Au Nanoparticles Immobilized on Honeycomb-Like Polymeric Films for Surface-Enhanced Raman Scattering (SERS) Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gold Nanoparticles (AuNPs)
2.3. Preparation of PU-PAZ Polymers
2.4. Preparation of PU-PAZ Honeycomb-Like Polymeric and Flat Films
2.5. Preparation of SERS Substrates
2.6. Characterization
2.6.1. Characterization of AuNPs
2.6.2. Characterization of Honeycomb-Like Polymeric Films
2.7. Measurement of Surface-Enhance Raman Scattering
3. Results and Discussion
3.1. Characterization of AuNPs
3.2. Morphology of Honeycomb-Like Films Based on PU-PAZ
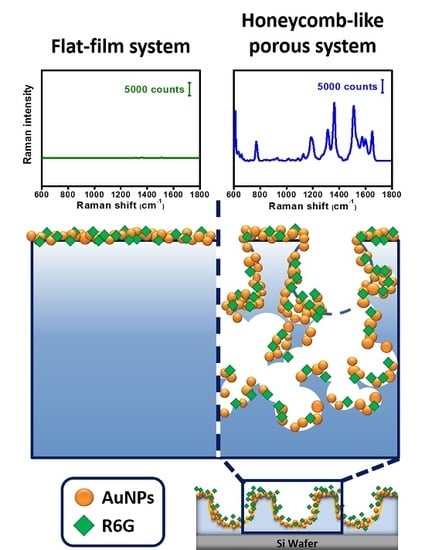
3.3. Hot-Spots Effect in the 3D Honeycomb-Like Polymeric SERS Substrate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

References
- Ko, H.; Singamaneni, S.; Tsukruk, V.V. Nanostructured surfaces and assemblies as sers media. Small 2008, 4, 1576–1599. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.D.; Lipert, R.J.; Siperko, L.M.; Wang, G.; Narayanan, R. Sers as a bioassay platform: Fundamentals, design, and applications. Chem. Soc. Rev. 2008, 37, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Bantz, K.C.; Meyer, A.F.; Wittenberg, N.J.; Im, H.; Kurtulus, O.; Lee, S.H.; Lindquist, N.C.; Oh, S.H.; Haynes, C.L. Recent progress in sers biosensing. Phys. Chem. Chem. Phys. 2011, 13, 11551–11567. [Google Scholar] [CrossRef] [PubMed]
- Cialla, D.; Marz, A.; Bohme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced raman spectroscopy (sers): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Manikas, A.C.; Romeo, G.; Papa, A.; Netti, P.A. Highly efficient surface-enhanced raman scattering substrate formulation by self-assembled gold nanoparticles physisorbed on poly (N-isopropylacrylamide) thermoresponsive hydrogels. Langmuir 2014, 30, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, P.; Stockburger, M. Surface-enhanced resonance raman spectroscopy of rhodamine 6G adsorbed on colloidal silver. J. Phys. Chem. 1984, 88, 5935–5944. [Google Scholar] [CrossRef]
- Wang, H.H.; Liu, C.Y.; Wu, S.B.; Liu, N.W.; Peng, C.Y.; Chan, T.H.; Hsu, C.F.; Wang, J.K.; Wang, Y.L. Highly raman-enhancing substrates based on silver nanoparticle arrays with tunable sub-10 nm gaps. Adv. Mater. 2006, 18, 491–495. [Google Scholar] [CrossRef]
- Zhao, J.; Pinchuk, A.O.; McMahon, J.M.; Li, S.; Ausman, L.K.; Atkinson, A.L.; Schatz, G.C. Methods for describing the electromagnetic properties of silver and gold nanoparticles. Acc. Chem. Res. 2008, 41, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.E.; McCourt, M.R. Sers enhancement by aggregated au colloids: Effect of particle size. Phys. Chem. Chem. Phys. 2009, 11, 7455–7462. [Google Scholar] [CrossRef] [PubMed]
- Doering, W.E.; Piotti, M.E.; Natan, M.J.; Freeman, R.G. Sers as a foundation for nanoscale, optically detected biological labels. Adv. Mater. 2007, 19, 3100–3108. [Google Scholar] [CrossRef]
- Xie, W.; Schlucker, S. Medical applications of surface-enhanced raman scattering. Phys. Chem. Chem. Phys. 2013, 15, 5329–5344. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-W.; Zhai, W.-L.; Li, Y.-T.; Long, Y.-T. Recent progress in surface enhanced raman spectroscopy for the detection of environmental pollutants. Microchim. Acta 2013, 181, 23–43. [Google Scholar] [CrossRef]
- Pang, S.; Yang, T.; He, L. Review of surface enhanced raman spectroscopic (sers) detection of synthetic chemical pesticides. TrAC Trends Anal. Chem. 2016, 85, 73–82. [Google Scholar] [CrossRef]
- Fan, M.; Andrade, G.F.; Brolo, A.G. A review on the fabrication of substrates for surface enhanced raman spectroscopy and their applications in analytical chemistry. Anal. Chim. Acta 2011, 693, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Bao, Y.; Qiu, C.; Jiang, C. Individual nanostructured materials: Fabrication and surface-enhanced raman scattering. Chem. Commun. (Camb.) 2012, 48, 7003–7018. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.C.; Sivashanmugan, K.; Liao, J.D.; Yao, C.K.; Peng, H.C. Nanofabricated sers-active substrates for single-molecule to virus detection in vitro: A review. Biosens. Bioelectron. 2014, 61, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Mevold, A.H.; Hsu, W.-W.; Hardiansyah, A.; Huang, L.-Y.; Yang, M.-C.; Liu, T.-Y.; Chan, T.-Y.; Wang, K.-S.; Su, Y.-A.; Jeng, R.-J. Fabrication of gold nanoparticles/graphene-pdda nanohybrids for bio-detection by sers nanotechnology. Nanoscale Res. Lett. 2015, 10, 397. [Google Scholar] [CrossRef] [PubMed]
- Shiohara, A.; Langer, J.; Polavarapu, L.; Liz-Marzan, L.M. Solution processed polydimethylsiloxane/gold nanostar flexible substrates for plasmonic sensing. Nanoscale 2014, 6, 9817–9823. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Haka, A.S.; Kneipp, H.; Badizadegan, K.; Yoshizawa, N.; Boone, C.; Shafer-Peltier, K.E.; Motz, J.T.; Dasari, R.R.; Feld, M.S. Surface-enhanced raman spectroscopy in single living cells using gold nanoparticles. Appl. Spectrosc. 2002, 56, 150–154. [Google Scholar] [CrossRef]
- Talley, C.E.; Jackson, J.B.; Oubre, C.; Grady, N.K.; Hollars, C.W.; Lane, S.M.; Huser, T.R.; Nordlander, P.; Halas, N.J. Surface-enhanced raman scattering from individual Au nanoparticles and nanoparticle dimer substrates. Nano Lett. 2005, 5, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J.; Gole, A.; Sau, T.K.; Murphy, C.J. Surface-enhanced raman spectroscopy of self-assembled monolayers: Sandwich architecture and nanoparticle shape dependence. Anal. Chem. 2005, 77, 3261–3266. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Graham, D. Molecularly-mediated assemblies of plasmonic nanoparticles for surface-enhanced raman spectroscopy applications. Chem. Soc. Rev. 2012, 41, 7085–7107. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Mourdikoudis, S.; Pastoriza-Santos, I.; Pérez-Juste, J. Nanocrystal engineering of noble metals and metal chalcogenides: Controlling the morphology, composition and crystallinity. CrystEngComm 2015, 17, 3727–3762. [Google Scholar] [CrossRef]
- Kleinman, S.L.; Frontiera, R.R.; Henry, A.-I.; Dieringer, J.A.; Van Duyne, R.P. Creating, characterizing, and controlling chemistry with sers hot spots. Phys. Chem. Chem. Phys. 2013, 15, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Braun, G.; Pavel, I.; Morrill, A.R.; Seferos, D.S.; Bazan, G.C.; Reich, N.O.; Moskovits, M. Chemically patterned microspheres for controlled nanoparticle assembly in the construction of sers hot spots. J. Am. Chem. Soc. 2007, 129, 7760–7761. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hao, J. Ordered patterns and structures via interfacial self-assembly: Superlattices, honeycomb structures and coffee rings. Chem. Soc. Rev. 2011, 40, 5457–5471. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lei, Y. Recent progress on surface pattern fabrications based on monolayer colloidal crystal templates and related applications. Nanoscale 2011, 3, 2768–2782. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lapsley, M.I.; Cao, B.; Zhao, C.; Zhao, Y.; Hao, Q.; Kiraly, B.; Scott, J.; Li, W.; Wang, L.; et al. Large-scale fabrication of three-dimensional surface patterns using template-defined electrochemical deposition. Adv. Funct. Mater. 2013, 23, 720–730. [Google Scholar] [CrossRef]
- Polavarapu, L.; Porta, A.L.; Novikov, S.M.; Coronado-Puchau, M.; Liz-Marzan, L.M. Pen-on-paper approach toward the design of universal surface enhanced raman scattering substrates. Small 2014, 10, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Govor, L.; Bashmakov, I.; Kaputski, F.; Pientka, M.; Parisi, J. Self-organized formation of low-dimensional network structures starting from a nitrocellulose solution. Macromol. Chem. Phys. 2000, 201, 2721–2728. [Google Scholar] [CrossRef]
- Widawski, G.; Rawiso, M.; Francois, B. Self-organized honeycomb morphology of star-polymer polystyrene films. Nature 1994, 369, 387–389. [Google Scholar] [CrossRef]
- Wu, C.-H.; Ting, W.-H.; Lai, Y.-W.; Dai, S.A.; Su, W.-C.; Tung, S.-H.; Jeng, R.-J. Tailored honeycomb-like polymeric films based on amphiphilic poly(urea/malonamide) dendrons. RSC Adv. 2016, 6, 91981–91990. [Google Scholar] [CrossRef]
- Stenzel, M.H.; Barner-Kowollik, C.; Davis, T.P. Formation of honeycomb-structured, porous films via breath figures with different polymer architectures. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2363–2375. [Google Scholar] [CrossRef]
- Chang, C.-C.; Juang, T.-Y.; Ting, W.-H.; Lin, M.-S.; Yeh, C.-M.; Dai, S.A.; Suen, S.-Y.; Liu, Y.-L.; Jeng, R.-J. Using a breath-figure method to self-organize honeycomb-like polymeric films from dendritic side-chain polymers. Mater. Chem. Phys. 2011, 128, 157–165. [Google Scholar] [CrossRef]
- Ma, H.; Hao, J. Evaporation-induced ordered honeycomb structures of gold nanoparticles at the air/water interface. Chemistry 2010, 16, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Dong, R.; Ma, H.; Hao, J. Au np honeycomb-patterned films with controllable pore size and their surface-enhanced raman scattering. Langmuir 2013, 29, 4235–4241. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Wang, L.-Y.; Zhu, L.-W.; Wan, L.-S.; Xu, Z.-K. In-situ immobilization of silver nanoparticles on self-assembled honeycomb-patterned films enables surface-enhanced raman scattering (sers) substrates. J. Phys. Chem. C 2014, 118, 11478–11484. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Liu, J.-L.; Xu, W.-C.; Wang, Z.-F.; Wang, C.-Y.; Zhang, M. Selective assembly of silver nanoparticles on honeycomb films and their surface-enhanced raman scattering. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 782–788. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Ho, J.-Y.; Wei, J.-C.; Cheng, W.-C.; Chen, I.H.; Shiue, J.; Wang, H.-H.; Wang, J.-K.; Wang, Y.-L.; Lin, J.-J. Label-free and culture-free microbe detection by three dimensional hot-junctions of flexible raman-enhancing nanohybrid platelets. J. Mater. Chem. B 2014, 2, 1136. [Google Scholar] [CrossRef]
- Ho, J.Y.; Liu, T.Y.; Wei, J.C.; Wang, J.K.; Wang, Y.L.; Lin, J.J. Selective sers detecting of hydrophobic microorganisms by tricomponent nanohybrids of silver-silicate-platelet-surfactant. ACS Appl. Mater. Interfaces 2014, 6, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-A.; Chen, W.-F.; Juang, T.-Y.; Ting, W.-H.; Liu, T.-Y.; Hsieh, C.-F.; Dai, S.A.; Jeng, R.-J. Honeycomb-like polymeric films from dendritic polymers presenting reactive pendent moieties. Polymer 2014, 55, 1481–1490. [Google Scholar] [CrossRef]
- Yabu, H.; Tanaka, M.; Ijiro, K.; Shimomura, M. Preparation of honeycomb-patterned polyimide films by self-organization. Langmuir 2003, 19, 6297–6300. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, B.; Xu, Y. A study on formation of regular honeycomb pattern in polysulfone film. Polymer 2005, 46, 713–717. [Google Scholar] [CrossRef]
- Karikari, A.S.; Williams, S.R.; Heisey, C.L.; Rawlett, A.M.; Long, T.E. Porous thin films based on photo-cross-linked star-shaped poly(d,l-lactide)s. Langmuir 2006, 22, 9687–9693. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Hao, J. Formation mechanism and morphology modulation of honeycomb hybrid films made of polyoxometalates/surfactants at the air/water interface. J. Colloid Interface Sci. 2009, 333, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Xia, X.; Ma, H.; Du, B.; Wei, Q. Honeycomb-patterned fluorescent films fabricated by self-assembly of surfactant-assisted porphyrin/polymer composites. J. Colloid Interface Sci. 2013, 402, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Grayson, S.M.; Frechet, J.M. Convergent dendrons and dendrimers: From synthesis to applications. Chem. Rev. 2001, 101, 3819–3868. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Dai, S.A.; Chang, H.L.; Su, W.C.; Jeng, R.J. Facile approach to polyurea/malonamide dendrons via a selective ring-opening addition reaction of azetidine-2,4-dione. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 682–688. [Google Scholar] [CrossRef]
- Chen, C.-P.; Dai, S.A.; Chang, H.-L.; Su, W.-C.; Wu, T.-M.; Jeng, R.-J. Polyurethane elastomers through multi-hydrogen-bonded association of dendritic structures. Polymer 2005, 46, 11849–11857. [Google Scholar] [CrossRef]
- Dai, S.A.; Chen, C.P.; Lin, C.C.; Chang, C.C.; Wu, T.M.; Su, W.C.; Chang, H.L.; Jeng, R.J. Novel side-chain dendritic polyurethanes based on hydrogen bonding rich polyurea/malonamide dendrons. Macromol. Mater. Eng. 2006, 291, 395–404. [Google Scholar] [CrossRef]
- Dai, S.A.; Juang, T.Y.; Chen, C.P.; Chang, H.Y.; Kuo, W.J.; Su, W.C.; Jeng, R.J. Synthesis of n-aryl azetidine-2,4-diones and polymalonamides prepared from selective ring-opening reactions. J. Appl. Polym. Sci. 2007, 103, 3591–3599. [Google Scholar] [CrossRef]
- Tomalia, D.A. Birth of a new macromolecular architecture: Dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog. Polym. Sci. 2005, 30, 294–324. [Google Scholar] [CrossRef]
- Ting, W.-H.; Chen, C.-C.; Dai, S.A.; Suen, S.-Y.; Yang, I.-K.; Liu, Y.-L.; Chen, F.M.; Jeng, R.-J. Superhydrophobic waxy-dendron-grafted polymer films via nanostructure manipulation. J. Mater. Chem. 2009, 19, 4819–4828. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Xia, H.; Bai, S.; Hartmann, J.; Wang, D. Synthesis of monodisperse quasi-spherical gold nanoparticles in water via silver(i)-assisted citrate reduction. Langmuir 2010, 26, 3585–3589. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jasinski, J.B.; Howell, J.L.; Patel, D.; Stephens, D.P.; Gobin, A.M. Tunability and stability of gold nanoparticles obtained from chloroauric acid and sodium thiosulfate reaction. Nanoscale Res. Lett. 2012, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Hernández-Guerrero, M.; Granville, A.M.; Davis, T.P.; Barner-Kowollik, C.; Stenzel, M.H. Water-assisted formation of honeycomb structured porous films. J. Porous Mater. 2006, 13, 213–223. [Google Scholar] [CrossRef]
- Sun, H.; Li, H.; Bu, W.; Xu, M.; Wu, L. Self-organized microporous structures based on surfactant-encapsulated polyoxometalate complexes. J. Phys. Chem. B 2006, 110, 24847–24854. [Google Scholar] [CrossRef] [PubMed]
- Kabuto, T.; Hashimoto, Y.; Karthaus, O. Thermally stable and solvent resistant mesoporous honeycomb films from a crosslinkable polymer. Adv. Funct. Mater. 2007, 17, 3569–3573. [Google Scholar] [CrossRef]
- Hsieh, S.-J.; Wang, C.-C.; Chen, C.-Y. Self-assembling microporous matrix from dendritic–linear copolymers based on a solvent-induced phase separation mechanism. Macromolecules 2009, 42, 4787–4794. [Google Scholar] [CrossRef]
- Yabu, H.; Takebayashi, M.; Tanaka, M.; Shimomura, M. Superhydrophobic and lipophobic properties of self-organized honeycomb and pincushion structures. Langmuir 2005, 21, 3235–3237. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, L.; Pérez-Juste, J.; Xu, Q.-H.; Liz-Marzán, L.M. Optical sensing of biological, chemical and ionic species through aggregation of plasmonic nanoparticles. J. Mater. Chem. C 2014, 2, 7460. [Google Scholar] [CrossRef]
- Chen, Y.W.; Liu, T.Y.; Chen, P.J.; Chang, P.H.; Chen, S.Y. A high-sensitivity and low-power theranostic nanosystem for cell sers imaging and selectively photothermal therapy using anti-egfr-conjugated reduced graphene oxide/mesoporous silica/aunps nanosheets. Small 2016, 12, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Pal, T. Anisotropic metal nanoparticles for use as surface-enhanced raman substrates. Adv. Mater. 2007, 19, 1761–1765. [Google Scholar] [CrossRef]












| Sample code | PU-PAZ conc. (mg/mL) | RH (%) | Pore size (μm) |
|---|---|---|---|
| HS5-L | 5 | 65 | 0.39 ± 0.08 |
| HS5-H | 5 | 95 | 0.73 ± 0.19 |
| HS10-L | 10 | 65 | 0.83 ± 0.14 |
| HS10-H | 10 | 95 | 1.46 ± 0.09 |
| HS20-L | 20 | 65 | 0.61 ± 0.10 |
| HS20-H | 20 | 95 | 1.24 ± 0.43 |
| FFS 1 | 20 | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.-Y.; Liu, T.-Y.; Su, Y.-A.; Wu, C.-H.; Cheng, Y.-W.; Cheng, H.-W.; Jeng, R.-J. Au Nanoparticles Immobilized on Honeycomb-Like Polymeric Films for Surface-Enhanced Raman Scattering (SERS) Detection. Polymers 2017, 9, 93. https://doi.org/10.3390/polym9030093
Chiang C-Y, Liu T-Y, Su Y-A, Wu C-H, Cheng Y-W, Cheng H-W, Jeng R-J. Au Nanoparticles Immobilized on Honeycomb-Like Polymeric Films for Surface-Enhanced Raman Scattering (SERS) Detection. Polymers. 2017; 9(3):93. https://doi.org/10.3390/polym9030093
Chicago/Turabian StyleChiang, Chia-Yen, Ting-Yu Liu, Yu-An Su, Chien-Hsin Wu, Yu-Wei Cheng, Ho-Wen Cheng, and Ru-Jong Jeng. 2017. "Au Nanoparticles Immobilized on Honeycomb-Like Polymeric Films for Surface-Enhanced Raman Scattering (SERS) Detection" Polymers 9, no. 3: 93. https://doi.org/10.3390/polym9030093
APA StyleChiang, C. -Y., Liu, T. -Y., Su, Y. -A., Wu, C. -H., Cheng, Y. -W., Cheng, H. -W., & Jeng, R. -J. (2017). Au Nanoparticles Immobilized on Honeycomb-Like Polymeric Films for Surface-Enhanced Raman Scattering (SERS) Detection. Polymers, 9(3), 93. https://doi.org/10.3390/polym9030093