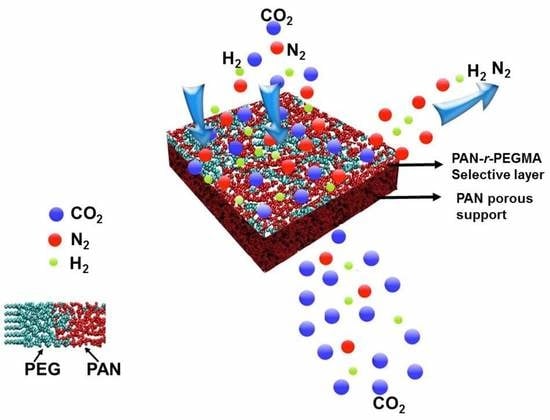
CO2-Philic Thin Film Composite Membranes: Synthesis and Characterization of PAN-r-PEGMA Copolymer
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Synthesis of Polyacrylonitrile-r-Polyethylene Glycol Methacrylate (PAN-r-PEGMA) Copolymer
2.3. Composite Membrane Fabrication
2.4. Characterization
2.5. Gas Permeation Measurements
3. Results and Discussion
3.1. Synthesis of PAN-r-PEGMA Copolymers
3.2. Membrane Preparation and Morphology
3.3. Gas Transport Properties of the Composite Membranes
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Descamps, C.; Bouallou, C.; Kanniche, M. Efficiency of an integrated gasification combined cycle (IGCC) power plant including CO2 removal. Energy 2008, 33, 874–881. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy 2008, 33, 554–570. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Powell, C.E.; Qiao, G.G. Polymeric CO2/N2 gas separation membranes for the capture of carbon dioxide from power plant flue gases. J. Membr. Sci. 2006, 279, 1–49. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Du, N.; Park, H.B.; Dal-Cin, M.M.; Guiver, M.D. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ. Sci. 2012, 5, 7306–7322. [Google Scholar] [CrossRef]
- Ismail, A.F.; Dunkin, I.R.; Gallivan, S.L.; Shilton, S.J. Production of super selective polysulfone hollow fiber membranes for gas separation. Polymer 1999, 40, 6499–6506. [Google Scholar] [CrossRef]
- Minhas, B.S.; Matsuura, T.; Sourirajan, S. Formation of asymmetric cellulose acetate membranes for the separation of carbon dioxide-methane gas mixtures. Ind. Eng. Chem. Res. 1987, 26, 2344–2348. [Google Scholar] [CrossRef]
- Peterson, J.; Peinemann, K.V. Novel polyamide composite membranes for gas separation prepared by interfacial polycondensation. J. Appl. Polym. Sci. 1997, 63, 1557–1563. [Google Scholar] [CrossRef]
- Wind, J.D.; Paul, D.R.; Koros, W.J. Natural gas permeation in polyimide membranes. J. Membr. Sci. 2004, 228, 227–236. [Google Scholar] [CrossRef]
- Nagai, K.; Masuda, T.; Nakagawa, T.; Freeman, B.D.; Pinnau, I. Poly(1-(trimethylsilyl)-1-propyne) and related polymers: Synthesis, properties and functions. Prog. Polym. Sci. 2001, 26, 721–798. [Google Scholar] [CrossRef]
- Stern, S.A. Polymers for gas separations: The next decade. J. Membr. Sci. 1994, 94, 1–65. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Liu, S.L.; Shao, L.; Chua, M.L.; Lau, C.H.; Wang, H.; Quan, S. Recent progress in the design of advanced PEO-containing membranes for CO2 removal. Prog. Polym. Sci. 2013, 38, 1089–1120. [Google Scholar] [CrossRef]
- Anderson, M.R.; Mattes, B.R.; Reiss, H.; Kaner, R.B. Conjugated polymer films for gas separations. Science 1991, 252, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Rana, D.; Matsuura, T.; Soltanieh, M.; Tabe, S. Novel surface modifying macromolecules (SMMs) blended polysulfone gas separation membranes by phase inversion technique. J. Appl. Polym. Sci. 2012, 124, 2287–2299. [Google Scholar] [CrossRef]
- Savoji, H.; Rana, D.; Matsuura, T.; Soltanieh, M.; Tabe, S. Influence of novel surface modifying macromolecules and coagulation media on the gas permeation properties of different polymeric gas separation membranes. J. Appl. Polym. Sci. 2012, 124, 2300–2310. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Oxygen-nitrogen separation. In Encyclopedia of Membrane Science and Technology; Hoek, E.M.V., Tarabara, V.V., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 3, pp. 1668–1692. [Google Scholar]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Okamoto, K.; Fuji, M.; Okamyo, S.; Suzuki, H.; Tanaka, K.; Kita, H. Gas permeation properties of poly(ether imide) segmented copolymers. Macromolecules 1995, 28, 6950–6956. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.V. Tailor-made polymeric membranes based on segmented block copolymers for CO2 separation. Adv. Funct. Mater. 2008, 18, 2815–2823. [Google Scholar] [CrossRef]
- Patel, R.; Kim, S.J.; Roh, D.K.; Kim, J.H. Synthesis of amphiphilic PCZ-r-PEG nanostructural copolymers and their use in CO2/N2 separation membranes. Chem. Eng. J. 2014, 254, 46–53. [Google Scholar] [CrossRef]
- Kim, H.W.; Park, H.B. Gas diffusivity, solubility and permeability in polysulfone-poly(ethylene oxide) random copolymer membranes. J. Membr. Sci. 2011, 372, 116–124. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Ijzer, A.C.; Nijmeijer, K.; Arun, A.; Gaymans, R.J.; Wessling, M.S. Subambient temperature CO2 and light gas permeation through segmented block copolymers with tailored soft phase. ACS Appl. Mater. Interface 2010, 2, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, J.H.; Jung, J.P.; Jung, B.; Kim, J.H. A highly selective PEGBEM-g-POEM comb copolymer membrane for CO2/N2 separation. J. Membr. Sci. 2015, 492, 452–460. [Google Scholar] [CrossRef]
- Talakesh, M.M.; Sadeghi, M.; Chenar, M.P.; Khosravi, A. Gas separation properties of poly(ethylene glycol)/poly(tetramethylene glycol) based polyurethane membranes. J. Membr. Sci. 2012, 415–416, 469–477. [Google Scholar] [CrossRef]
- Yave, W.; Szymczyk, A.; Yave, N.; Roslanie, Z. Design, synthesis, characterization and optimization of PTT-b-PEO copolymers: A new membrane material for CO2 separation. J. Membr. Sci. 2010, 362, 407–416. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, J.P.; Jang, E.; Lee, K.B.; Kang, Y.S.; Kim, J.H. CO2-philic PBEM-g-POEM comb copolymer membranes: Synthesis, characterization and CO2/N2 separation. J. Membr. Sci. 2016, 502, 191–201. [Google Scholar] [CrossRef]
- Feng, H.; Hong, T.; Mahurin, S.M.; Vogiatzis, K.D.; Gmernicki, K.R.; Long, B.K.; Mays, J.W.; Sokolov, A.P.; Kang, N.G.; Saito, T. Gas separation mechanism of CO2 selective amidoxime-poly(1-trimethylsilyl-1-propyne) membranes. Polym. Chem. 2017, 8, 3341–3350. [Google Scholar] [CrossRef]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas sorption and characterization of poly(ether-b-amide) segmented block copolymers. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2463–2475. [Google Scholar] [CrossRef]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas transport properties of poly(ether-b-amide) segmented block copolymers. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2051–2062. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.V. Pebax®/polyethylene glycol blend thin film composite membranes for CO2 separation: Performance with mixed gases. Sep. Purif. Technol. 2008, 62, 110–117. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.; Peinemann, K.V.; Lai, Z. Carbon dioxide selective mixed matrix composite membrane containing ZIF-7 nano-fillers. J. Membr. Sci. 2013, 425–426, 235–242. [Google Scholar] [CrossRef]
- Fam, W.; Mansouri, J.; Li, H.; Chen, V. Improving CO2 separation performance of thin film composite hollow fiber with Pebax®1657/ionic liquid gel membranes. J. Membr. Sci. 2017, 537, 54–68. [Google Scholar] [CrossRef]
- Asatekin, A.; Kang, S.; Elimelech, M.; Mayes, A.M. Anti-fouling ultrafiltration membranes containing polyacrylonitrile-graft-poly(ethylene oxide) comb copolymer additives. J. Membr. Sci. 2007, 298, 136–146. [Google Scholar] [CrossRef]
- Janevieve, A.J.; Novo, N.; Kendra, F.; Pagnucco, C.D.; Carew, S.; Cheong, C.; Kong, X.Z.; Burke, N.A.D.; Stover, H.D.H. Thermoresponsive copolymers of methacrylic acid and poly(ethylene glycol) methyl ether methacrylate. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6095–6104. [Google Scholar]
- Beevers, R.B. Dependence of the glass transition temperature of polyacrylonitrile on molecular weight. J. Polym. Sci. Part A Polym. Chem. 1964, 2, 5257–5265. [Google Scholar] [CrossRef]












| Sample Code | Mol (%) | PEGMA (Da) | PEGMA wt % | Mw (Da) | Mw/Mn (PDI) | |
|---|---|---|---|---|---|---|
| AN | PEGMA | |||||
| PAN-r-PEGMA40 | 89.3 | 10.7 | 300 | 40 | 56,052 | 2.15 |
| PAN-r-PEGMA60 | 79.0 | 21.0 | 300 | 60 | 53,669 | 2.39 |
| PAN-r-PEGMA64 | 83.6 | 16.4 | 475 | 64 | 43,918 | 1.96 |
| PAN-r-PEGMA67 | 89.9 | 10.1 | 950 | 67 | 52,397 | 3.60 |
| Membranes | Selective Layer | Selectivity (α) | |||
|---|---|---|---|---|---|
| Thickness (μm) | (Barrer) | CO2/N2 | CO2/CH4 | CO2/H2 | |
| PAN-r-PEGMA40 | 1.32 | 2 | 52 | 28 | 1.6 |
| PAN-r-PEGMA60 | 1.35 | 10 | 53 | 25 | 3.7 |
| PAN-r-PEGMA64 | 1.23 | 20 | 60 | 23 | 5.9 |
| PAN-r-PEGMA67 | 1.36 | 69 | 65 | 20 | 9.8 |
| PEBAX1657 | 1.38 | 64 | 51 | 18 | 7.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karunakaran, M.; Kumar, M.; Shevate, R.; Akhtar, F.H.; Peinemann, K.-V. CO2-Philic Thin Film Composite Membranes: Synthesis and Characterization of PAN-r-PEGMA Copolymer. Polymers 2017, 9, 219. https://doi.org/10.3390/polym9070219
Karunakaran M, Kumar M, Shevate R, Akhtar FH, Peinemann K-V. CO2-Philic Thin Film Composite Membranes: Synthesis and Characterization of PAN-r-PEGMA Copolymer. Polymers. 2017; 9(7):219. https://doi.org/10.3390/polym9070219
Chicago/Turabian StyleKarunakaran, Madhavan, Mahendra Kumar, Rahul Shevate, Faheem Hassan Akhtar, and Klaus-Viktor Peinemann. 2017. "CO2-Philic Thin Film Composite Membranes: Synthesis and Characterization of PAN-r-PEGMA Copolymer" Polymers 9, no. 7: 219. https://doi.org/10.3390/polym9070219
APA StyleKarunakaran, M., Kumar, M., Shevate, R., Akhtar, F. H., & Peinemann, K. -V. (2017). CO2-Philic Thin Film Composite Membranes: Synthesis and Characterization of PAN-r-PEGMA Copolymer. Polymers, 9(7), 219. https://doi.org/10.3390/polym9070219