Electrical and Electrochemical Properties of Conducting Polymers
Abstract
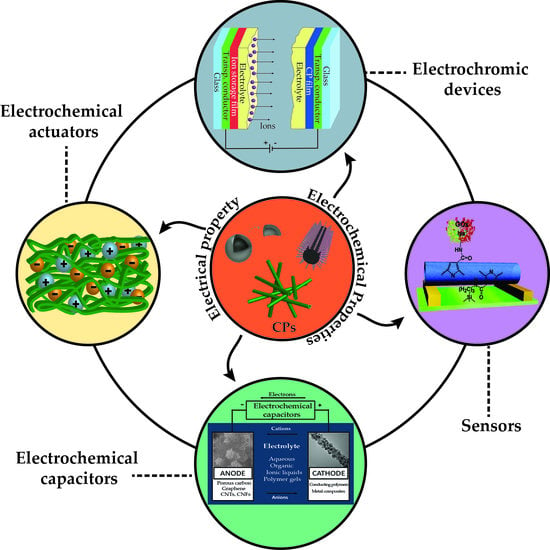
:1. Introduction
2. Conductive Mechanism
2.1. Inherent Molecular Structure
2.2. Doping
3. Electrical Properties
3.1. Tunable Conductivity
3.2. Charge Carrier Transport Models
3.3. Temperature Dependence
4. Electrochemical Properties
4.1. Reversible Oxidation/Reduction
4.2. Pseudocapacitance
4.3. Swelling and De-Swelling
4.4. Electrochromism
5. Applications
5.1. Electrochemical Capacitors
5.2. CP Sensors
5.2.1. Chemical Sensors
5.2.2. Biosensors
6. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CPs | Conducting polymers |
| PPy | Polypyrrole |
| PANI | Polyaniline |
| PT | Polythiophene |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PPV | Poly(p-phenylene vinylene) |
| HOMO | Highest occupied molecular orbital |
| LUMO | Lowest unoccupied molecular orbital |
| CSA | Camphor sulfonic acid |
| CV | Cyclic voltammetry |
| EDLCs | Electrochemical double layer capacitors |
| RGO | Graphene oxide |
| NCFC | Nitrogen-doped carbon fiber cloth |
| CNTs | Carbon nanotubes |
| PPCL | PPy/cellulose |
| FET | Field-effect transistor |
| CPNTs | Carboxylated polypyrrole nanotubes |
| GOx | Glucose oxidase |
| PSA | Prostate-specific antigen |
| BRCA1 | Breast cancer susceptibility gene |
References
- Hall, N. Twenty-five years of conducting polymers. Chem. Commun. 2003, 7, 1–4. [Google Scholar]
- Huang, W.S.; Humphrey, B.D.; MacDiarmid, A.G. Polyaniline, a novel conducting polymer. Morphology and chemistry of its oxidation and reduction in aqueous electrolytes. J. Chem. Soc. Faraday Trans. 1 1986, 82, 2385–2400. [Google Scholar] [CrossRef]
- McCullough, R.D.; Lowe, R.D.; Jayaraman, M.; Anderson, D.L. Design, synthesis, and control of conducting polymer architectures: Structurally homogeneous poly(3-alkylthiophenes). J. Org. Chem. 1993, 58, 904–912. [Google Scholar] [CrossRef]
- Wang, J.; Dai, J.; Yarlagadda, T. Carbon nanotube conducting polymer composite nanowires. Langmuir 2005, 21, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lee, J.H.; Kahng, Y.H.; Kim, N.; Kim, Y.J.; Lee, J.; Lee, T.; Lee, K. Graphene-conducting polymer hybrid transparent electrodes for efficient organic optoelectronic devices. Adv. Funct. Mater. 2014, 24, 1847–1856. [Google Scholar] [CrossRef]
- Gupta, S.; McDonald, B.; Carrizosa, S.B.; Price, C. Microstructure, residual stress, and intermolecular force distribution maps of graphene/polymer hybrid composites: Nanoscale morphology-promoted synergistic effects. Compos. Part B 2016, 92, 175–192. [Google Scholar] [CrossRef]
- Gupta, S.; Price, C.; Heintzman, E. Conducting polymer nanostructures and nanocomposites with carbon nanotubes: Hierarchical assembly by molecular electrochemistry, growth aspects and property characterization. J. Nanosci. Nanotechnol. 2016, 16, 374–391. [Google Scholar] [CrossRef] [PubMed]
- Cantwell, W.J.; Morton, J. The impact resistance of composite materials—A review. Composites 1991, 22, 347–362. [Google Scholar] [CrossRef]
- Gribkova, O.L.; Omelchenko, O.D.; Tameev, A.R.; Lypenko, D.A.; Nekrasov, A.A.; Posudievskii, O.Y.; Koshechko, V.G.; Vannikov, A.V. The specific effect of graphene additives in polyaniline-based nanocomposite layers on performance characteristics of electroluminescent and photovoltaic devices. High Energy Chem. 2016, 50, 134–138. [Google Scholar] [CrossRef]
- Biju, P.; Jining, X.; Jose, K.A.; Vijay, K.V. A new synthetic route to enhance polyaniline assembly on carbon nanotubes in tubular composites. Smart Mater. Struct. 2004, 13, N105. [Google Scholar]
- Kuo, C.T.; Chiou, W.H. Field-effect transistor with polyaniline thin film as semiconductor. Synth. Met. 1997, 88, 23–30. [Google Scholar] [CrossRef]
- Tang, H.; Kumar, P.; Zhang, S.; Yi, Z.; Crescenzo, G.D.; Santato, C.; Soavi, F.; Cicoira, F. Conducting polymer transistors making use of activated carbon gate electrodes. ACS Appl. Mater. Interfaces 2015, 7, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhao, L.; Xu, Y.; Qiu, T.; Zhi, L.; Shi, G. Polyaniline electrochromic devices with transparent graphene electrodes. Electrochim. Acta 2009, 55, 491–497. [Google Scholar] [CrossRef]
- Shen, K.Y.; Hu, C.W.; Chang, L.C.; Ho, K.C. A complementary electrochromic device based on carbon nanotubes/conducting polymers. Sol. Energy Mater. Sol. Cells 2012, 98, 294–299. [Google Scholar] [CrossRef]
- García-Gallegos, J.C.; Martín-Gullón, I.; Conesa, J.A.; Vega-Cantú, Y.I.; Rodríguez-Macías, F.J. The effect of carbon nanofillers on the performance of electromechanical polyaniline-based composite actuators. Nanotechnology 2016, 27, 015501. [Google Scholar] [CrossRef] [PubMed]
- May, T.; Van-Tan, T.; Geoffrey, M.S.; Gordon, G.W. Carbon nanotube and polyaniline composite actuators. Smart Mater. Struct. 2003, 12, 626. [Google Scholar]
- Jiang, X.; Setodoi, S.; Fukumoto, S.; Imae, I.; Komaguchi, K.; Yano, J.; Mizota, H.; Harima, Y. An easy one-step electrosynthesis of graphene/polyaniline composites and electrochemical capacitor. Carbon 2014, 67, 662–672. [Google Scholar] [CrossRef]
- Memon, M.A.; Bai, W.; Sun, J.; Imran, M.; Phulpoto, S.N.; Yan, S.; Huang, Y.; Geng, J. Conjunction of conducting polymer nanostructures with macroporous structured graphene thin films for high-performance flexible supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 11711–11719. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, H.; Kee, S.; Lee, S.H.; Jeong, S.Y.; Kim, G.; Kim, J.; Hong, S.; Back, H.; Lee, K. Long-term stable recombination layer for tandem polymer solar cells using self-doped conducting polymers. ACS Appl. Mater. Interfaces 2016, 8, 6144–6151. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.M.J.; Yang, L.; Gabrielsson, E.; Lohse, P.W.; Boschloo, G.; Sun, L.; Hagfeldt, A. Combining a small hole-conductor molecule for efficient dye regeneration and a hole-conducting polymer in a solid-state dye-sensitized solar cell. J. Phys. Chem. C 2012, 116, 18070–18078. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials 2013, 3, 524. [Google Scholar] [CrossRef] [PubMed]
- Stafström, S.; Chao, K.A. Polaron-bipolaron–soliton doping in polyacetylene. Phys. Rev. B 1984, 30, 2098–2103. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, bipolarons, and solitons in conducting polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Ravichandran, R.; Sundarrajan, S.; Venugopal, J.R.; Mukherjee, S.; Ramakrishna, S. Applications of conducting polymers and their issues in biomedical engineering. J. R. Soc. Interface 2010, 7, S559–S579. [Google Scholar] [CrossRef] [PubMed]
- Wise, D.L.; Wnek, G.E.; Trantolo, D.J.; Cooper, T.M.; Gresser, J.D.; Marcel, D. Electrical and Optical Polymer Systems: Fundamentals, Methods and Application; CRC Press: Boca Raton, FL, USA, 1998; pp. 1031–1040. [Google Scholar]
- Saxena, V.; Malhotra, B.D.; Menon, R. Charge transport and electrical properties of doped conjugated polymers. In Handbook of Polymers in Electronics; Malhotra, B.D., Ed.; Rapra Technology Limited: Shrewsbury, Shropshire, UK, 2002; pp. 3–65. [Google Scholar]
- Su, W.P.; Schrieffer, J.R.; Heeger, A.J. Solitons in polyacetylene. Phys. Rev. Lett. 1979, 42, 1698–1701. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Mammone, R.J.; Kaner, R.B.; Porter, S.J.; Pethig, R.; Heeger, A.J.; Rosseinsky, D.R. The concept of ‘doping’ of conducting polymers: The role of reduction potentials. Phil. Trans. R. Soc. A 1985, 314, 3–15. [Google Scholar] [CrossRef]
- Cuuran, S.; Hauser, A.S.; Roth, S. Conductive polymers: Synthesis and electrical properties. In Handbook of Organic Conductive Molecules and Polymers; Hari, S.N., Ed.; John Wiley & Sons: New York, NY, USA, 1997; Volume 2, pp. 1–60. [Google Scholar]
- Wan, M. Conducting Polymers with Micro or Nanometer Structure; Springer: New York, NY, USA, 2008; pp. 1–13. [Google Scholar]
- Li, Y. Conducting polymer. In Organic Optoelectronic Materials; Li, Y., Ed.; Springer International Publishing: New York, NY, USA, 2015; pp. 23–50. [Google Scholar]
- Zhang, Y.; de Boer, B.; Blom, P.W.M. Controllable molecular doping and charge transport in solution-processed polymer semiconducting layers. Adv. Funct. Mater. 2009, 19, 1901–1905. [Google Scholar] [CrossRef]
- Zhang, Y.; Blom, P.W.M. Electron and hole transport in poly(fluorene-benzothiadiazole). Appl. Phys. Lett. 2011, 98, 143504. [Google Scholar] [CrossRef]
- Nollau, A.; Pfeiffer, M.; Fritz, T.; Leo, K. Controlled n-type doping of a molecular organic semiconductor: Naphthalenetetracarboxylic dianhydride (NTCDA) doped with bis(ethylenedithio)-tetrathiafulvalene (BEDT-TTF). J. Appl. Phys. 2000, 87, 4340–4343. [Google Scholar] [CrossRef]
- Bajpai, M.; Srivastava, R.; Dhar, R.; Tiwari, R.S. Review on optical and electrical properties of conducting polymers. Indian J. Eng. Mater. Sci. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Patil, A.O.; Heeger, A.J.; Wudl, F. Optical properties of conducting polymers. Chem. Rev. 1988, 88, 183–200. [Google Scholar] [CrossRef]
- Borchert, H. Solar Cells Based on Colloidal Nanocrystals; Springer International Publishing: New York, NY, USA, 2014; pp. 39–60. [Google Scholar]
- Roth, S.; Bleier, H. Solitons in polyacetylene. Adv. Phys. 1987, 36, 385–462. [Google Scholar] [CrossRef]
- Heeger, A.J.; Kivelson, S.; Schrieffer, J.R.; Su, W.P. Solitons in conducting polymers. Rev. Mod. Phys. 1988, 60, 781–850. [Google Scholar] [CrossRef]
- Tsukamoto, J. Recent advances in highly conductive polyacetylene. Adv. Phys. 1992, 41, 509–546. [Google Scholar] [CrossRef]
- Tsukamoto, J.; Takahashi, A.; Kawasaki, K. Structure and electrical properties of polyacetylene yielding a conductivity of 105 S/cm. Jpn. J. Appl. Phys. 1990, 29, 125. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E. Electronic Processes in Non-Crystalline Materials; Oxford University Press: New York, NY, USA, 1979; pp. 7–97. [Google Scholar]
- Lee, P.A.; Ramakrishnan, T.V. Disordered electronic systems. Rev. Mod. Phys. 1985, 57, 287–337. [Google Scholar] [CrossRef]
- Markus, A.; Reghu, M. The localization-interaction model applied to the direct-current conductivity of metallic conducting polymers. J. Phys. Condens. Matter 1998, 10, 7171. [Google Scholar]
- Anderson, P.W. Absence of diffusion in certain random lattices. Phys. Rev. 1958, 109, 1492–1505. [Google Scholar] [CrossRef]
- Epstein, A.J.; Joo, J.; Kohlman, R.S.; Du, G.; MacDiarmid, A.G.; Oh, E.J.; Min, Y.; Tsukamoto, J.; Kaneko, H.; Pouget, J.P. Inhomogeneous disorder and the modified Drude metallic state of conducting polymers. Synth. Met. 1994, 65, 149–157. [Google Scholar] [CrossRef]
- Kohlman, R.S.; Zibold, A.; Tanner, D.B.; Ihas, G.G.; Ishiguro, T.; Min, Y.G.; MacDiarmid, A.G.; Epstein, A.J. Limits for metallic conductivity in conducting polymers. Phys. Rev. Lett. 1997, 78, 3915–3918. [Google Scholar] [CrossRef]
- Kohlman, R.S.; Joo, J.; Min, Y.G.; MacDiarmid, A.G.; Epstein, A.J. Crossover in electrical frequency response through an insulator-metal transition. Phys. Rev. Lett. 1996, 77, 2766–2769. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Long, S.M.; Pouget, J.P.; Oh, E.J.; MacDiarmid, A.G.; Epstein, A.J. Charge transport of the mesoscopic metallic state in partially crystalline polyanilines. Phys. Rev. B 1998, 57, 9567–9580. [Google Scholar] [CrossRef]
- Zuo, F.; Angelopoulos, M.; MacDiarmid, A.G.; Epstein, A.J. Transport studies of protonated emeraldine polymer: A granular polymeric metal system. Phys. Rev. B 1987, 36, 3475–3478. [Google Scholar] [CrossRef]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Wang, P.C.; MacDiarmid, A.G. Vapor phase secondary doping of polyaniline (emeraldine salt) thin films with o-chlorophenol investigated by UV-VIS-NIR: Effects of primary dopants, substrate surfaces, and pre-treatments of organic vapors. React. Funct. Polym. 2008, 68, 201–207. [Google Scholar] [CrossRef]
- Kulszewicz-Bajer, I.; Proń, A.; Abramowicz, J.; Jeandey, C.; Oddou, J.L.; Sobczak, J.W. Lewis acid doped polyaniline: Preparation and spectroscopic Characterization. Chem. Mater. 1999, 11, 552–556. [Google Scholar] [CrossRef]
- Chiang, C.K.; Blubaugh, E.A.; Yap, W.T. Electrochemical studies on doping of polyacetylene. Polymer 1984, 25, 1112–1116. [Google Scholar] [CrossRef]
- Chiang, C.K.; Druy, M.A.; Gau, S.C.; Heeger, A.J.; Louis, E.J.; MacDiarmid, A.G.; Park, Y.W.; Shirakawa, H. Synthesis of highly conducting films of derivatives of polyacetylene, (CH)x. J. Am. Chem. Soc. 1978, 100, 1013–1015. [Google Scholar] [CrossRef]
- Ivory, D.M.; Miller, G.G.; Sowa, J.M.; Shacklette, L.W.; Chance, R.R.; Baughman, R.H. Highly conducting charge-transfer complexes of poly(p-phenylene). J. Chem. Phys 1979, 71, 1506–1507. [Google Scholar] [CrossRef]
- Han, C.C.; Elsenbaumer, R.L. Protonic acids: Generally applicable dopants for conducting polymers. Synth. Met. 1989, 30, 123–131. [Google Scholar] [CrossRef]
- Wnek, G.E.; Chien, J.C.W.; Karasz, F.E.; Lillya, C.P. Electrically conducting derivative of poly(p-phenylene vinylene). Polymer 1979, 20, 1441–1443. [Google Scholar] [CrossRef]
- Jeon, I.-R.; Noma, N.; Claridge, R.F.C.; Shirota, Y. Electrochemical doping of poly(3-vinylperylene) and electrical properties of doped polymers. Polym. J. 1992, 24, 273–279. [Google Scholar] [CrossRef]
- Salmon, M.; Diaz, A.F.; Logan, A.J.; Krounbi, M.; Bargon, J. Chemical modification of conducting polypyrrole films. Mol. Cryst. Liq. Cryst. 1982, 83, 265–276. [Google Scholar] [CrossRef]
- Gupta, S. Template-free synthesis of conducting-polymer polypyrrole micro/nanostructures using electrochemistry. Appl. Phys. Lett. 2006, 88, 063108. [Google Scholar] [CrossRef]
- Brie, M.; Turcu, R.; Neamtu, C.; Pruneanu, S. The effect of initial conductivity and doping anions on gas sensitivity of conducting polypyrrole films to NH3. Sens. Actuators B 1996, 37, 119–122. [Google Scholar] [CrossRef]
- Maddison, D.S.; Jenden, C.M. Dopant exchange in conducting polypyrrole films. Polym. Int. 1992, 27, 231–235. [Google Scholar] [CrossRef]
- Arribas, C.; Rueda, D. Preparation of conductive polypyrrole-polystyrene sulfonate by chemical polymerization. Synth. Met. 1996, 79, 23–26. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, Y.; Ahn, K.J.; Huh, J.; Shim, H.W.; Sampath, G.; Im, W.B.; Huh, Y.I.; Yoon, H. Role of co-vapors in vapor deposition polymerization. Sci. Rep. 2015, 5, 8420. [Google Scholar] [CrossRef] [PubMed]
- Taunk, M.; Kapil, A.; Chand, S. Chemical synthesis and low temperature electrical transport in polypyrrole doped with sodium bis(2-ethylhexyl) sulfosuccinate. J. Mater. Sci. Mater. Electron. 2011, 22, 136–142. [Google Scholar] [CrossRef]
- Holland, E.R.; Pomfret, S.J.; Adams, P.N.; Monkman, A.P. Conductivity studies of polyaniline doped with CSA. J. Phys. Condens. Matter 1996, 8, 2991. [Google Scholar] [CrossRef]
- Macdiarmid, A.G.; Chiang, J.-C.; Halpern, M.; Huang, W.-S.; Mu, S.-L.; Nanaxakkara, L.D.; Wu, S.W.; Yaniger, S.I. “Polyaniline”: Interconversion of metallic and insulating forms. Mol. Cryst. Liq. Cryst. 1985, 121, 173–180. [Google Scholar] [CrossRef]
- Lee, Y.W.; Do, K.; Lee, T.H.; Jeon, S.S.; Yoon, W.J.; Kim, C.; Ko, J.; Im, S.S. Iodine vapor doped polyaniline nanoparticles counter electrodes for dye-sensitized solar cells. Synth. Met. 2013, 174, 6–13. [Google Scholar] [CrossRef]
- Palaniappan, S.; Devi, S.L. Novel chemically synthesized polyaniline electrodes containing a fluoroboric acid dopant for supercapacitors. J. Appl. Polym. Sci. 2008, 107, 1887–1892. [Google Scholar] [CrossRef]
- Kao, C.Y.; Lee, B.; Wielunski, L.S.; Heeney, M.; McCulloch, I.; Garfunkel, E.; Feldman, L.C.; Podzorov, V. Doping of conjugated polythiophenes with alkyl silanes. Adv. Funct. Mater. 2009, 19, 1906–1911. [Google Scholar] [CrossRef]
- Glaudell, A.M.; Cochran, J.E.; Patel, S.N.; Chabinyc, M.L. Impact of the doping method on conductivity and thermopower in semiconducting polythiophenes. Adv. Energy Mater. 2015, 5, 1401072. [Google Scholar] [CrossRef]
- Chayer, M.; Faïd, K.; Leclerc, M. Highly conducting water-soluble polythiophene derivatives. Chem. Mater. 1997, 9, 2902–2905. [Google Scholar] [CrossRef]
- Dillingham, T.R.; Cornelison, D.M.; Townsend, S.W. Structural and chemical characterization of vapor-deposited polythiophene films. J. Vac. Sci. Technol. A 1996, 14, 1494–1498. [Google Scholar] [CrossRef]
- Su, N. Improving electrical conductivity, thermal stability, and solubility of polyaniline-polypyrrole nanocomposite by doping with anionic spherical polyelectrolyte brushes. Nanoscale Res. Lett. 2015, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, M.; Reghu, M.; Heeger, A.J. The temperature dependence of the conductivity in the critical regime of the metal-insulator transition in conducting polymers. J. Phys. Condens. Matter 1997, 9, 4145. [Google Scholar] [CrossRef]
- Ahlskog, M.; Reghu, M.; Heeger, A.J.; Noguchi, T.; Ohnishi, T. Electronic transport in the metallic state of oriented poly(p-phenylenevinylene). Phys. Rev. B 1996, 53, 15529–15537. [Google Scholar] [CrossRef]
- Chiang, C.K.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G. Conducting polymers: Halogen doped polyacetylene. J. Chem. Phys 1978, 69, 5098–5104. [Google Scholar] [CrossRef]
- Roth, S.; Bleier, H.; Pukacki, W. Charge transport in conducting polymers. Farad. Discuss. 1989, 88, 223–233. [Google Scholar] [CrossRef]
- Roth, S.; Carroll, D. One-Dimensional Metals: Conjugated Polymers, Organic Crystals, Carbon Nanotubes; Wiley-VCH: Weinheim, Germany, 2004; pp. 200–300. [Google Scholar]
- Aleshin, A.; Kiebooms, R.; Menon, R.; Wudl, F.; Heeger, A.J. Metallic conductivity at low temperatures in poly(3,4-ethylenedioxythiophene) doped with PF6. Phys. Rev. B 1997, 56, 3659–3663. [Google Scholar] [CrossRef]
- Lee, K.; Cho, S.; Heum Park, S.; Heeger, A.J.; Lee, C.-W.; Lee, S.-H. Metallic transport in polyaniline. Nature 2006, 441, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.F.; Castillo, J.I.; Logan, J.A.; Lee, W.-Y. Electrochemistry of conducting polypyrrole films. J. Electroanal. Chem. Interfacial Electrochem. 1981, 129, 115–132. [Google Scholar] [CrossRef]
- Guay, J.; Paynter, R.; Dao, L.H. Synthesis and characterization of poly(diarylamines): A new class of electrochromic conducting polymers. Macromolecules 1990, 23, 3598–3605. [Google Scholar] [CrossRef]
- Park, H.W.; Kim, T.; Huh, J.; Kang, M.; Lee, J.E.; Yoon, H. Anisotropic growth control of polyaniline nanostructures and their morphology-dependent electrochemical characteristics. ACS Nano 2012, 6, 7624–7633. [Google Scholar] [CrossRef] [PubMed]
- Bull, R.A.; Fan, F.R.F.; Bard, A.J. Polymer films on electrodes: VII. Electrochemical behavior at polypyrrole-coated platinum and tantalum electrodes. J. Electrochem. Soc. 1982, 129, 1009–1015. [Google Scholar] [CrossRef]
- Beck, F.; Braun, P.; Oberst, M. Organic electrochemistry in the solid state-overoxidation of polypyrrole. Ber. Bunsenges. Phys. Chem. 1987, 91, 967–974. [Google Scholar] [CrossRef]
- Novák, P.; Rasch, B.; Vielstich, W. Overoxidation of polypyrrole in propylene carbonate: An in situ FTIR study. J. Electrochem. Soc. 1991, 138, 3300–3304. [Google Scholar] [CrossRef]
- Lewis, T.W.; Wallace, G.G.; Kim, C.Y.; Kim, D.Y. Studies of the overoxidation of polypyrrole. Synth. Met. 1997, 84, 403–404. [Google Scholar] [CrossRef]
- Li, Y.; Qian, R. Electrochemical overoxidation of conducting polypyrrole nitrate film in aqueous solutions. Electrochim. Acta 2000, 45, 1727–1731. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Idrees, F.; Ma, X. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Xie, D.H.; Cui, P.; Chen, X.Y. Conversion of a zinc salicylate complex into porous carbons through a template carbonization process as a superior electrode material for supercapacitors. RSC Adv. 2014, 4, 6664–6671. [Google Scholar] [CrossRef]
- Hu, F.; Li, W.; Zhang, J.; Meng, W. Effect of graphene oxide as a dopant on the electrochemical performance of graphene oxide/polyaniline composite. J. Mater. Sci. Technol. 2014, 30, 321–327. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Pieta, P.; Obraztsov, I.; D’Souza, F.; Kutner, W. Composites of conducting polymers and various carbon nanostructures for electrochemical supercapacitors. ECS J. Solid State Sci. Technol. 2013, 2, M3120–M3134. [Google Scholar] [CrossRef]
- Tang, C.; Chen, N.; Hu, X. Conducting polymer nanocomposites: Recent developments and future prospects. In Conducting Polymer Hybrids, 1st ed.; Kumar, V., Kalia, S., Swart, H.C., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 1–45. [Google Scholar]
- Pei, Q.; Inganaes, O. Electrochemical applications of the bending beam method. 1. Mass transport and volume changes in polypyrrole during redox. J. Phys. Chem. 1992, 96, 10507–10514. [Google Scholar] [CrossRef]
- Lizarraga, L.; Marı́a Andrade, E.; Victor Molina, F. Swelling and volume changes of polyaniline upon redox switching. J. Electroanal. Chem. 2004, 561, 127–135. [Google Scholar] [CrossRef]
- Otero, T.F.; Angulo, E.; Rodríguez, J.; Santamaría, C. Electrochemomechanical properties from a bilayer: Polypyrrole/non-conducting and flexible material-artificial muscle. J. Electroanal. Chem. 1992, 341, 369–375. [Google Scholar] [CrossRef]
- Bay, L.; Jacobsen, T.; Skaarup, S.; West, K. Mechanism of actuation in conducting polymers: Osmotic expansion. J. Phys. Chem. B 2001, 105, 8492–8497. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Travas-Sejdic, J. Nanostructural aspects of conducting-polymer actuators. In Nanostructured Conductive Polymers, 1st ed.; Eftekhari, A., Ed.; John Wiley & Sons: New York, NY, USA, 2010; pp. 599–630. [Google Scholar]
- Wataru, T.; Shyam, S.P.; Masaki, F.; Keiichi, K. Cyclic step-voltammetric analysis of cation-driven and anion-driven actuation in polypyrrole films. Jpn. J. Appl. Phys. 2002, 41, 7532. [Google Scholar]
- Maw, S.; Smela, E.; Yoshida, K.; Stein, R.B. Effects of monomer and electrolyte concentrations on actuation of PPy(DBS) bilayers. Synth. Met. 2005, 155, 18–26. [Google Scholar] [CrossRef]
- Aydemir, N.; Kilmartin, P.A.; Travas-Sejdic, J.; Kesküla, A.; Peikolainen, A.-L.; Parcell, J.; Harjo, M.; Aabloo, A.; Kiefer, R. Electrolyte and solvent effects in PPy/DBS linear actuators. Sens. Actuators B 2015, 216, 24–32. [Google Scholar] [CrossRef]
- Pagès, H.; Topart, P.; Lemordant, D. Wide band electrochromic displays based on thin conducting polymer films. Electrochim. Acta 2001, 46, 2137–2143. [Google Scholar] [CrossRef]
- Barnes, A.; Despotakis, A.; Wong, T.C.P.; Anderson, A.P.; Chambers, B.; Wright, P.V. Towards a ‘smart window’ for microwave applications. Smart Mater. Struct. 1998, 7, 752. [Google Scholar] [CrossRef]
- Stafström, S.; Brédas, J.L.; Epstein, A.J.; Woo, H.S.; Tanner, D.B.; Huang, W.S.; MacDiarmid, A.G. Polaron lattice in highly conducting polyaniline: Theoretical and optical studies. Phys. Rev. Lett. 1987, 59, 1464–1467. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Richter, A.F.; MacDiarmid, A.G.; Epstein, A.J. Polyaniline: Protonation/deprotonation of amine and imine sites. Synth. Met. 1989, 29, 151–156. [Google Scholar] [CrossRef]
- Banerjee, A.; Bhatnagar, S.; Upadhyay, K.K.; Yadav, P.; Ogale, S. Hollow Co0.85Se nanowire array on carbon fiber paper for high rate pseudocapacitor. ACS Appl. Mater. Interfaces 2014, 6, 18844–18852. [Google Scholar] [CrossRef] [PubMed]
- Schwendeman, I.; Hwang, J.; Welsh, D.M.; Tanner, D.B.; Reynolds, J.R. Combined visible and infrared electrochromism using dual polymer devices. Adv. Mater. 2001, 13, 634–637. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. High performance electrochemical supercapacitor from electrochemically synthesized nanostructured polyaniline. Mater. Lett. 2006, 60, 1466–1469. [Google Scholar] [CrossRef]
- Kalaji, M.; Murphy, P.J.; Williams, G.O. The study of conducting polymers for use as redox supercapacitors. Synth. Met. 1999, 102, 1360–1361. [Google Scholar] [CrossRef]
- Nagamuthu, S.; Vijayakumar, S.; Muralidharan, G. Biopolymer-assisted synthesis of λ-MnO2 nanoparticles as an electrode material for aqueous symmetric supercapacitor devices. Ind. Eng. Chem. Res. 2013, 52, 18262–18268. [Google Scholar] [CrossRef]
- Singh, A.K.; Sarkar, D.; Khan, G.G.; Mandal, K. Hydrogenated NiO nanoblock architecture for high performance pseudocapacitor. ACS Appl. Mater. Interfaces 2014, 6, 4684–4692. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.A.; Cook, J.B.; Kim, H.-S.; Tolbert, S.H.; Dunn, B. High Performance Pseudocapacitor Based on 2D Layered Metal Chalcogenide Nanocrystals. Nano Lett. 2015, 15, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Fang, G.; Pan, A.; Liang, S. Oxygen-incorporated MoS2 nanosheets with expanded interlayers for hydrogen evolution reaction and pseudocapacitor applications. ACS Appl. Mater. Interfaces 2016, 8, 33681–33689. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.; Sasmal, A.K.; Ray, C.; Dutta, S.; Pal, A.; Pal, T. Suitable morphology makes CoSn(OH)6 nanostructure a superior electrochemical pseudocapacitor. ACS Appl. Mater. Interfaces 2016, 8, 17987–17998. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Rakhi, R.B.; Alshareef, H.N. Morphology-dependent enhancement of the pseudocapacitance of template-guided tunable polyaniline nanostructures. J. Phys. Chem. C 2013, 117, 15009–15019. [Google Scholar] [CrossRef]
- Wang, Z.L.; He, X.J.; Ye, S.H.; Tong, Y.X.; Li, G.R. Design of polypyrrole/polyaniline double-walled nanotube arrays for electrochemical energy storage. ACS Appl. Mater. Interfaces 2014, 6, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Rakhi, R.B.; Alshareef, H.N. Facile synthesis of polyaniline nanotubes using reactive oxide templates for high energy density pseudocapacitors. J. Mater. Chem. A 2013, 1, 3315–3324. [Google Scholar] [CrossRef]
- Wang, K.; Huang, J.; Wei, Z. Conducting polyaniline nanowire arrays for high performance supercapacitors. J. Phys. Chem. C 2010, 114, 8062–8067. [Google Scholar] [CrossRef]
- Lee, Y.; Noh, S.; Kim, M.S.; Kong, H.J.; Im, K.; Kwon, O.S.; Kim, S.; Yoon, H. The effect of nanoparticle packing on capacitive electrode performance. Nanoscale 2016, 8, 11940–11948. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Lee, J.E.; Shim, H.W.; Jeong, M.S.; Im, W.B.; Yoon, H. Intrinsically conductive polymer binders for electrochemical capacitor application. RSC Adv. 2014, 4, 27939–27945. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Mishra, J.; Hatui, G.; Nayak, G.C. Conductive polymer composites based on carbon nanomaterials. In Conducting Polymer Hybrids, 1st ed.; Kumar, V., Kalia, S., Swart, H.C., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 117–142. [Google Scholar]
- Choi, H.; Ahn, K.J.; Lee, Y.; Noh, S.; Yoon, H. Free-standing, multilayered graphene/polyaniline-glue/graphene nanostructures for flexible, solid-state electrochemical capacitor application. Adv. Mater. Interfaces 2015, 2, 1500117. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.; Zhao, X.; Wu, L.; Zhang, Q. Graphene-wrapped polyaniline nanowire arrays on nitrogen-doped carbon fabric as novel flexible hybrid electrode materials for high-performance supercapacitor. Langmuir 2014, 30, 5306–5313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, G.; Wang, Z.; Yang, Y.; Shi, Z.; Gu, Z. Tube-covering-tube nanostructured polyaniline/carbon nanotube array composite electrode with high capacitance and superior rate performance as well as good cycling stability. Electrochem. Commun. 2008, 10, 1056–1059. [Google Scholar] [CrossRef]
- Huang, Z.H.; Song, Y.; Xu, X.X.; Liu, X.X. Ordered polypyrrole nanowire arrays grown on a carbon cloth substrate for a high-performance pseudocapacitor electrode. ACS Appl. Mater. Interfaces 2015, 7, 25506–25513. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xu, Q.; Wang, K.; Chen, J.; Chen, Z. Fabrication of free-standing hierarchical carbon nanofiber/graphene oxide/polyaniline films for supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Price, C. Investigating graphene/conducting polymer hybrid layered composites as pseudocapacitors: Interplay of heterogeneous electron transfer, electric double layers and mechanical stability. Compos. Part B 2016, 105, 46–59. [Google Scholar] [CrossRef]
- Lee, T.; Yun, T.; Park, B.; Sharma, B.; Song, H.-K.; Kim, B.-S. Hybrid multilayer thin film supercapacitor of graphene nanosheets with polyaniline: Importance of establishing intimate electronic contact through nanoscale blending. J. Mater. Chem. 2012, 22, 21092–21099. [Google Scholar] [CrossRef]
- Liu, T.; Finn, L.; Yu, M.; Wang, H.; Zhai, T.; Lu, X.; Tong, Y.; Li, Y. Polyaniline and polypyrrole pseudocapacitor electrodes with excellent cycling stability. Nano Lett. 2014, 14, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Xia, Q.; Yang, M.; Xia, H. Unique core-shell nanorod arrays with polyaniline deposited into mesoporous NiCo2O4 support for high-performance supercapacitor electrodes. ACS Appl. Mater. Interfaces 2016, 8, 6093–6100. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Lee, P.S. Redox active polyaniline-h-MoO3 hollow nanorods for improved pseudocapacitive performance. J. Phys. Chem. C 2015, 119, 9041–9049. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Mishra, S.; Shimpi, N.G. Synthesis and sensing applications of polyaniline nanocomposites: A review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Talaie, A. Conducting polymer based pH detector: A new outlook to pH sensing technology. Polymer 1997, 38, 1145–1150. [Google Scholar] [CrossRef]
- Chandrasekhar, P. Conducting Polymers, Fundamentals and Applications; Springer International Publishing: New York, NY, USA, 1999; pp. 483–508. [Google Scholar]
- Forzani, E.S.; Li, X.; Tao, N. Hybrid amperometric and conductometric chemical sensor based on conducting polymer nanojunctions. Anal. Chem. 2007, 79, 5217–5224. [Google Scholar] [CrossRef] [PubMed]
- Athawale, A.A.; Bhagwat, S.V.; Katre, P.P. Nanocomposite of Pd-polyaniline as a selective methanol sensor. Sens. Actuators B 2006, 114, 263–267. [Google Scholar] [CrossRef]
- Choudhury, A. Polyaniline/silver nanocomposites: Dielectric properties and ethanol vapour sensitivity. Sens. Actuators B 2009, 138, 318–325. [Google Scholar] [CrossRef]
- Sharma, S.; Nirkhe, C.; Pethkar, S.; Athawale, A.A. Chloroform vapour sensor based on copper/polyaniline nanocomposite. Sens. Actuators B 2002, 85, 131–136. [Google Scholar] [CrossRef]
- Barkade, S.S.; Pinjari, D.V.; Singh, A.K.; Gogate, P.R.; Naik, J.B.; Sonawane, S.H.; Ashokkumar, M.; Pandit, A.B. Ultrasound assisted miniemulsion polymerization for preparation of polypyrrole-zinc oxide (PPy/ZnO) functional latex for liquefied petroleum gas sensing. Ind. Eng. Chem. Res. 2013, 52, 7704–7712. [Google Scholar] [CrossRef]
- Suri, K.; Annapoorni, S.; Sarkar, A.K.; Tandon, R.P. Gas and humidity sensors based on iron oxide-polypyrrole nanocomposites. Sens. Actuators B 2002, 81, 277–282. [Google Scholar] [CrossRef]
- Geng, L.; Wang, S.; Zhao, Y.; Li, P.; Zhang, S.; Huang, W.; Wu, S. Study of the primary sensitivity of polypyrrole/r-Fe2O3 to toxic gases. Mater. Chem. Phys. 2006, 99, 15–19. [Google Scholar] [CrossRef]
- Tongpool, R. Effect of nitrogen dioxide and temperature on the properties of lead phthalocyanine in polypyrrole. Thin Solid Films 2003, 438–439, 14–19. [Google Scholar] [CrossRef]
- Kwon, O.S.; Hong, J.Y.; Park, S.J.; Jang, Y.; Jang, J. Resistive gas sensors based on precisely size-controlled polypyrrole nanoparticles: Effects of particle size and deposition method. J. Phys. Chem. C 2010, 114, 18874–18879. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Yoon, H.; Jang, J. Highly sensitive and selective chemiresistive sensors based on multidimensional polypyrrole nanotubes. Chem. Commun. 2012, 48, 10526–10528. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Park, S.J.; Lee, J.S.; Park, E.; Kim, T.; Park, H.-W.; You, S.A.; Yoon, H.; Jang, J. Multidimensional conducting polymer nanotubes for ultrasensitive chemical nerve agent sensing. Nano Lett. 2012, 12, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Park, C.S.; Park, S.J.; Noh, S.; Kim, S.; Kong, H.J.; Bae, J.; Lee, C.S.; Yoon, H. Carboxylic acid-functionalized conducting-polymer nanotubes as highly sensitive nerve-agent chemiresistors. Sci. Rep. 2016, 6, 33724. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Shim, H.W.; Kwon, O.S.; Huh, Y.-I.; Yoon, H. Real-time detection of metal ions using conjugated polymer composite papers. Analyst 2014, 139, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, S.; Kong, H.J.; Kwon, O.S.; Yoon, H. Tunable electrical-sensing performance of random-alternating layered graphene/polyaniline nanoarchitectures. J. Phys. Chem. C 2016, 120, 18289–18295. [Google Scholar] [CrossRef]
- Yoon, H.; Ko, S.; Jang, J. Field-effect-transistor sensor based on enzyme-functionalized polypyrrole nanotubes for glucose detection. J. Phys. Chem. B 2008, 112, 9992–9997. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Jang, J. A field-effect-transistor sensor based on polypyrrole nanotubes coupled with heparin for thrombin detection. Mol. Cryst. Liq. Cryst. 2008, 491, 21–31. [Google Scholar] [CrossRef]
- Park, S.J.; Song, H.S.; Kwon, O.S.; Chung, J.H.; Lee, S.H.; An, J.H.; Ahn, S.R.; Lee, J.E.; Yoon, H.; Park, T.H.; et al. Human dopamine receptor nanovesicles for gate-potential modulators in high-performance field-effect transistor biosensors. Sci. Rep. 2014, 4, 4342. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Ahn, S.R.; Park, S.J.; Song, H.S.; Lee, S.H.; Lee, J.S.; Hong, J.Y.; Lee, J.S.; You, S.A.; Yoon, H.; et al. Ultrasensitive and selective recognition of peptide hormone using close-packed arrays of hPTHR-conjugated polymer nanoparticles. ACS Nano 2012, 6, 5549–5558. [Google Scholar] [CrossRef] [PubMed]
- Song, H.S.; Kwon, O.S.; Lee, S.H.; Park, S.J.; Kim, U.K.; Jang, J.; Park, T.H. Human taste receptor-functionalized field effect transistor as a human-like nanobioelectronic tongue. Nano Lett. 2013, 13, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Lee, S.H.; Kwon, O.S.; Song, H.S.; Oh, E.H.; Park, T.H.; Jang, J. Polypyrrole nanotubes conjugated with human olfactory receptors: High-performance transducers for FET-type bioelectronic noses. Angew. Chem. Int. Ed. 2009, 48, 2755–2758. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Kaushik, A.; Arya, S.K.; Bhansali, S. Mediator free highly sensitive polyaniline-gold hybrid nanocomposite based immunosensor for prostate-specific antigen (PSA) detection. J. Mater. Chem. 2012, 22, 14763–14772. [Google Scholar] [CrossRef]
- Hui, N.; Sun, X.; Niu, S.; Luo, X. PEGylated polyaniline nanofibers: Antifouling and conducting biomaterial for electrochemical DNA sensing. ACS Appl. Mater. Interfaces 2017, 9, 2914–2923. [Google Scholar] [CrossRef] [PubMed]

















| CP | Repeat Unit | Chain Orientation | Conductivity (S·cm−1) |
|---|---|---|---|
| Polyacetylene | C2H2 | High | 104–105 |
| PPV | C6H4–C2H2 | High | 104 |
| PPy | C5H2N | Low | 400 |
| PANI | C6H4–NH | Low | 400 |
| Poly(3-methylthiophene) | C5H2S–CH3 | Low | 400 |
| PEDOT | C7H4O2S | Low | 300 |
| CP Type | Dopant | Chemical Source | Doping Method | Conductivity | References |
|---|---|---|---|---|---|
| Trans-polyacetylene | Na+ | (C10H8)Na | Solution doping | 80 | [56] |
| Poly(p-phenylene) | Vapor phase doping | 1.5 × 104 | [57] | ||
| Poly(p-phenylene vinylene) | CH3SO3H | CH3SO3H | Non-redox doping | 10.7 | [58] |
| Vapor phase doping | 57 | [59] | |||
| Poly(3-vinylperylene) | (C4H9)4N(ClO4) | Electrochemical doping | 10−5 | [60] | |
| PPy | C16H36AsF6N, (CH3)4N(PF6), (C2H5)4N(BF4) | Electrochemical doping | 30–100 | [61] | |
| NSA | 2-naphthalene sulfonic acid (NSA) | Electrochemical doping | 1–50 | [62] | |
| LiClO4 | Electrochemical doping | 65 | [63] | ||
| NaCl | Electrochemical doping | 10 | [64] | ||
| PSS/ | PSS/FeCl3 | Solution doping | 4 | [65] | |
| MeOH | MeOH | Vapor phase doping | 0.74 | [66] | |
| (C4H9)4N(HSO4) | Electrochemical doping | 0.3 | [61] | ||
| C20H37O4SO3− | C20H37O4SO3Na | Solution doping | 4.5 | [67] | |
| PANI | C10H15OSO3− | C10H16O4S | Solution doping | 300 | [68] |
| HC1 | HC1 | Non-redox doping | 10 | [69] | |
| I3− | I2 | Vapor phase doping | 9.3 | [70] | |
| BF4− | HBF4 | Solution doping | (2.3 × 10−1) | [71] | |
| PBTTT 1 | FTS 2 | C8H4F13SiCl3 | Vapor phase doping | 604–1.1 × 103 | [72,73] |
| Poly(2-(3-thienyloxy)ethanesulfonate) | Na2SO3 | Na2SO3 | Solution doping | 5 | [74] |
| PT | FeCl3 | Vapor phase doping | 10–25 | [75] | |
| PANI-PPy | ASPB | Anionic spherical polyelectrolyte brushes (ASPB) | Electrochemical doping | 8.3 | [76] |
| Doping Method | Controlled Variables | Advantages | Disadvantages |
|---|---|---|---|
| Chemical doping | Vapor pressure, Exposure time to dopant | Simple way to obtain doping upon exposure of the sample to a vapor of the dopant or immersion into a solution with the dopant | Performed as slowly as possible to avoid inhomogeneous doping |
| The doping levels obtained are not stable with respect to time | |||
| Unexpected structural distortion may cause electrical conductivity decay | |||
| Doping/de-doping shows low reversibility | |||
| Electrochemical doping | Amount of current passed | Doping level can be easily controlled by using an electrochemical cell with a controlled amount of current passed | Unexpected structural distortion may cause electrical conductivity decay |
| Doping/de-doping is highly reversible and clean polymer can be retrieved | |||
| Can be achieved with many dopant species | |||
| Photo doping | Radiation energy of light beam | Charge carrier is formed without chemical compound (dopants) | The electrical conductivity disappears rapidly when irradiation is discontinued due to recombination of electrons and holes |
| No distortion of the material structure | |||
| Non-redox doping | Protonic acid strength | Number of electrons generally does not change | Depends on the degree of oxidation of CPs and degree of protonation of the material |
| Low conductivities are observed for some CPs | |||
| Charge-injection doping | Applying an appropriate potential on the polymer structure | Does not generate counter ions. Minimized distortion | Coulombic interaction between charge and dopant ion is very strong and can lead to change in the energetics of the system |
| CP Type | Metallic | Critical | Insulating | |||
|---|---|---|---|---|---|---|
| σ (S·cm−1) | σ (S·cm−1) | σ (S·cm−1) | ||||
| Polyacetylene-I2 | <10 | >5000 | 10–20 | 3–5 × 104 | >20 | <3000 |
| Polyacetylene-I2 | <5 | >5 × 104 | 9.8–165 | 2–5 × 104 | >400 | <2 × 104 |
| Polyacetylene-FeCl3 | <2 | >2 × 104 | 2.6–11.4 | 1–2 × 104 | >27 | <104 |
| PPV-AsF5 | <5 | 300–2400 | 9.7–34 | 100–300 | >50 | <100 |
| PPV-H2SO4 | <2 | >4 × 103–104 | 4.7–27 | 1000–4000 | >60 | <1000 |
| PPy | <2 | 300–400 | 2–10 | 200–300 | >10 | <200 |
| PANI | <2 | 250–350 | 2–5 | 200–250 | >10 | <200 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. https://doi.org/10.3390/polym9040150
Le T-H, Kim Y, Yoon H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers. 2017; 9(4):150. https://doi.org/10.3390/polym9040150
Chicago/Turabian StyleLe, Thanh-Hai, Yukyung Kim, and Hyeonseok Yoon. 2017. "Electrical and Electrochemical Properties of Conducting Polymers" Polymers 9, no. 4: 150. https://doi.org/10.3390/polym9040150
APA StyleLe, T.-H., Kim, Y., & Yoon, H. (2017). Electrical and Electrochemical Properties of Conducting Polymers. Polymers, 9(4), 150. https://doi.org/10.3390/polym9040150