A High-Performance Soy Protein Isolate-Based Nanocomposite Film Modified with Microcrystalline Cellulose and Cu and Zn Nanoclusters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
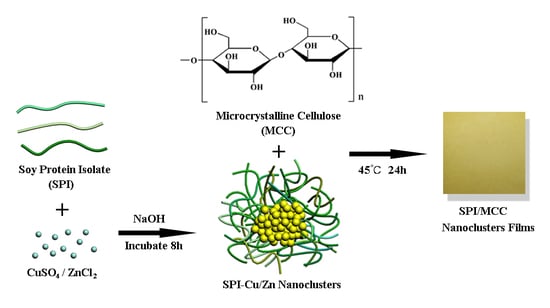
2.2. Fabrication of SPI-Cu NCs and SPI-Zn NCs
2.3. Preparation of SPI-MCC Nanocomposite Films
2.4. Characterization of SPI–MCC Nanocomposite Films
2.4.1. High Resolution Transmission Electron Microscopy (HRTEM)
2.4.2. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy
2.4.3. X-ray Diffraction Analysis
2.4.4. Scanning Electron Microscope
2.4.5. Mechanical Properties
2.4.6. Thermogravimetric Analysis
2.4.7. Contact Angles Determination
2.4.8. Water Resistance
3. Results
3.1. Characterization of SPI-Based Cu NCs and Zn NCs
3.2. Structural Analysis
3.3. Micromorphology
3.4. Physical and Mechanical Properties
3.5. Thermal Stabilities
3.6. Water Resistance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Rawdkuen, S. Effect of protein concentrations on the properties of fish myofibrillar protein based film compared with PVC film. J. Food Sci. Technol. 2016, 53, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Dong, Y.; Zhang, W.; Zhang, S.; Li, L.; Li, J. Preparation of cross-linked soy protein isolate-based environmentally-friendly films enhanced by PTGE and PAM. Ind. Crops Prod. 2015, 67, 373–380. [Google Scholar] [CrossRef]
- Li, K.; Chen, H.; Li, Y.; Li, J.; He, J. Endogenous Cu and Zn nanocluster-regulated soy protein isolate films: Excellent hydrophobicity and flexibility. RSC Adv. 2015, 5, 66543–66548. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yang, H.-J.; Lee, K.-Y.; Song, K.B. Preparation and application of a flaxseed meal protein film containing lemongrass (Cymbopogon citratus) oil. Int. J. Food Sci. Technol. 2016, 51, 1473–1480. [Google Scholar] [CrossRef]
- Pan, H.; Jiang, B.; Chen, J.; Jin, Z. Blend-modification of soy protein/lauric acid edible films using polysaccharides. Food Chem. 2014, 151, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, Y.; Yao, J.; Shao, Z.; Chen, X. Robust soy protein films obtained by slight chemical modification of polypeptide chains. Polym. Chem. 2013, 4, 5425–5431. [Google Scholar] [CrossRef]
- Kang, H.; Song, X.; Wang, Z.; Zhang, W.; Zhang, S.; Li, J. High-performance and fully renewable soy protein isolate-based film from microcrystalline cellulose via bio-inspired poly(dopamine) surface modification. ACS Sustain. Chem. Eng. 2016, 4, 4354–4360. [Google Scholar] [CrossRef]
- Coupry, D.E.; Butson, J.; Petkov, P.S.; Saunders, M.; O'Donnell, K.; Kim, H.; Buckley, C.; Addicoat, M.; Heine, T.; Szilagyi, P.A. Controlling embedment and surface chemistry of nanoclusters in metal-organic frameworks. Chem. Commun. 2016, 52, 5175–5178. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, G.U. Metal nanoclusters: Protein corona formation and implications for biological applications. Int. J. Biochem. Cell. B 2016, 75, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Li, M.; Ren, J.; Qu, X. Metal nanoclusters: Novel probes for diagnostic and therapeutic applications. Chem. Soc. Rev. 2015, 44, 8636–8663. [Google Scholar] [CrossRef] [PubMed]
- Jin, R. Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 2015, 7, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, J.; Gao, Y.; Liu, H.; Li, T.; Zou, H.; Wang, Z.; Zhang, K.; Wang, Y.; Zhang, H.; Yang, B. Assembly-induced enhancement of Cu nanoclusters luminescence with mechanochromic property. J. Am. Chem. Soc. 2015, 137, 12906–12913. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lin, L.; Li, H.; Li, J.; Lin, J.-M. Aggregation-induced structuretransition of protein-stabilized zinc/copper nanoclusters for amplified chemiluminescence. ACS Nano 2015, 9, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-L.; Jo, Y.; Cha, M.; Cha, Y.; Yoon, D.; Sanandiya, N.; Prajatelistia, E.; Oh, D.; Hwang, D. Mussel-inspired anisotropic nanocellulose and silver nanoparticle composite with improved mechanical properties, electrical conductivity and antibacterial activity. Polymers 2016, 8, 102. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Shukla, M. Physico-Chemical Characterization of Cellulose Extracted from Ficus Leaves. J. Biobased Mater. Bioenergy 2013, 7, 496–499. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Cellulose reinforced polymer composites and nanocomposites: A critical review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of microcrystalline cellulose on the microrheological property and freeze-thaw stability of soybean protein hydrolysate stabilized curcumin emulsion. LWT-Food Sci. Technol. 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Da Silva, J.R.T.; de O. Farias, E.A.; Filho, E.C.S.; Eiras, C. Development and characterization of composites based on polyaniline and modified microcrystalline cellulose with anhydride maleic as platforms for electrochemical trials. Colloid Polym. Sci. 2014, 293, 1049–1058. [Google Scholar] [CrossRef]
- Vanhatalo, K.; Lundin, T.; Koskimäki, A.; Lillandt, M.; Dahl, O. Microcrystalline cellulose property–structure effects in high-pressure fluidization: Microfibril characteristics. J. Mater. Sci. 2016, 51, 6019–6034. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Chand, N.; Ahuja, S.; Roy, M.K. Vapor induced phase inversion technique to prepare chitosan/microcrystalline cellulose composite films: Synthesis, characterization and moisture absorption study. Cellulose 2015, 22, 3825–3837. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.-X.; Lian, Z.-X.; Wang, X.-X.; Zhou, J.; Ma, Z.-S. The effects of ultrasonic/microwave assisted treatment on the properties of soy protein isolate/microcrystalline wheat-bran cellulose film. J. Food Eng. 2013, 114, 183–191. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Dong, Y.; Li, K.; Li, L.; Li, J. Carbon nanoparticles/soy protein isolate bio-films with excellent mechanical and water barrier properties. Ind. Crops Prod. 2016, 82, 133–140. [Google Scholar] [CrossRef]
- González, A.; Strumia, M.C.; Alvarez Igarzabal, C.I. Cross-linked soy protein as material for biodegradable films: Synthesis, characterization and biodegradation. J. Food Eng. 2011, 106, 331–338. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Song, F.; Zhang, M.; Wang, X.-L.; Wang, Y.-Z. Roles of Soft Segment Length in Structure and Property of Soy Protein Isolate/Waterborne Polyurethane Blend Films. Ind. Eng. Chem. Res. 2016, 55, 1229–1235. [Google Scholar] [CrossRef]
- Koshy, R.R.; Mary, S.K.; Thomas, S.; Pothan, L.A. Environment friendly green composites based on soy protein isolate—A review. Food Hydrocoll. 2015, 50, 174–192. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.; Li, C.; Gan, L.; He, M.; He, X.; Tian, W.; Li, M.; Xu, L.; Li, Y.; Chen, Y. Improvement in physical and biological properties of chitosan/soy protein films by surface grafted heparin. Int. J. Biol. Macromol. 2016, 83, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Luo, J.; Qin, Z.; Chen, H.; Gao, Q.; Li, J. Mechanical and thermal properties of microcrystalline cellulose-reinforced soy protein isolate–gelatin eco-friendly films. RSC Adv. 2015, 5, 56518–56525. [Google Scholar] [CrossRef]
- Carpiné, D.; Dagostin, J.L.A.; de Andrade, E.F.; Bertan, L.C.; Mafra, M.R. Effect of the natural surfactant Yucca schidigera extract on the properties of biodegradable emulsified films produced from soy protein isolate and coconut oil. Ind. Crop. Prod. 2016, 83, 364–371. [Google Scholar] [CrossRef]
- Pang, J.; Wu, M.; Zhang, Q.; Tan, X.; Xu, F.; Zhang, X.; Sun, R. Comparison of physical properties of regenerated cellulose films fabricated with different cellulose feedstocks in ionic liquid. Carbohydr. Polym. 2015, 121, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yan, C.; Lei, X.; Qin, Z.; Yao, J. Novel approach to extract thermally stable cellulose nanospheres with high yield. Mater. Lett. 2014, 131, 12–15. [Google Scholar] [CrossRef]
- Ghaderi, M.; Mousavi, M.; Yousefi, H.; Labbafi, M. All-cellulose nanocomposite film made from bagasse cellulose nanofibers for food packaging application. Carbohyd. Polym. 2014, 104, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, W.; Zhang, S.; Li, L.; Li, J.; Zhang, Y. Preparation and characterization of poly(vinyl alcohol) and 1,2,3-propanetriol diglycidyl ether incorporated soy protein isolate-based films. J. Appl. Polym. Sci. 2015, 132, 42578. [Google Scholar] [CrossRef]
- Jensen, A.; Lim, L.T.; Barbut, S.; Marcone, M. Development and characterization of soy protein films incorporated with cellulose fibers using a hot surface casting technique. LWT-Food Sci. Technol. 2015, 60, 162–170. [Google Scholar] [CrossRef]
- Xiong, R.; Zhang, X.; Tian, D.; Zhou, Z.; Lu, C. Comparing microcrystalline with spherical nanocrystalline cellulose from waste cotton fabrics. Cellulose 2012, 19, 1189–1198. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Zhang, B.; Liu, J. Enhanced antibacterial activity of silver nanoparticles/halloysite nanotubes/graphene nanocomposites with sandwich-like structure. Sci. Rep. 2014, 4, 4551. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Yang, H.-J.; Lee, K.-Y.; Song, K.B. Physical properties and application of a red pepper seed meal protein composite film containing oregano oil. Food Hydrocoll. 2016, 55, 136–143. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Chen, Y.; Xia, W.; Xiong, Y.L.; Wang, H. Enhanced physicochemical properties of chitosan/whey protein isolate composite film by sodium laurate-modified TiO2 nanoparticles. Carbohydr. Polym. 2016, 138, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Anandjiwala, R.D.; Kumar, A. Thermal and mechanical properties of mandelic acid-incorporated soy protein films. J. Therm. Anal. Calorim. 2015, 123, 1273–1279. [Google Scholar] [CrossRef]
- Lee, J.-A.; Yoon, M.-J.; Lee, E.-S.; Lim, D.-Y.; Kim, K.-Y. Preparation and characterization of cellulose nanofibers (CNFs) from microcrystalline cellulose (MCC) and CNF/polyamide 6 composites. Macromol. Res. 2014, 22, 738–745. [Google Scholar] [CrossRef]
- Song, X.; Zhou, C.; Fu, F.; Chen, Z.; Wu, Q. Effect of high-pressure homogenization on particle size and film properties of soy protein isolate. Ind. Crops Prod. 2013, 43, 538–544. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, C.; Dong, Y.; Yan, Y.; Li, J.; Shi, S.Q.; Cai, L. Soy protein isolate-based films reinforced by surface modified cellulose nanocrystal. Ind. Crops Prod. 2016, 80, 207–213. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- González, A.; Alvarez Igarzabal, C.I. Nanocrystal-reinforced soy protein films and their application as active packaging. Food Hydrocoll. 2015, 43, 777–784. [Google Scholar] [CrossRef]
- Sharma, L.; Singh, C. Composite film developed from the blends of sesame protein isolate and gum rosin and their properties thereof. Polym. Compos. 2016. [Google Scholar] [CrossRef]
- Kang, H.; Wang, Z.; Zhang, W.; Li, J.; Zhang, S. Physico-chemical properties improvement of soy protein isolate films through caffeic acid incorporation and tri-functional aziridine hybridization. Food Hydrocoll. 2016, 61, 923–932. [Google Scholar] [CrossRef]








| Films | Thickness (mm) | TS (MPa) | EB (%) | E (MPa) |
|---|---|---|---|---|
| SPI | 0.158 (0.023) a | 2.91 (0.27) | 164.90 (0.07) | 55.48 (3.62) |
| SPI-Cu | 0.271 (0.015) | 4.78 (0.30) | 69.13 (0.03) | 144.00 (3.35) |
| SPI-Zn | 0.260 (0.017) | 4.56 (0.16) | 168.30 (0.12) | 118.50 (2.14) |
| SPI-MCC | 0.255 (0.027) | 4.04 (0.40) | 29.57 (0.07) | 154.90 (2.31) |
| SPI-MCC-Cu | 0.335 (0.019) | 13.95 (0.09) | 17.12 (0.15) | 554.70 (4.64) |
| SPI-MCC-Zn | 0.248 (0.020) | 6.52 (0.30) | 26.82 (0.10) | 258.40 (3.73) |
| Films | Ti1 (°C) | Tmax1 (°C) | Ti2 (°C) | Tmax2 (°C) |
|---|---|---|---|---|
| SPI | 146.72 | 239.98 | 286.55 | 304.85 |
| SPI-Cu | 155.83 | 241.63 | 291.94 | 308.17 |
| SPI-Zn | 178.68 | 251.38 | 295.28 | 312.84 |
| SPI-MCC | 168.42 | 235.85 | 304.94 | 328.39 |
| SPI-MCC-Cu | 183.59 | 243.26 | 309.54 | 329.06 |
| SPI-MCC-Zn | 170.44 | 243.28 | 305.16 | 310.81 |
| Films | Contact Angles (°) | MC (%) | TSM (%) | WA (%) |
|---|---|---|---|---|
| SPI | 48.36 (1.8) a | 15.80 (1.2) | 36.79 (1.0) | 197.32 (8.3) |
| SPI-Cu | 34.54 (2.8) | 15.44 (1.8) | 38.99 (0.9) | 210.21 (6.9) |
| SPI-Zn | 51.49 (2.8) | 15.49 (1.5) | 36.91 (0.8) | 200.06 (6.7) |
| SPI-MCC | 42.37 (1.1) | 13.40 (1.7) | 29.63 (1.2) | 119.86 (7.0) |
| SPI-MCC-Cu | 58.03 (1.9) | 11.68 (2.1) | 10.13 (0.8) | 94.86 (6.5) |
| SPI-MCC-Zn | 47.11 (2.1) | 13.96 (1.8) | 27.36 (1.0) | 162.88 (5.0) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Jin, S.; Chen, H.; He, J.; Li, J. A High-Performance Soy Protein Isolate-Based Nanocomposite Film Modified with Microcrystalline Cellulose and Cu and Zn Nanoclusters. Polymers 2017, 9, 167. https://doi.org/10.3390/polym9050167
Li K, Jin S, Chen H, He J, Li J. A High-Performance Soy Protein Isolate-Based Nanocomposite Film Modified with Microcrystalline Cellulose and Cu and Zn Nanoclusters. Polymers. 2017; 9(5):167. https://doi.org/10.3390/polym9050167
Chicago/Turabian StyleLi, Kuang, Shicun Jin, Hui Chen, Jing He, and Jianzhang Li. 2017. "A High-Performance Soy Protein Isolate-Based Nanocomposite Film Modified with Microcrystalline Cellulose and Cu and Zn Nanoclusters" Polymers 9, no. 5: 167. https://doi.org/10.3390/polym9050167
APA StyleLi, K., Jin, S., Chen, H., He, J., & Li, J. (2017). A High-Performance Soy Protein Isolate-Based Nanocomposite Film Modified with Microcrystalline Cellulose and Cu and Zn Nanoclusters. Polymers, 9(5), 167. https://doi.org/10.3390/polym9050167