Tannin-Based Copolymer Resins: Synthesis and Characterization by Solid State 13C NMR and FT-IR Spectroscopy
Abstract
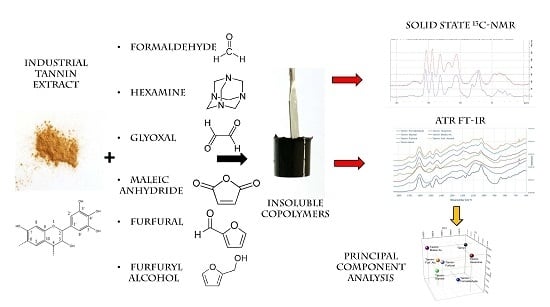
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Formulation Preparation and Hardening
2.2.2. Leachability Tests
2.2.3. 13C NMR Analysis
2.2.4. FT-IR Investigation
2.2.5. Principal Component Analysis (PCA)
3. Results and Discussion
3.1. Copolymer Preparation
3.2. Leaching Resistance
3.3. 13C NMR Investigation
3.3.1. Tannin–Formaldehyde (pH = 4.5)
3.3.2. Tannin–Hexamine (pH = 9.0)
3.3.3. Tannin–Glyoxal (pH = 4.5)
3.3.4. Tannin–Maleic Anhydride (pH = 4.5)
3.3.5. Tannin–Furfural (pH = 9.0)
3.3.6. Tannin–Furfuryl Alcohol (pH = 2.0)
3.4. FT–IR Spectroscopy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Douglas, G.B.; Wang, Y.; Waghorn, G.C.; Barry, T.N.; Purchas, R.W.; Foote, A.G.; Wilson, G.F. Liveweight gain and wool production of sheep grazing Lotus corniculatus and lucerne (Medicago sativa). N. Z. J. Agric. Res. 1995, 38, 95–104. [Google Scholar] [CrossRef]
- Meier, H.; Buchs, L.; Buchala, A.J.; Homewood, T. (1→3)-β-d-Glucan (callose) is a probable intermediate in biosynthesis of cellulose of cotton fibres. Nature 1981, 289, 821–822. [Google Scholar] [CrossRef]
- Woodings, C. Regenerated Cellulose Fibres; Woodhead Publishing: Abington, UK, 2001. [Google Scholar]
- Arbenz, A.; Avérous, L. Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem. 2015, 17, 2626–2646. [Google Scholar] [CrossRef]
- Pizzi, A. Phenolic and tannin-based adhesive resins by reactions of coordinated metal ligands. II. Tannin adhesive preparation, characteristics, and application. J. Appl. Polym. Sci. 1979, 24, 1257–1268. [Google Scholar] [CrossRef]
- Bisanda, E.T.N.; Ogola, W.O.; Tesha, J.V. Characterisation of tannin resin blends for particle board applications. Cem. Concr. Compos. 2003, 25, 593–598. [Google Scholar] [CrossRef]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: Opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Jahanshahi, S.; Pizzi, A.; Abdulkhani, A.; Shakeri, A. Analysis and testing of bisphenol A—Free bio-based tannin epoxy-acrylic adhesives. Polymers 2016, 8, 143. [Google Scholar] [CrossRef]
- Khanbabaee, K.; van Ree, T. Tannins: classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [PubMed]
- Bate-Smith, E.C.; Swain, T. Flavonoid compounds. Comp. Biochem. 1962, 3, 755–809. [Google Scholar]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Sen, S.; Tascioglu, C.; Tırak, K. Fixation, leachability, and decay resistance of wood treated with some commercial extracts and wood preservative salts. Int. Biodeterior. Biodegrad. 2009, 63, 135–141. [Google Scholar] [CrossRef]
- Zhan, K.; Ejima, H.; Yoshie, N. Antioxidant and adsorption properties of bioinspired phenolic polymers: A comparative study of catechol and gallol. ACS Sustain. Chem. Eng. 2016, 4, 3857–3863. [Google Scholar] [CrossRef]
- Haslam, E. Plant Polyphenols: Vegetable Tannins Revisited; CUP Archive: Oakleigh, Australia, 1989. [Google Scholar]
- Pizzi, A. Advanced Wood Adhesives Technology; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Pasch, H.; Pizzi, A.; Rode, K. MALDI–TOF mass spectrometry of polyflavonoid tannins. Polymer 2001, 42, 7531–7539. [Google Scholar] [CrossRef]
- Hemingway, R.W.; Karchesy, J.J. Chemistry and Significance of Condensed Tannins; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Pizzi, A.; Mittal, K.L. Wood Adhesives; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Theis, M.; Grohe, B. Biodegradable lightweight construction boards based on tannin/hexamine bonded hemp shaves. Holz als Roh und Werkstoff 2002, 60, 291–296. [Google Scholar] [CrossRef]
- Kain, G.; Güttler, V.; Barbu, M.C.; Petutschnigg, A.; Richter, K.; Tondi, G. Density related properties of bark insulation boards bonded with tannin hexamine resin. Eur. J. Wood Wood Prod. 2014, 72, 417–424. [Google Scholar] [CrossRef]
- Ballerini, A.; Despres, A.; Pizzi, A. Non-toxic, zero emission tannin-glyoxal adhesives for wood panels. Holz als Roh und Werkstoff 2005, 63, 477–478. [Google Scholar] [CrossRef]
- Santiago-Medina, F.J.; Pizzi, A.; Basso, M.C.; Delmotte, L.; Celzard, A. Polycondensation Resins by Flavonoid Tannins Reaction with Amines. Polymers 2017, 9, 37. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Bridson, J.H.; Lomas, C.; Elliot, J.A. Esterification of condensed tannins and their impact on the properties of poly (lactic acid). Polymers 2013, 5, 344–360. [Google Scholar] [CrossRef]
- Foo, L.Y.; Hemingway, R.W. Condensed tannins: Reactions of model compounds with furfuryl alcohol and furfuraldehyde. J. Wood Chem. Technol. 1985, 5, 135–158. [Google Scholar] [CrossRef]
- Hauptmann, M.; Gindl-Altmutter, W.; Hansmann, C.; Bacher, M.; Rosenau, T.; Liebner, F.; Schwanninger, M. Wood modification with tricine. Holzforschung 2015, 69, 985–991. [Google Scholar] [CrossRef]
- Banfi, D.; Patiny, L. Resurrecting and Processing NMR Spectra On-line. CHIMIA Int. J. Chem. 2008, 62, 280–281. [Google Scholar] [CrossRef]
- Castillo, A.M.; Patiny, L.; Wist, J. Fast and accurate algorithm for the simulation of NMR spectra of large spin systems. J. Magn. Reson. 2011, 209, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, C.; Krause, S.; Kuhn, S. NMRShiftDB constructing a free chemical information system with open-source components. J. Chem. Inf. Comput. Sci. 2003, 43, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Pichelin, F.; Kamoun, C.; Pizzi, A. Hexamine hardener behaviour: Effects on wood glueing, tannin and other wood adhesives. Eur. J. Wood Wood Prod. 1999, 57, 305–317. [Google Scholar] [CrossRef]
- Pilato, L. Phenolic Resins: A Century of Progress; Springer: New York, NY, USA, 2010; pp. 121–123. [Google Scholar]
- Roux, D.G.; Ferreira, D.; Hundt, H.K.; Malan, E. Structure, stereochemistry, and reactivity of natural condensed tannins as basis for their extended industrial application. Appl. Polym. Symp. 1975, 28, 335–353. [Google Scholar]
- Grenier-Loustalot, M.F.; Larroque, S.; Grenier, P.; Bedel, D. Phenolic resins: 4. Self-condensation of methylolphenols in formaldehyde-free media. Polymer 1996, 37, 955–964. [Google Scholar] [CrossRef]
- Rego, R.; Adriaensens, P.J.; Carleer, R.A.; Gelan, J.M. Fully quantitative carbon-13 NMR characterization of resol phenol–formaldehyde prepolymer resins. Polymer 2004, 45, 33–38. [Google Scholar] [CrossRef]
- Pizzi, A.; Stephanou, A. A comparative C13 NMR study of polyflavonoid tannin extracts for phenolic polycondensates. J. Appl. Polym. Sci. 1993, 50, 2105–2113. [Google Scholar] [CrossRef]
- Pizzi, A.; Scharfetter, H.O. The chemistry and development of tannin-based adhesives for exterior plywood. J. Appl. Polym. Sci. 1978, 22, 1745–1761. [Google Scholar] [CrossRef]
- Kiatgrajai, P.; Wellons, J.D.; Gollob, L.; White, J.D. Kinetics of polymerization of (+)-catechin with formaldehyde. J. Org. Chem. 1982, 47, 2913–2917. [Google Scholar] [CrossRef]
- Pizzi, A.; Valenezuela, J.; Westermeyer, C. Low formaldehyde emission, fast pressing, pine and pecan tannin adhesives for exterior particleboard. Eur. J. Wood Wood Prod. 1994, 52, 311–315. [Google Scholar] [CrossRef]
- Thevenon, M.F.; Tondi, G.; Pizzi, A. High performance tannin resin-boron wood preservatives for outdoor end-uses. Eur. J. Wood Wood Prod. 2009, 67, 89–93. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Pizzi, A.; Stauber, M.; Celzard, A. A new method for preparing tannin-based foams. Ind. Crop. Prod. 2014, 54, 40–53. [Google Scholar] [CrossRef]
- Pena, C.; De la Caba, K.; Retegi, A.; Ocando, C.; Labidi, J.; Echeverria, J.; Mondragon, I. Mimosa and chestnut tannin extracts reacted with hexamine in solution. J. Therm. Anal. Calorim. 2009, 96, 515–521. [Google Scholar] [CrossRef]
- Pichelin, F.; Nakatani, M.; Pizzi, A.; Wieland, S. Structural beams from thick wood panels bonded industrially with formaldehyde-free tannin adhesives. For. Prod. J. 2006, 56, 31–36. [Google Scholar]
- Pizzi, A.; Tekely, P. Mechanism of polyphenolic tannin resin hardening by hexamethylenetetramine: CP–MAS 13C NMR. J. Appl. Polym. Sci. 1995, 56, 1645–1650. [Google Scholar] [CrossRef]
- Ramires, E.C.; Megiatto, J.D.; Gardrat, C.; Castellan, A.; Frollini, E. Biobased composites from glyoxal–phenolic resins and sisal fibers. Bioresour. Technol. 2010, 101, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, B. Maleic Anhydride; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Rossouw, D.D.T.; Pizzi, A.; McGillivray, G. The kinetics of condensation of phenolic polyflavonoid tannins with aldehydes. J. Polym. Sci. Polym. Chem. Ed. 1980, 18, 3323–3343. [Google Scholar] [CrossRef]
- Gandini, A.; Belgacem, M.N. Furans in polymer chemistry. Prog. Polym. Sci. 1997, 22, 1203–1379. [Google Scholar] [CrossRef]
- Abdullah, U.H.B.; Pizzi, A. Tannin-furfuryl alcohol wood panel adhesives without formaldehyde. Eur. J. Wood Wood Prod. 2013, 71, 131–132. [Google Scholar] [CrossRef]
- Luckeneder, P.; Gavino, J.; Kuchernig, R.; Petutschnigg, A.; Tondi, G. Sustainable Phenolic Fractions as Basis for Furfuryl Alcohol-Based Co-Polymers and Their Use as Wood Adhesives. Polymers 2016, 8, 396. [Google Scholar] [CrossRef]
- Link, M.; Kolbitsch, C.; Tondi, G.; Ebner, M.; Wieland, S.; Petutschnigg, A. Formaldehyde-free tannin based foams and their use as lightweight panels. BioResources 2011, 6, 4218–4228. [Google Scholar]
- Basso, M.C.; Pizzi, A.; Lacoste, C.; Delmotte, L.; Al-Marzouki, F.M.; Abdalla, S.; Celzard, A. MALDI-TOF and 13C NMR analysis of tannin-furanic-polyurethane foams adapted for industrial continuous lines application. Polymers 2014, 6, 2985–3004. [Google Scholar] [CrossRef]
- Tondi, G.; Link, M.; Oo, C.W.; Petutschnigg, A. A simple approach to distinguish classic and formaldehyde-free tannin based rigid foams by ATR FT-IR. J. Spectrosc. 2015, 2015, 90234. [Google Scholar] [CrossRef]
- Reyer, A.; Tondi, G.; Berger, R.J.F.; Petutschnigg, A.; Musso, M. Raman spectroscopic investigation of tannin-furanic rigid foams. Vib. Spectrosc. 2016, 84, 58–66. [Google Scholar] [CrossRef]
- Pizzi, A.; Tondi, G.; Pasch, H.; Celzard, A. Matrix-assisted laser desorption/ionization time-of-flight structure determination of complex thermoset networks: Polyflavonoid tannin–furanic rigid foams. J. Appl. Polym. Sci. 2008, 110, 1451–1456. [Google Scholar] [CrossRef]
- Tondi, G.; Petutschnigg, A. Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts. Ind. Crop. Prod. 2015, 65, 422–428. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Wiley Online Library: Hoboken, NJ, USA, 2000. [Google Scholar]
















| Family of Hardeners | Hardener | Amount (% s./s. tannin) | pH |
|---|---|---|---|
| Known | Formaldehyde, Hexamine | 4%, 6%, 8% and 25% | 2, 4.5, 7, 9 |
| Aldehydes | Glyoxal, Glutaraldehyde, p-phthaldialdehyde | 6%, 12.5%, 25% and 50% | 2, 4.5, 7, 9 |
| Nitrogen compound | Caprolactam, Dicyandiamide, Chitosan | 6%, 12.5%, 25% and 50% | 2, 4.5, 7, 9 |
| Acids | Citric acid, Maleic anhydride, Phthalic acid | 6%, 12.5%, 25% and 50% | 2, 4.5, 7, 9 |
| Furanics | Furfural, Furfuryl alcohol | 6%, 12.5%, 25% and 50% | 2, 4.5, 7, 9 |
| Hardener | Amount | pH | Hardening Temp. (°C) | Observations |
|---|---|---|---|---|
| Formaldehyde | 4%, 6%, 8%; 25% | Every | 20 to 103 | pH 2 and 9 hardens at lower T |
| Hexamine | 4%, 6%, 8%; 25% | 9, 7, 9 | 50 to 90 | Homogeneous polymers |
| Glyoxal | 12.5%, 25% | Every | 90 to 103 | Elastic solids |
| Glutaraldehyde | 50% | 9 | 70 | Weak gel |
| p-Phthaldialdehyde | 12.5% | 9 | 50 | Two phases |
| Caprolactam | Any | Any | No hardening | Remains liquid |
| Chitosan | Any | Any | No hardening | Remains liquid/clumps |
| Dicyandiamide | Any | Any | No hardening | Remains liquid |
| Citric acid | Any | Every | No hardening | Remains liquid |
| Maleic anhydride | 12.5% to 50% | 2, 4.5 | 100 | Elastic solid |
| Phthalic acid | Any | Every | No hardening | Remains liquid |
| Furfural | 25%, 37.5% | Every | 103, 70, 50 | High amount/pH → Solid |
| Furfuryl alcohol | 12.5%, 25% | 2, (4.5) | 90, 103 | Hard, black solid |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tondi, G. Tannin-Based Copolymer Resins: Synthesis and Characterization by Solid State 13C NMR and FT-IR Spectroscopy. Polymers 2017, 9, 223. https://doi.org/10.3390/polym9060223
Tondi G. Tannin-Based Copolymer Resins: Synthesis and Characterization by Solid State 13C NMR and FT-IR Spectroscopy. Polymers. 2017; 9(6):223. https://doi.org/10.3390/polym9060223
Chicago/Turabian StyleTondi, Gianluca. 2017. "Tannin-Based Copolymer Resins: Synthesis and Characterization by Solid State 13C NMR and FT-IR Spectroscopy" Polymers 9, no. 6: 223. https://doi.org/10.3390/polym9060223
APA StyleTondi, G. (2017). Tannin-Based Copolymer Resins: Synthesis and Characterization by Solid State 13C NMR and FT-IR Spectroscopy. Polymers, 9(6), 223. https://doi.org/10.3390/polym9060223