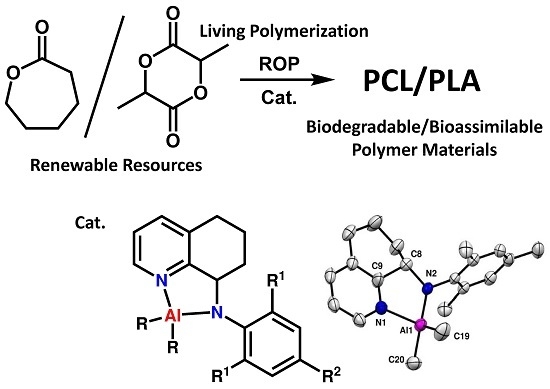
Synthesis of Aluminum Complexes Bearing 8-Anilide-5,6,7-trihydroquinoline Ligands: Highly Active Catalyst Precursors for Ring-Opening Polymerization of Cyclic Esters
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Considerations
2.2. Synthesis of 8-(2,6-R1-4-R2-anilide)-5,6,7-trihydroquinoline (LH1–LH4)
2.3. Synthesis of Aluminum Complexes (Al1–Al5)
2.4. The ROP of ε-CL and rac-LA
2.5. Crystal Structure Determinations
3. Results and Discussion
3.1. Synthesis and Characterization of the Ligands and Complexes
3.2. Ring-opening Polymerization of ε-CL and rac-LA
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arbaoui, A.; Redshaw, C. Metal catalysts for ε-caprolactone polymerisation. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Winzenburg, G.; Schmidt, C.; Fuchs, S.; Kissel, T. Biodegradable polymers and their potential use in parenteral veterinary drug delivery systems. Adv. Drug Deliv. Rev. 2004, 56, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.A.; Kalra, B. Biodegradable polymers for the environment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Marlier, E.E.; Macaranas, J.A.; Marell, D.J.; Dunbar, C.R.; Johnson, M.A.; DePorre, Y.; Miranda, M.O.; Neisen, B.D.; Cramer, C.J.; Hillmyer, M.A.; et al. Mechanistic studies of ε-caprolactone polymerization by (salen)alor complexes and a predictive model for cyclic ester polymerizations. ACS Catal. 2016, 6, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, W.; Yuan, D.; Zhang, Y.; Shen, Q.; Yao, Y. Syntheses of mononuclear and dinuclear aluminum complexes stabilized by phenolato ligands and their applications in the polymerization of ε-caprolactone: A comparative study. Inorg. Chem. 2015, 54, 4699–4708. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, B.; Liu, D.; Wu, C.; Li, S.; Liu, B.; Cui, D. Copolymerization of ε-caprolactone and l-lactide catalyzed by multinuclear aluminum complexes: An immortal approach. Organometallics 2014, 33, 6474–6480. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.-X. Aluminum and zinc complexes supported by functionalized phenolate ligands: Synthesis, characterization and catalysis in the ring-opening polymerization of ε-caprolactone and rac-lactide. J. Organomet. Chem. 2008, 693, 3151–3158. [Google Scholar] [CrossRef]
- Ovitt, T.M.; Coates, G.W. Stereochemistry of lactide polymerization with chiral catalysts: New opportunities for stereocontrol using polymer exchange mechanisms. J. Am. Chem. Soc. 2002, 124, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Klitzke, J.S.; Roisnel, T.; Kirillov, E.; Casagrande, O.D.L.; Carpentier, J.-F. Yttrium– and aluminum–bis(phenolate)pyridine complexes: Catalysts and model compounds of the intermediates for the stereoselective ring-opening polymerization of racemic lactide and β-butyrolactone. Organometallics 2014, 33, 309–321. [Google Scholar] [CrossRef]
- Fang, J.; Tschan, M.J.L.; Roisnel, T.; Trivelli, X.; Gauvin, R.M.; Thomas, C.M.; Maron, L. Yttrium catalysts for syndioselective β-butyrolactone polymerization: On the origin of ligand-induced stereoselectivity. Polym. Chem. 2013, 4, 360–367. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, S.; Yang, W.; Hao, X.; Glaser, R.; Sun, W.-H. Chloroyttrium 2-(1-(Arylimino)alkyl)quinolin-8-olate Complexes: Synthesis, characterization, and catalysis of the ring-opening polymerization of epsilon-caprolactone. Organometallics 2012, 31, 8178–8188. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, S.; Sun, W.-H.; Hao, X.; Redshaw, C. Trimetallic yttrium N-(2-methylquinolin-8-yl)benzamides: Synthesis, structure and use in ring-opening polymerization (ROP) of [varepsilon]-caprolactone. New J. Chem. 2012, 36, 2392–2396. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, Y.; Rodriguez-Delgado, A.; Chen, E.Y.X. Neutral metallocene ester enolate and non-metallocene alkoxy complexes of zirconium for catalytic ring-opening polymerization of cyclic esters. Organometallics 2008, 27, 5632–5640. [Google Scholar] [CrossRef]
- Takeuchi, D.; Nakamura, T.; Aida, T. Bulky titanium bis(phenolate) complexes as novel initiators for living anionic polymerization of ε-caprolactone. Macromolecules 2000, 33, 725–729. [Google Scholar] [CrossRef]
- Honrado, M.; Otero, A.; Fernandez-Baeza, J.; Sanchez-Barba, L.F.; Garces, A.; Lara-Sanchez, A.; Rodriguez, A.M. Copolymerization of cyclic esters controlled by chiral NNO-scorpionate zinc initiators. Organometallics 2016, 35, 189–197. [Google Scholar] [CrossRef]
- Kremer, A.B.; Osten, K.M.; Yu, I.; Ebrahimi, T.; Aluthge, D.C.; Mehrkhodavandi, P. Dinucleating ligand platforms supporting indium and zinc catalysts for cyclic ester polymerization. Inorg. Chem. 2016, 55, 5365–5374. [Google Scholar] [CrossRef] [PubMed]
- Kronast, A.; Reiter, M.; Altenbuchner, P.T.; Jandl, C.; Pöthig, A.; Rieger, B. Electron-deficient β-diiminato-zinc-ethyl complexes: Synthesis, structure, and reactivity in ring-opening polymerization of lactones. Organometallics 2016, 35, 681–685. [Google Scholar] [CrossRef]
- Rieth, L.R.; Moore, D.R.; Lobkovsky, E.B.; Coates, G.W. Single-site β-diiminate zinc catalysts for the ring-opening polymerization of β-butyrolactone and β-valerolactone to poly(3-hydroxyalkanoates). J. Am. Chem. Soc. 2002, 124, 15239–15248. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Moore, D.R.; Reczek, J.J.; Chamberlain, B.M.; Lobkovsky, E.B.; Coates, G.W. Single-Site β-Diiminate Zinc Catalysts for the Alternating Copolymerization of CO2 and Epoxides: Catalyst Synthesis and Unprecedented Polymerization Activity. J. Am. Chem. Soc. 2001, 123, 8738–8749. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, B.M.; Cheng, M.; Moore, D.R.; Ovitt, T.M.; Lobkovsky, E.B.; Coates, G.W. Polymerization of Lactide with Zinc and Magnesium β-Diiminate Complexes: Stereocontrol and Mechanism. J. Am. Chem. Soc. 2001, 123, 3229–3238. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Attygalle, A.B.; Lobkovsky, E.B.; Coates, G.W. Single-site catalysts for ring-opening polymerization: Synthesis of heterotactic poly(lactic acid) from rac-lactide. J. Am. Chem. Soc. 1999, 121, 11583–11584. [Google Scholar] [CrossRef]
- Qi, C.-Y.; Wang, Z.-X. Synthesis and characterization of Aluminum(III) and Tin(II) complexes supported by diiminophosphinate ligands and their application in ring-opening polymerization catalysis of ε-caprolactone. J. Polym. Sci. Part A 2006, 44, 4621–4631. [Google Scholar] [CrossRef]
- Kowalski, A.; Libiszowski, J.; Duda, A.; Penczek, S. Polymerization of l,l-Dilactide Initiated by Tin(II) Butoxide. Macromolecules 2000, 33, 1964–1971. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Liu, M.-Y.; Sutar, A.K.; Lin, C.-C. Synthesis and structural studies of heterobimetallic alkoxide complexes supported by bis(phenolate) ligands: Efficient catalysts for ring-opening polymerization of l-lactide. Inorg. Chem. 2010, 49, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-Z.; Sun, H.-M.; Li, W.-F.; Wang, Z.-G.; Shen, Q.; Zhang, Y. Synthesis, structure of functionalized N-heterocyclic carbene complexes of Fe(II) and their catalytic activity for ring-opening polymerization of ε-caprolactone. J. Organomet. Chem. 2006, 691, 2489–2494. [Google Scholar] [CrossRef]
- O'Keefe, B.J.; Monnier, S.M.; Hillmyer, M.A.; Tolman, W.B. Rapid and controlled polymerization of lactide by structurally characterized ferric alkoxides. J. Am. Chem. Soc. 2001, 123, 339–340. [Google Scholar] [CrossRef] [PubMed]
- O'Keefe, B.J.; Breyfogle, L.E.; Hillmyer, M.A.; Tolman, W.B. Mechanistic comparison of cyclic ester polymerizations by novel iron(iii)−alkoxide complexes: Single vs. multiple site catalysis. J. Am. Chem. Soc. 2002, 124, 4384–4393. [Google Scholar] [CrossRef] [PubMed]
- Hormnirun, P.; Marshall, E.L.; Gibson, V.C.; Pugh, R.I.; White, A.J.P. Study of ligand substituent effects on the rate and stereoselectivity of lactide polymerization using aluminum salen-type initiators. Proc. Natl. Acad. Sci. USA 2006, 103, 15343–15348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, N.; Akita, A.; Ishii, R.; Mizuno, M. random copolymerization of epsilon-caprolactone with lactide using a homosalen-al complex. J. Am. Chem. Soc. 2010, 132, 1750–1751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, S.; Zuo, W.; Ye, H.; Li, Z. Synthesis of dinuclear aluminum complexes bearing bis-phenolate ligand and application in ring-opening polymerization of ε-caprolactone. New J. Chem. 2017. [Google Scholar] [CrossRef]
- Sun, W.-H.; Shen, M.; Zhang, W.; Huang, W.; Liu, S.; Redshaw, C. Methylaluminium 8-quinolinolates: Synthesis, characterization and use in ring-opening polymerization (ROP) of caprolactone. Dalton Trans. 2011, 40, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Sun, W.-H.; Wang, L.; Redshaw, C. Dimethylaluminium aldiminophenolates: Synthesis, characterization and ring-opening polymerization behavior towards lactides. Dalton Trans. 2012, 41, 11587–11596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Wang, L.; Redshaw, C.; Sun, W.-H. Dialkylaluminium 2-imidazolylphenolates: Synthesis, characterization and ring-opening polymerization behavior towards lactides. J. Organomet. Chem. 2014, 750, 65–73. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Cao, J.; Wang, L.; Pan, Y.; Redshaw, C.; Sun, W.-H. Synthesis and characterization of dialkylaluminum amidates and their ring-opening polymerization of ε-caprolactone. Organometallics 2011, 30, 6253–6261. [Google Scholar] [CrossRef]
- Hou, X.; Cai, Z.; Chen, X.; Wang, L.; Redshaw, C.; Sun, W.-H. N-(5,6,7-Trihydroquinolin-8-ylidene)-2-benzhydrylbenzenaminonickel halide complexes: Synthesis, characterization and catalytic behavior towards ethylene polymerization. Dalton Trans. 2012, 41, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hao, X.; Sun, W.-H.; Redshaw, C. Synthesis, characterization, and ethylene polymerization behavior of 8-(nitroarylamino)-5,6,7-trihydroquinolylnickel dichlorides: Influence of the nitro group and impurities on catalytic activity. ACS Catal. 2011, 1, 1213–1220. [Google Scholar] [CrossRef]
- Yu, J.; Hu, X.; Zeng, Y.; Zhang, L.; Ni, C.; Hao, X.; Sun, W.-H. Synthesis, characterisation and ethylene oligomerization behaviour of N-(2-substituted-5,6,7-trihydroquinolin-8-ylidene)arylaminonickel dichlorides. New J. Chem. 2011, 35, 178–183. [Google Scholar] [CrossRef]
- Yu, J.; Zeng, Y.; Huang, W.; Hao, X.; Sun, W.-H. N-(5,6,7-trihydroquinolin-8-ylidene)arylaminonickel dichlorides as highly active single-site pro-catalysts in ethylene polymerization. Dalton Trans. 2011, 40, 8436–8443. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-Y.; Igarashi, A.; Sun, W.-H.; Inagaki, A.; Liu, J.; Zhang, W.; Li, Y.-S.; Nomura, K. Synthesis of (imido)vanadium(v) complexes containing 8-(2,6-dimethylanilide)-5,6,7-trihydroquinoline ligands: Highly active catalyst precursors for ethylene dimerization. Organometallics 2014, 33, 1053–1060. [Google Scholar] [CrossRef]
- Save, M.; Schappacher, M.; Soum, A. Controlled ring-opening polymerization of lactones and lactides initiated by lanthanum isopropoxide, 1. General aspects and kinetics. Macromol. Chem. Phys. 2002, 203, 889–899. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXTL PC, Version 5.0, An Integrated System for Solving, Refining, and Displaying Crystal Structures from Diffraction Data, 6th ed.; Bruker AXS: Madison, WI, USA, 2000. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2: A complete structure solution, refinement and analysis program. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, W.; Nomura, K.; Sun, W.H. Synthesis and characterization of organoaluminum compounds containing quinolin-8-amine derivatives and their catalytic behaviour for ring-opening polymerization of ε-caprolactone. Dalton Trans. 2009, 9000–9009. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Velders, A.H.; Dijkstra, P.J.; Zhong, Z.; Chen, X.; Feijen, J. Polymerization of lactide using achiral bis(pyrrolidene) schiff base aluminum complexes. Macromolecules 2009, 42, 1058–1066. [Google Scholar] [CrossRef]
- Haddad, M.; Laghzaoui, M.; Welter, R.; Dagorne, S. Synthesis and structure of neutral and cationic aluminum complexes supported by bidentate o,p-phosphinophenolate ligands and their reactivity with propylene oxide and ε-caprolactone. Organometallics 2009, 28, 4584–4592. [Google Scholar] [CrossRef]
- Pang, X.; Chen, X.; Du, H.; Wang, X.; Jing, X. Enolic Schiff-base aluminum complexes and their application in lactide polymerization. J. Organomet. Chem. 2007, 692, 5605–5613. [Google Scholar] [CrossRef]
- Ma, H.; Okuda, J. Kinetics and mechanism of l-Lactide polymerization by rare earth metal silylamido complexes: Effect of alcohol addition. Macromolecules 2005, 38, 2665–2673. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Liu, X.; Cui, D. Facile synthesis of pendant- and α,ϖ-chain-end-functionalized polycarbonates via immortal polymerization by using a salan lutetium alkyl precursor. Chem. Commun. 2012, 48, 4588–4590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, Y.; Liu, X.; Chen, X.; Cui, D.; Chen, E.Y.X. Protic compound mediated living cross-chain-transfer polymerization of rac-lactide: Synthesis of isotactic (crystalline)-heterotactic (amorphous) stereomultiblock polylactide. Chem. Commun. 2012, 48, 6375–6377. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cui, D.; Liu, X.; Chen, X. Facile synthesis of hydroxyl-ended, highly stereoregular, star-shaped poly(lactide) from immortal rop of rac-lactide and kinetics study. Macromolecules 2010, 43, 6678–6684. [Google Scholar] [CrossRef]
- Helou, M.; Miserque, O.; Brusson, J.-M.; Carpentier, J.-F.; Guillaume, S. M. Ultraproductive, zinc-mediated, immortal ring-opening polymerization of trimethylene carbonate. Chem. Eur. J. 2008, 14, 8772–8775. [Google Scholar] [CrossRef] [PubMed]
- Amgoune, A.; Thomas, C.M.; Carpentier, J.-F. Yttrium Complexes as catalysts for living and immortal polymerization of lactide to highly heterotactic PLA. Macromol. Rapid Commun. 2007, 28, 693–697. [Google Scholar] [CrossRef]
- Inoue, S. Immortal polymerization: The outset, development, and application. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 2861–2871. [Google Scholar] [CrossRef]
- Koller, J.; Bergman, R.G. Highly efficient aluminum-catalyzed ring-opening polymerization of cyclic carbonates, lactones, and lactides, including a unique crystallographic snapshot of an intermediate. Organometallics 2011, 30, 3217–3224. [Google Scholar] [CrossRef]
- Pappalardo, D.; Annunziata, L.; Pellecchia, C. Living Ring-opening homo- and copolymerization of ε-caprolactone and l- and d,l-lactides by dimethyl(salicylaldiminato)aluminum compounds. Macromolecules 2009, 42, 6056–6062. [Google Scholar] [CrossRef]
- Sun, Z.; Duan, R.; Yang, J.; Zhang, H.; Li, S.; Pang, X.; Chen, W.; Chen, X. Bimetallic Schiff base complexes for stereoselective polymerisation of racemic-lactide and copolymerisation of racemic-lactide with ε-caprolactone. RSC Adv. 2016, 6, 17531–17538. [Google Scholar] [CrossRef]
- Gilmour, D.J.; Webster, R.L.; Perry, M.R.; Schafer, L.L. Titanium pyridonates for the homo- and copolymerization of rac-lactide and ε-caprolactone. Dalton Trans. 2015, 44, 12411–12419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darensbourg, D.J.; Karroonnirun, O. Ring-opening polymerization of l-lactide and ε-caprolactone utilizing biocompatible Zinc catalysts. Macromolecules 2010, 43, 8880–8886. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Bornhorst, K.; Hachmann-Thiessen, H. Bismuth(III) n-hexanoate and tin(II) 2-ethylhexanoate initiated copolymerizations of ε-caprolactone and l-lactide. Macromolecules 2005, 38, 5017–5024. [Google Scholar] [CrossRef]
- Florczak, M.; Duda, A. Effect of the configuration of the active center on comonomer reactivities: The case of ε-caprolactone/l,l-lactide copolymerization. Angew. Chem. Int. Ed. 2008, 47, 9088–9091. [Google Scholar] [CrossRef] [PubMed]






| run | Com. | CL:Al:BnOH | t min | conv. (%) b | Mn c × 10−4 | Ðc | Mn d (cal.) × 10−4 |
|---|---|---|---|---|---|---|---|
| 1 | Al2 | 250:1:0 | 30 | 98 | 3.79 | 1.59 | - |
| 2 | Al1 | 250:1:1 | 30 | 99 | 2.27 | 1.20 | 2.83 |
| 3 | Al2 | 250:1:1 | 30 | 99 | 2.76 | 1.18 | 2.83 |
| 4 | Al3 | 250:1:1 | 30 | 99 | 2.86 | 1.19 | 2.83 |
| 5 | Al4 | 250:1:1 | 30 | 100 | 2.50 | 1.23 | 2.86 |
| 6 | Al5 | 250:1:1 | 30 | 99 | 2.43 | 1.24 | 2.83 |
| 7 | Al2 | 250:1:1 | 5 | 48 | 1.33 | 1.10 | 1.37 |
| 8 | Al2 | 250:1:1 | 10 | 76 | 2.20 | 1.10 | 2.16 |
| 9 | Al2 | 250:1:1 | 20 | 94 | 2.59 | 1.12 | 2.68 |
| 10 | Al2 | 125:1:1 | 30 | 100 | 1.44 | 1.14 | 1.43 |
| 11 | Al2 | 500:1:1 | 30 | 80 | 3.59 | 1.46 | 4.57 |
| 12 | Al2 | 250:1:5 | 30 | 99 | 0.54 | 1.07 | 0.51 |
| 13 | Al2 | 250:1:10 | 30 | 99 | 0.31 | 1.13 | 0.29 |
| run | Com. | t/h | conv. (%) b | Mn c × 10−4 | Ðc | Mn d (cal.) × 10−4 |
|---|---|---|---|---|---|---|
| 1 | Al1 | 12 | 96 | 2.65 | 1.31 | 3.46 |
| 2 | Al2 | 12 | 96 | 2.96 | 1.26 | 3.33 |
| 3 | Al3 | 12 | 94 | 2.34 | 1.38 | 3.39 |
| 4 | Al4 | 12 | 95 | 3.02 | 1.32 | 3.42 |
| 5 | Al5 | 12 | 96 | 2.89 | 1.36 | 3.46 |
| 6 | Al2 | 1 | 42 | 1.46 | 1.12 | 1.52 |
| 7 | Al2 | 3 | 67 | 2.06 | 1.15 | 2.42 |
| 8 | Al2 | 6 | 87 | 2.87 | 1.32 | 3.13 |
| 9 e | Al2 | 12 | 100/6.1 f | 3.58 | 1.05 | 3.78 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, J.; Zuo, W.; Zhang, W.; Sun, W.-H.; Ye, H.; Li, Z. Synthesis of Aluminum Complexes Bearing 8-Anilide-5,6,7-trihydroquinoline Ligands: Highly Active Catalyst Precursors for Ring-Opening Polymerization of Cyclic Esters. Polymers 2017, 9, 83. https://doi.org/10.3390/polym9030083
Liu S, Zhang J, Zuo W, Zhang W, Sun W-H, Ye H, Li Z. Synthesis of Aluminum Complexes Bearing 8-Anilide-5,6,7-trihydroquinoline Ligands: Highly Active Catalyst Precursors for Ring-Opening Polymerization of Cyclic Esters. Polymers. 2017; 9(3):83. https://doi.org/10.3390/polym9030083
Chicago/Turabian StyleLiu, Shaofeng, Jie Zhang, Weiwei Zuo, Wenjuan Zhang, Wen-Hua Sun, Hongqi Ye, and Zhibo Li. 2017. "Synthesis of Aluminum Complexes Bearing 8-Anilide-5,6,7-trihydroquinoline Ligands: Highly Active Catalyst Precursors for Ring-Opening Polymerization of Cyclic Esters" Polymers 9, no. 3: 83. https://doi.org/10.3390/polym9030083
APA StyleLiu, S., Zhang, J., Zuo, W., Zhang, W., Sun, W. -H., Ye, H., & Li, Z. (2017). Synthesis of Aluminum Complexes Bearing 8-Anilide-5,6,7-trihydroquinoline Ligands: Highly Active Catalyst Precursors for Ring-Opening Polymerization of Cyclic Esters. Polymers, 9(3), 83. https://doi.org/10.3390/polym9030083