Synthesis of Dimethyl-Substituted Polyviologen and Control of Charge Transport in Electrodes for High-Resolution Electrochromic Displays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Dimethyl-Substituted Polyviologen 2
2.3. Measurements
3. Results and Discussion
3.1. Synthesis of Dimethyl-Substituted Polyviologen and Electrochemical Properties
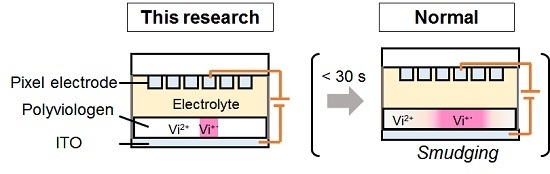
3.2. Suppression of Smudging for Electrochromic Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gracia, R.; Mecerreyes, D. Polymers with redox properties: Materials for batteries, biosensors and more. Polym. Chem. 2013, 4, 2206. [Google Scholar] [CrossRef]
- Casado, N.; Hernandez, G.; Sardon, H.; Mecerreyes, D. Progress in polymer science current trends in redox polymers for energy and medicine. Prog. Polym. Sci. 2016, 52, 107–135. [Google Scholar] [CrossRef]
- Sato, K.; Yamasaki, T.; Mizuma, T.; Oyaizu, K.; Nishide, H. Dynamic switching of ionic conductivity by cooperative interaction of polyviologen and liquid crystals for efficient charge storage. J. Mater. Chem. A 2016, 4, 3249–3252. [Google Scholar] [CrossRef]
- Tokue, H.; Kakitani, K.; Nishide, H.; Oyaizu, K. Electrochemical current rectification with cross reaction at a TEMPO/viologen-substituted polymer thin-layer heterojunction. RSC Adv. 2016, 6, 99195–99201. [Google Scholar] [CrossRef]
- Sasada, Y.; Langford, S.J.; Oyaizu, K.; Nishide, H. Poly(norbornyl-NDIs) as a potential cathode-active material in rechargeable charge storage devices. RSC Adv. 2016, 6, 42911–42916. [Google Scholar] [CrossRef]
- Kato, R.; Yoshimasa, K.; Egashira, T.; Oya, T.; Oyaizu, K.; Nishide, H. A ketone/alcohol polymer for cycle of electrolytic hydrogen-fixing with water and releasing under mild conditions. Nat. Commun. 2016, 7, 13032. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, K.; Ando, Y.; Konishi, H.; Nishide, H. Nernstian adsorbate-like bulk layer of organic radical polymers for high-density charge storage purposes. J. Am. Chem. Soc. 2008, 130, 14459–14461. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Tomita, W.; Hara, S.; Min, C.M.; Lee, J.S.; Oyaizu, K.; Nishide, H. Polyviologen hydrogel with high-rate capability for anodes toward an aqueous electrolyte-type and organic-based rechargeable device. ACS Appl. Mater. Interfaces 2013, 5, 1355–1361. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Hayashi, N.; Oyaizu, K.; Honda, K.; Nishide, H. Totally organic polymer-based electrochromic cell using tempo-substituted polynorbornene as a counter electrode-active material. Polym. J. 2008, 40, 763–767. [Google Scholar] [CrossRef]
- Argun, A.A.; Aubert, P.H.; Thompson, B.C.; Schwendeman, I.; Gaupp, C.L.; Hwang, J.; Pinto, N.J.; Tanner, D.B.; Macdiarmid, A.G.; Reynolds, J.R. Multicolored electrochromism in polymers: Structures and devices. Chem. Mater. 2004, 16, 4401–4412. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Displays 2006, 27, 2–18. [Google Scholar] [CrossRef]
- Vasilyeva, S.V.; Beaujuge, P.M.; Wang, S.; Babiarz, J.E.; Ballarotto, V.W.; Reynolds, J.R. Material strategies for black-to-transmissive window-type polymer electrochromic devices. ACS Appl. Mater. 2011, 4, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, S.V.; Unur, E.; Walczak, R.M.; Donoghue, E.P.; Rinzler, A.G.; Reynolds, J.R. Color purity in polymer electrochromic window devices on indium-tin oxide and single-walled carbon nanotube electrodes. ACS Appl. Mater. Interfaces 2009, 1, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Sato, T.; Zhang, J.; Moriyama, S.; Higuchi, M. Three-dimensional Fe(II)-based metallo-supramolecular polymers with electrochromic properties of quick switching, large contrast, and high coloration efficiency. ACS Appl. Mater. Interfaces 2014, 6, 9118–9125. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M. Stimuli-responsive metallo-supramolecular polymer films: Design, synthesis and device fabrication. J. Mater. Chem. C 2014, 2, 9331–9341. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Solution-processable triarylamine-based electroactive high performance polymers for anodically electrochromic applications. Polym. Chem. 2012, 3, 255. [Google Scholar] [CrossRef]
- Kline, W.M.; Lorenzini, R.G.; Sotzing, G.A. A review of organic electrochromic fabric devices. Color. Technol. 2014, 130, 73–80. [Google Scholar] [CrossRef]
- Li, Y.; Michinobu, T. Click synthesis and reversible electrochromic behaviors of novel polystyrenes bearing aromatic amine units. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2111–2120. [Google Scholar] [CrossRef]
- Gonzalo, C.P.; Garcia, R.M.; Telleria, M.S.; Alonso, J.A.P.; Telleria, H.J.G. Viologen-based electrochromic compositions which can be formulated and applied at room temperature. U.S. Pat. Appl. 2008, 12, 865–869. [Google Scholar]
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic Systems and the prospects for devices. Adv. Mater. 2001, 13, 783–793. [Google Scholar] [CrossRef]
- Yashiro, T.; Okada, Y.; Naijoh, Y.; Hirano, S.; Sagisaka, T.; Gotoh, D.; Inoue, M.; Kim, S.; Tsuji, K.; Takahashi, H.; Fujimura, K. Flexible electrochromic display. Proc. IDW 2013, 13, 1300–1303. [Google Scholar]
- Naijoh, Y.; Yashiro, T.; Hirano, S.; Okada, Y.; Kim, S.; Tsuji, K.; Takahashi, H.; Fujimura, K.; Kondoh, H. Multi-layered electrochromic display. Proc. IDW 2011, 13, 375–378. [Google Scholar]
- Heikenfeld, J.; Drzaic, P.; Yeo, J.S.; Koch, T. Review paper: A critical review of the present and future prospects for electronic paper. J. Soc. Inf. Disp. 2011, 19, 129–148. [Google Scholar] [CrossRef]
- Meng, X.; Tang, F.; Peng, B.; Ren, J. Monodisperse hollow tricolor pigment particles for electronic paper. Nanoscale Res. Lett. 2010, 5, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Qiang, L.; Su, X.; Ren, J.; Tang, F. Synthesis of black magnetic electrophoretic particles for magnetic-electric dual-driven electronic paper. ACS Appl. Mater. Interfaces 2013, 5, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Monk, P.M.S. The Viologens: Physicochemical Properties, Synthesis and Applications of the Salts of 4,4′-Bipyridine, 1st ed.; Wiley-VCH: Weinheim, UK, 1999. [Google Scholar]
- Keshtov, M.L.; Udum, Y.A.; Toppare, L.; Kochurov, V.S.; Khokhlov, A.R. Synthesis of aromatic poly(pyridinium salt)s and their electrochromic properties. Mater. Chem. Phys. 2013, 139, 936–943. [Google Scholar] [CrossRef]
- Pozo-Gonzalo, C.; Salsamendi, M.; Vinuales, A.; Pomposo, J.A.; Grande, H.J. Highly transparent electrochromic plastic device that changes to purple and to blue by increasing the potential. Sol. Energy Mater. Sol. Cells 2009, 93, 2093–2097. [Google Scholar] [CrossRef]
- Bar, G.; Larina, N.; Grinis, L.; Lokshin, V.; Gvishi, R.; Kiryuschev, I.; Zaban, A.; Khodorkovsky, V. RGB organic electrochromic cells. Sol. Energy Mater. Sol. Cells 2012, 99, 123–128. [Google Scholar] [CrossRef]
- Beneduci, A.; Cospito, S.; La Deda, M.; Veltri, L.; Chidichimo, G. Electrofluorochromism in π-conjugated ionic liquid crystals. Nat. Commun. 2014, 5, 3105. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Nagashima, T.; Nakamura, K.; Kobayashi, N. Solar energy materials and solar cells continuous-tone images obtained using three primary-color electrochromic cells containing gel electrolyte. Sol. Energy Mater. Sol. Cells 2012, 104, 140–145. [Google Scholar] [CrossRef]
- Andersson, P.; Kawahara, J.; Berggren, M. Printed passive matrix addressed electrochromic displays. Org. Electron. 2013, 14, 3371–3378. [Google Scholar] [CrossRef]
- Cao, X.; Lau, C.; Liu, Y.; Wu, F.; Gui, H.; Liu, Q.; Wan, H.; Zhou, C. Fully screen-printed, large-area, and flexible active-matrix electrochromic displays using carbon nanotube thin-film transistors. ACS Nano 2016, 10, 9816–9822. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Bard, L.R.F. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Evgenij, B.J.; Ross, M. Impedance Spectroscopy Theory, Experiment, and Application, 2nd ed.; Wiley: New Jersey, NY, USA, 2005. [Google Scholar]
- Ue, M.; Ida, K.; Mori, S. Electrochemical properties of organic liquid electrolytes based on quaternary onium salts for electrical double-layer capacitors. J. Electrochem. Soc. 1994, 141, 2989. [Google Scholar] [CrossRef]
- Seki, S.; Serizawa, N.; Hayamizu, K.; Tsuzuki, S.; Umebayashi, Y.; Takei, K.; Miyashiro, H. Physicochemical and electrochemical properties of 1-ethyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate and 1-ethyl-3-methylimidazolium tetracyanoborate. J. Electrochem. Soc. 2012, 159, 967–971. [Google Scholar] [CrossRef]
- Porter, W.W.; Vaid, T.P. Isolation and characterization of phenyl viologen as a radical cation and neutral molecule. J. Org. Chem. 2005, 70, 5028–5035. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, N.; Mercier, N.; Toma, O.; Kassiba, A.H.; Zorina, L.; Auban-Senzier, P.; Pasquier, C. Unprecedented stacking of MV2+ dications and MV+ radical cations in the mixed-valence viologen salt (MV)2(BF4)3 (MV = methylviologen). Chem. Commun. 2013, 49, 10272–10274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iehl, J.; Frasconi, M.; Jacquot de Rouville, H.P.; Renaud, N.; Dyar, S.M.; Strutt, N.L.; Carmieli, R.; Wasielewski, M.R.; Ratner, M.A.; Nierengarten, J.F.; Stoddart, J.F. π-Dimerization of viologen subunits around the core of C60 from twelve to six directions. Chem. Sci. 2013, 4, 1462. [Google Scholar] [CrossRef]
- Lisowski, M.; Skopec, A. Effective area of thin guarded electrode in determining of permittivity and volume resistivity. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 24–31. [Google Scholar] [CrossRef]
- Chmielewski, A.G. Viscosity coefficients of some nematic liquid crystals. Mol. Cryst. Liq. Cryst. 1986, 132, 339–352. [Google Scholar] [CrossRef]
- Tachikawa, N.; Katayama, Y.; Miura, T. Electrode kinetics of ferrocenium/ferrocene in some amide-based room-temperature ionic liquids. Electrochem. Solid-State Lett. 2009, 3, F39–F41. [Google Scholar] [CrossRef]
- Shah, R.R.; Abbott, N.L. Coupling of the orientations of liquid crystals to electrical double layers formed by the dissociation of surface-immobilized salts. J. Phys. Chem. B 2001, 105, 4936–4950. [Google Scholar] [CrossRef]











| Entry | Solvent | Electrolyte salt | Concentration (mM) | Suppression of smudging |
|---|---|---|---|---|
| 1 | 5CB | EMIM TCB | 35 | Yes |
| 2 | 7BN | EMIM TCB | 35 | Yes |
| 3 | MBBA | EMIM TCB | 35 | Yes |
| 4 | 5CB | EMIM TCB | 350 | No |
| 5 | - | EMIM TCB | - | No |
| 6 | GBL | EMIM TCB | 35 | No |
| 7 | GBL | LiCF3SO3 | 1000 | No |
| 8 | GBL | LiCF3SO3 | 10 | No |
| 9 | GBL | - | 0 | No |
| 10 | Ethyl acetate | EMIM TCB | 35 | No |
| 11 | Benzonitrile | EMIM TCB | 35 | No |
| 12 | Hexane | EMIM TCB | Immiscible | - |
| 13 | Toluene | EMIM TCB | Immiscible | - |
| 14 | Chlorobenzene | EMIM TCB | Immiscible | - |
| 15 | Dichlorobenzene | EMIM TCB | Immiscible | - |
| 16 | 4-Pentylbiphenyl | EMIM TCB | Immiscible | - |
| Entry | Solvent | Electrolyte salt | Concentration (mM) | Ionic conductivity (mS/cm) | Viscosity (mPa∙s) 1 | Exchange current density i0 (mA/cm2) | Suppression of smudging |
|---|---|---|---|---|---|---|---|
| 1 | 5CB | EMIM TCB | 35 | 0.012 | 25 [42] | 0.00083 | Yes |
| 2 | 7BN | EMIM TCB | 35 | 0.41 | 6 | 0.0074 | Yes |
| 3 | - | EMIM TCB | - | 5.50 | 23 2 | 2.1 | No |
| 4 | GBL | LiCF3SO3 | 1000 | 5.8 | 1.7 2 | 2.3 | No |
| 5 | GBL | LiCF3SO3 | 10 | 0.95 | 1.7 2 | 1.4 | No |
| 6 | GBL | - | 0 | - | 1.7 2 | - | No |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, K.; Mizukami, R.; Mizuma, T.; Nishide, H.; Oyaizu, K. Synthesis of Dimethyl-Substituted Polyviologen and Control of Charge Transport in Electrodes for High-Resolution Electrochromic Displays. Polymers 2017, 9, 86. https://doi.org/10.3390/polym9030086
Sato K, Mizukami R, Mizuma T, Nishide H, Oyaizu K. Synthesis of Dimethyl-Substituted Polyviologen and Control of Charge Transport in Electrodes for High-Resolution Electrochromic Displays. Polymers. 2017; 9(3):86. https://doi.org/10.3390/polym9030086
Chicago/Turabian StyleSato, Kan, Ryusuke Mizukami, Takahiro Mizuma, Hiroyuki Nishide, and Kenichi Oyaizu. 2017. "Synthesis of Dimethyl-Substituted Polyviologen and Control of Charge Transport in Electrodes for High-Resolution Electrochromic Displays" Polymers 9, no. 3: 86. https://doi.org/10.3390/polym9030086
APA StyleSato, K., Mizukami, R., Mizuma, T., Nishide, H., & Oyaizu, K. (2017). Synthesis of Dimethyl-Substituted Polyviologen and Control of Charge Transport in Electrodes for High-Resolution Electrochromic Displays. Polymers, 9(3), 86. https://doi.org/10.3390/polym9030086