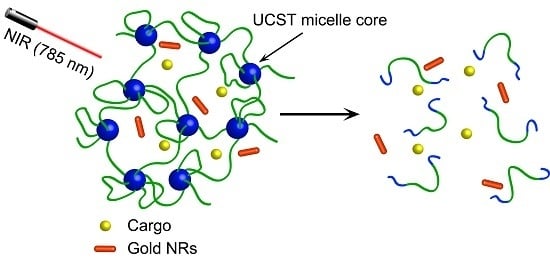
A Near-Infrared Light-Responsive Hybrid Hydrogel Based on UCST Triblock Copolymer and Gold Nanorods
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Statement
References
- Woodcock, J.W.; Wright, R.A.E.; Jiang, X.; O’Lenick, T.G.; Zhao, B. Dually responsive aqueous gels from thermo- and light-sensitive hydrophilic ABA triblock copolymers. Soft Matter 2010, 6, 3325–3336. [Google Scholar] [CrossRef]
- Peng, K.; Tomatsu, I.; Kros, A. Light controlled protein release from a supramolecular hydrogel. Chem. Commun. 2010, 46, 4094–4096. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.R.; Kasko, A.M. Photodegradable Macromers and Hydrogels for Live Cell Encapsulation and Release. J. Am. Chem. Soc. 2012, 134, 13103–13107. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Tong, X.; Zhao, Y. Photoresponsive Nanogels Based on Photocontrollable Cross-Links. Macromolecules 2009, 42, 4845–4852. [Google Scholar] [CrossRef]
- Tamesue, S.; Takashima, Y.; Yamaguchi, H.; Shinkai, S.; Harada, A. Photoswitchable Supramolecular Hydrogels Formed by Cyclodextrins and Azobenzene Polymers. Angew. Chem. Int. Ed. 2010, 49, 7461–7464. [Google Scholar] [CrossRef] [PubMed]
- Charati, M.B.; Lee, I.; Hribar, K.C.; Burdick, J.A. Light-Sensitive Polypeptide Hydrogel and Nanorod Composites. Small 2010, 6, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Gorelikov, I.; Field, L.M.; Kumacheva, E. Hybrid Microgels Photoresponsive in the Near-Infrared Spectral Range. J. Am. Chem. Soc. 2004, 126, 15938–15939. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Sanson, N.; Fava, D.; Kumacheva, E. Microgels Loaded with Gold Nanorods: Photothermally Triggered Volume Transitions under Physiological Conditions. Langmuir 2007, 23, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Oishi, M.; Nakamura, T.; Jinji, Y.; Matsuishi, K.; Nagasaki, Y. Multi-stimuli-triggered release of charged dye from smart PEGylated nanogels containing gold nanoparticles to regulate fluorescence signals. J. Mater. Chem. 2009, 19, 5909–5912. [Google Scholar] [CrossRef]
- Suzuki, A.; Tanaka, T. Phase transition in polymer gels induced by visible light. Nature 1990, 346, 345–347. [Google Scholar] [CrossRef]
- Nayak, S.; Lyon, L.A. Photoinduced Phase Transitions in Poly(N-isopropylacrylamide) Microgels. Chem. Mater. 2004, 16, 2623–2627. [Google Scholar] [CrossRef]
- Shiotani, A.; Mori, T.; Niidome, T.; Niidome, Y.; Katayama, Y. Stable Incorporation of Gold Nanorods into N-Isopropylacrylamide Hydrogels and Their Rapid Shrinkage Induced by Near-Infrared Laser Irradiation. Langmuir 2007, 23, 4012–4018. [Google Scholar] [CrossRef] [PubMed]
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, P.; Babu, T.; Liu, Y.; Gong, J.; Nie, Z. Near-infrared light-responsive vesicles of Au nanoflowers. Chem. Commun. 2012, 49, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, I.; Peng, K.; Kros, A. Photoresponsive hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2011, 63, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Gohy, J.-F.; Zhao, Y. Photo-responsive block copolymer micelles: Design and behavior. Chem. Soc. Rev. 2013, 42, 7117–7129. [Google Scholar] [CrossRef] [PubMed]
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Fomina, N.; McFearin, C.; Sermsakdi, M.; Edigin, O.; Almutairi, A. UV and Near-IR Triggered Release from Polymeric Nanoparticles. J. Am. Chem. Soc. 2010, 132, 9540–9542. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Boyer, J.-C.; Habault, D.; Branda, N.R.; Zhao, Y. Near Infrared Light Triggered Release of Biomacromolecules from Hydrogels Loaded with Upconversion Nanoparticles. J. Am. Chem. Soc. 2012, 134, 16558–16561. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, J.; Ren, J.; Qu, X. 3D Graphene Oxide-Polymer Hydrogel: Near-Infrared Light-Triggered Active Scaffold for Reversible Cell Capture and On-Demand Release. Adv. Mater. 2013, 25, 6737–6743. [Google Scholar] [CrossRef] [PubMed]
- Strong, L.E.; West, J.L. Hydrogel-Coated Near Infrared Absorbing Nanoshells as Light-Responsive Drug Delivery Vehicles. ACS Biomater. Sci. Eng. 2015, 1, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Chai, R.; Ma, P.; Dai, Y.; Kang, X.; Lian, H.; Hou, Z.; Li, C.; Lin, J. Multiwalled Carbon Nanotubes and NaYF4:Yb3+/Er3+ Nanoparticle-Doped Bilayer Hydrogel for Concurrent NIR-Triggered Drug Release and Up-Conversion Luminescence Tagging. Langmuir 2013, 29, 9573–9580. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, K.; Huang, S.; Yang, C.; Wang, M. Near-Infrared Light-Responsive Semiconductor Polymer Composite Hydrogels: Spatial/Temporal-Controlled Release via a Photothermal “Sponge” Effect. ACS Appl. Mater. Interfaces 2017, 9, 13602–13610. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhang, Y.; Lu, X.; Wang, K.; Wang, Z.; Zhang, H. Polydopamine Nanoparticles Modulating Stimuli-Responsive PNIPAM Hydrogels with Cell/Tissue Adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Zhang, Q.; Cheng, Y. Near infrared light-responsive and injectable supramolecular hydrogels for on-demand drug delivery. Chem. Commun. 2016, 52, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wei, C.; Liang, J.; Liu, T.; Kong, D.; Lv, F. Thermosensitive hydrogel loaded with chitosan-carbon nanotubes for near infrared light triggered drug delivery. Colloids Surf. B 2017, 154, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.T.; Filla, D.; Shea, R. Functional Polymers from Novel Carboxyl-Terminated Trithiocarbonates as Highly Efficient RAFT Agents. Macromolecules 2002, 35, 6754–6756. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. First Example of a Universal and Cost-Effective Approach: Polymers with Tunable Upper Critical Solution Temperature in Water and Electrolyte Solution. Macromolecules 2012, 45, 3910–3918. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Guo, Z.R.; Gu, C.R.; Fan, X.; Bian, Z.P.; Wu, H.F.; Yang, D.; Gu, N.; Zhang, J.N. Fabrication of Anti-human Cardiac Troponin I Immunogold Nanorods for Sensing Acute Myocardial Damage. Nanoscale Res. Lett. 2009, 4, 1428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tong, X.; Zhao, Y. Diverse Thermoresponsive Behaviors of Uncharged UCST Block Copolymer Micelles in Physiological Medium. Langmuir 2014, 30, 11433–11441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, S.; Fan, W.; Zhao, Y. Ultrasensitive pH-Induced Water Solubility Switch Using UCST Polymers. Macromolecules 2016, 49, 1424–1433. [Google Scholar] [CrossRef]
- Taribagil, R.R.; Hillmyer, M.A.; Lodge, T.P. Hydrogels from ABA and ABC Triblock Polymers. Macromolecules 2010, 43, 5396–5404. [Google Scholar] [CrossRef]
- Nguyen-Misra, M.; Mattice, W.L. Micellization and Gelation of Symmetric Triblock Copolymers with Insoluble End Blocks. Macromolecules 1995, 28, 1444–1457. [Google Scholar] [CrossRef]
- Ge, Z.; Zhou, Y.; Tong, Z.; Liu, S. Thermogelling of Double Hydrophilic Multiblock and Triblock Copolymers of N,N-Dimethylacrylamide and N-Isopropylacrylamide: Chain Architectural and Hofmeister Effects. Langmuir 2011, 27, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, S.E.; Hensarling, R.M.; McConaughy, S.D.; Guo, Y.; Jarrett, W.L.; McCormick, C.L. Thermoreversible Hydrogels from RAFT-Synthesized BAB Triblock Copolymers: Steps toward Biomimetic Matrices for Tissue Regeneration. Biomacromolecules 2008, 9, 481–486. [Google Scholar] [CrossRef] [PubMed]
- O’Lenick, T.G.; Jiang, X.; Zhao, B. Thermosensitive Aqueous Gels with Tunable Sol–Gel Transition Temperatures from Thermo- and pH-Responsive Hydrophilic ABA Triblock Copolymer. Langmuir 2010, 26, 8787–8796. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Boissiere, O.; Kumar, S.; Tong, X.; Tremblay, L.; Zhao, Y. Two-Way CO2-Switchable Triblock Copolymer Hydrogels. Macromolecules 2012, 45, 7440–7445. [Google Scholar] [CrossRef]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Guo, S.; Fu, S.; Zhao, Y. A Near-Infrared Light-Responsive Hybrid Hydrogel Based on UCST Triblock Copolymer and Gold Nanorods. Polymers 2017, 9, 238. https://doi.org/10.3390/polym9060238
Zhang H, Guo S, Fu S, Zhao Y. A Near-Infrared Light-Responsive Hybrid Hydrogel Based on UCST Triblock Copolymer and Gold Nanorods. Polymers. 2017; 9(6):238. https://doi.org/10.3390/polym9060238
Chicago/Turabian StyleZhang, Hu, Shengwei Guo, Shangyi Fu, and Yue Zhao. 2017. "A Near-Infrared Light-Responsive Hybrid Hydrogel Based on UCST Triblock Copolymer and Gold Nanorods" Polymers 9, no. 6: 238. https://doi.org/10.3390/polym9060238
APA StyleZhang, H., Guo, S., Fu, S., & Zhao, Y. (2017). A Near-Infrared Light-Responsive Hybrid Hydrogel Based on UCST Triblock Copolymer and Gold Nanorods. Polymers, 9(6), 238. https://doi.org/10.3390/polym9060238