T.; Kuo, S.-W. Unusual Emission of Polystyrene-Based Alternating Copolymers Incorporating Aminobutyl Maleimide Fluorophore-Containing Polyhedral Oligomeric Silsesquioxane Nanoparticles. Polymers 2017, 9, 103. https://doi.org/10.3390/polym9030103
Mohamed MG, Jheng Y-R, Yeh S-L, Chen T, Kuo S-W. Unusual Emission of Polystyrene-Based Alternating Copolymers Incorporating Aminobutyl Maleimide Fluorophore-Containing Polyhedral Oligomeric Silsesquioxane Nanoparticles. Polymers. 2017; 9(3):103. https://doi.org/10.3390/polym9030103
Chicago/Turabian StyleMohamed, Mohamed Gamal, Yu-Ru Jheng, Shu-Ling Yeh, Tao Chen, and Shiao-Wei Kuo. 2017. "Unusual Emission of Polystyrene-Based Alternating Copolymers Incorporating Aminobutyl Maleimide Fluorophore-Containing Polyhedral Oligomeric Silsesquioxane Nanoparticles" Polymers 9, no. 3: 103. https://doi.org/10.3390/polym9030103
APA StyleMohamed, M. G., Jheng, Y. -R., Yeh, S. -L., Chen, T., & Kuo, S. -W. (2017). Unusual Emission of Polystyrene-Based Alternating Copolymers Incorporating Aminobutyl Maleimide Fluorophore-Containing Polyhedral Oligomeric Silsesquioxane Nanoparticles. Polymers, 9(3), 103. https://doi.org/10.3390/polym9030103