A New Polymer-Based Fluorescent Chemosensor Incorporating Propane-1,3-Dione and 2,5-Diethynylbenzene Moieties for Detection of Copper(II) and Iron(III)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Measurements
2.2. Preparation of the Conjugated Polymer PDBDBM
2.3. Metal Ion Titration Procedure
3. Results and Discussion
3.1. Synthesis and Characterization of PDBDBM
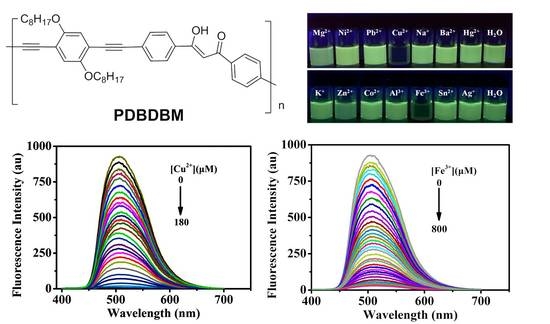
3.2. Selectivity and Sensitivity of the PDBDBM Sensor for Detection of Cu2+ and Fe3+
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yin, J.; Hu, Y.; Yoon, J. Fluorescent probes and bioimaging: Alkali metals, alkaline earth metals and pH. Chem. Soc. Rev. 2015, 44, 4619–4644. [Google Scholar] [CrossRef] [PubMed]
- Festa, R.A.; Thiele, D.J. Copper: An essential metal in biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef] [PubMed]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, F.; Luo, Z.; Bao, B.; Hu, Y.; Weng, L.; Cheng, Y.; Wang, L. Conjugated polymer nanoparticles with aggregation induced emission characteristics for intracellular Fe3+ sensing. J. Polym. Sci. Polym. Chem. 2016, 54, 1686–1693. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Wang, H.; Lin, Q.; Chen, X.; Chen, Y. The influence of soil heavy metals pollution on soil microbial biomass, enzyme activity, and community composition near a copper smelter. Ecotoxicol. Environ. Saf. 2007, 67, 75–81. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, R. The adsorption of lead and other heavy metals on oxides of manganese and iron. Soil Res. 1980, 18, 61–73. [Google Scholar] [CrossRef]
- Dong, Y.; Koken, B.; Ma, X.; Wang, L.; Cheng, Y.; Zhu, C. Polymer-based fluorescent sensor incorporating 2,2′-bipyridyl and benzo[2,1,3]thiadiazole moieties for Cu2+ detection. Inorg. Chem. Commun. 2011, 14, 1719–1722. [Google Scholar] [CrossRef]
- Ajlec, R.; Stupar, J. Determination of iron species in wine by ion-exchange chromatography-flame atomic absorption spectrometry. Analyst 1989, 114, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Teng, Z.; Wang, C.; Wang, G. Ultra-high sensitive voltammetric sensor modified by largely oxygenous functionalized ultrathin carbon nitride nanosheets for detection of Cu(II). Sens. Actuators B 2017, 242, 897–903. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, C.; Yuan, H.; Liu, L.; Lv, F.; Wang, S. Conjugated polymer nanoparticles: Preparation, properties, functionalization and biological applications. Chem. Soc. Rev. 2013, 42, 6620–6633. [Google Scholar] [CrossRef] [PubMed]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated polymer-based chemical sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Song, F.; Wei, G.; Cheng, Y.; Zhu, C. A highly selective and sensitive polymer-based off-on fluorescent sensor for Hg2+ detection incorporating salen and perylenyl moieties. J. Mater. Chem. 2012, 22, 478–482. [Google Scholar] [CrossRef]
- Li, F.; Meng, F.; Wang, Y.; Zhu, C.; Cheng, Y. Polymer-based fluorescence sensor incorporating thiazole moiety for direct and visual detection of Hg2+ and Ag+. Tetrahedron 2015, 71, 1700–1704. [Google Scholar] [CrossRef]
- Kim, H.N.; Guo, Z.; Zhu, W.; Yoon, J.; Tian, H. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem. Soc. Rev. 2011, 40, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Huang, N.; Gao, J.; Xu, F.; Xu, Y.; Jiang, D. Controlled synthesis of conjugated microporous polymer films: Versatile platforms for highly sensitive and label-free chemo- and biosensing. Angew. Chem. Int. Ed. 2014, 53, 4850–4855. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Huang, N.; Wu, Y.; Xu, H.; Jiang, D. Design of highly photofunctional porous polymer films with controlled thickness and prominent microporosity. Angew. Chem. Int. Ed. 2015, 54, 11540–11544. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Taranekar, P.; Reynolds, J.R.; Schanze, K.S. Conjugated polyelectrolytes: Synthesis, photophysics, and applications. Angew. Chem. Int. Ed. 2009, 48, 4300–4316. [Google Scholar] [CrossRef] [PubMed]
- Lange, U.; Roznyatovskaya, N.V.; Mirsky, V.M. Conducting polymers in chemical sensors and arrays. Anal. Chim. Acta 2008, 614, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.F.; Shi, Z.Q.; Yu, M.H.; Wang, S. Cationic conjugated polyelectrolyte-based fluorometric detection of copper(II) ions in aqueous solution. Polymer 2008, 49, 2698–2703. [Google Scholar] [CrossRef]
- Chen, X.L.; Zeng, W.F.; Yang, X.D.; Lu, X.W.; Qu, J.Q.; Liu, R.Y. Thiourea based conjugated polymer fluorescent chemosensor for Cu+ and its use for the detection of hydrogen peroxide and glucose. Chin. J. Polym. Sci. 2016, 34, 324–331. [Google Scholar] [CrossRef]
- Zeng, Q.; Cai, P.; Li, Z.; Qin, J.; Tang, B.Z. An imidazole-functionalized polyacetylene: Convenient synthesis and selective chemosensor for metal ions and cyanide. Chem. Commun. 2008, 1094–1096. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Yuwen, L.; Zhan, X.; Wang, L. Water-soluble hyperbranched polyelectrolytes with high fluorescence quantum yield: Facile synthesis and selective chemosensor for Hg2+ and Cu2+ ions. J. Polym. Sci. Polym. Chem. 2010, 48, 3431–3439. [Google Scholar] [CrossRef]
- Guo, C.X.; Jiang, S.X.; Zhu, W.X.; Yang, X.X.; Pei, M.S.; Zhang, G.Y. Polythiophene based fluorescent probe for copper ions with high sensitivity. J. Appl. Polym. Sci. 2015, 132, 7. [Google Scholar] [CrossRef]
- Maiti, C.; Banerjee, R.; Maiti, S.; Dhara, D. Water-soluble polymeric chemosensor for detection of Cu2+ ions with high selectivity and sensitivity. Des. Monomers Polym. 2016, 19, 669–678. [Google Scholar] [CrossRef]
- Li, N.; Xu, Q.; Xia, X.; Wang, L.; Lu, J.; Wen, X. A polymeric chemosensor for Fe3+ based on fluorescence quenching of polymer with quinoline derivative in the side chain. Mater. Chem. Phys. 2009, 114, 339–343. [Google Scholar] [CrossRef]
- Wu, X.; Xu, B.; Tong, H.; Wang, L. Phosphonate-functionalized polyfluorene film sensors for sensitive detection of iron(III) in both organic and aqueous media. Macromolecules 2010, 43, 8917–8923. [Google Scholar] [CrossRef]
- Dwivedi, A.K.; Saikia, G.; Iyer, P.K. Aqueous polyfluorene probe for the detection and estimation of Fe3+ and inorganic phosphate in blood serum. J. Mater. Chem. 2011, 21, 2502–2507. [Google Scholar] [CrossRef]
- Geng, T.M.; Huang, R.Y.; Wu, D.Y. Turn-on fluorogenic and chromogenic detection of Fe3+ and Cr3+ in a completely water medium with polyacrylamide covalently bonding to rhodamine B using diethylenetriamine as a linker. RSC Adv. 2014, 4, 46332–46339. [Google Scholar] [CrossRef]
- Jin, L.; Liu, C.; An, N.Q.; Zhang, Q.; Wang, J.; Zhao, L.L.; Lu, Y. Fluorescence turn-on detection of Fe3+ in pure water based on a cationic poly(perylene diimide) derivative. RSC Adv. 2016, 6, 58394–58400. [Google Scholar] [CrossRef]
- Luo, C.X.; Liu, Y.T.; Zhang, Q.; Cai, X.D. Hyperbranched conjugated polymers containing 1,3-butadiene units: Metal-free catalyzed synthesis and selective chemosensors for Fe3+ ions. RSC Adv. 2017, 7, 12269–12276. [Google Scholar] [CrossRef]
- Feng, N.; Xie, J.; Zhang, D. Synthesis, characterization, photophysical and oxygen-sensing properties of a novel europium(III) complex. Spectrochim. Acta A 2010, 77, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, Z.; Zou, Y.; Yang, C.; Qin, J.; Cao, Y. Synthesis, characterization, and photophysics of electroluminescent copolymers with a quinoline-based iridium complex in the main chain: A versatile method for constructing metal-containing copolymers. Organometallics 2007, 26, 3699–3707. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, S.; Hu, Y.; Chen, J.; Bao, B.; Yuwen, L.; Weng, L.; Cheng, Y.; Wang, L. AIE-active conjugated polymer nanoparticles with red-emission for in vitro and in vivo imaging. RSC Adv. 2016, 6, 114580–114586. [Google Scholar] [CrossRef]
- Hou, J.; Song, F.; Wang, L.; Wei, G.; Cheng, Y.; Zhu, C. In situgenerated 1:1 Zn(II)-containing polymer complex sensor for highly enantioselective recognition of n-boc-protected alanine. Macromolecules 2012, 45, 7835–7842. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, X.; Jiang, Y.; Quan, Y.; Cheng, Y.; Zhu, C. Fluorescence study of chiral β-ketoiminate-based newly synthesized boron hybrid polymers. Macromol. Chem. Phys. 2014, 215, 358–364. [Google Scholar] [CrossRef]
- Han, C.; Huang, T.; Liu, Q.; Xu, H.; Zhuang, Y.; Li, J.; Hu, J.; Wang, A.; Xu, K. Design and synthesis of a highly sensitive "turn-on" fluorescent organic nanoprobe for iron(III) detection and imaging. J. Mater. Chem. C 2014, 2, 9077–9082. [Google Scholar] [CrossRef]
- McDonagh, C.; Bowe, P.; Mongey, K.; MacCraith, B.D. Characterisation of porosity and sensor response times of sol–gel-derived thin films for oxygen sensor applications. J. Non-Cryst. Solids 2002, 306, 138–148. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, H.; Wu, F.; Hao, G.; Chen, Y.; Tan, C.; Tan, Y.; Jiang, Y. A dual-response quinoline-based fluorescent sensor for the detection of copper(II) and iron(III) ions in aqueous medium. Sens. Actuators B 2017, 243, 765–774. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, B.; Wen, Y.; Lu, L.; Xu, J. Facile fabrication of a cost-effective, water-soluble, and electrosynthesized poly(9-aminofluorene) fluorescent sensor for the selective and sensitive detection of Fe(III) and inorganic phosphates. Sens. Actuators B 2012, 171, 786–794. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Sharma, D.; Bera, R.K.; Crisponi, G.; Callan, J.F. Iron(III) selective molecular and supramolecular fluorescent probes. Chem. Soc. Rev. 2012, 41, 7195–7227. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.K.; Yin, C.X.; Huo, F.J. Highly sensitive and selective fluorescent probe for determination of Cu(II) in aqueous solution. J. Coord. Chem. 2014, 67, 2039–2047. [Google Scholar] [CrossRef]
- Xu, Z.C.; Xiao, Y.; Qian, X.H.; Cui, J.N.; Cui, D.W. Ratiometric and selective fluorescent sensor for Cu(II) based on internal charge transfer (ICT). Org. Lett. 2005, 7, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Lohar, S.; Pal, S.; Maji, A.; Chattopadhyay, P. A new chemosensor selective for Cu2+ ions through fluorescence quenching approach applicable to real samples. J. Ind. Chem. Soc. 2015, 92, 1–8. [Google Scholar]
- Chen, A.; Wu, W.; Fegley, M.; Pinnock, S.; Duffy-Matzner, J.; Bernier, W.; Jones, W. Pentiptycene-derived fluorescence turn-off polymer chemosensor for copper(II) cation with high selectivity and sensitivity. Polymers 2017, 9, 118. [Google Scholar] [CrossRef]
- Choi, Y.W.; Park, G.J.; Na, Y.J.; Jo, H.Y.; Lee, S.A.; You, G.R.; Kim, C. A single schiff base molecule for recognizing multiple metal ions: A fluorescence sensor for Zn(II) and Al(III) and colorimetric sensor for Fe(II) and Fe(III). Sens. Actuators B 2014, 194, 343–352. [Google Scholar] [CrossRef]
- Bao, X.; Cao, X.; Nie, X.; Xu, Y.; Guo, W.; Zhou, B.; Zhang, L.; Liao, H.; Pang, T. A new selective fluorescent chemical sensor for Fe3+ based on rhodamine b and a 1,4,7,10-tetraoxa-13-azacyclopentadecane conjugate and its imaging in living cells. Sens. Actuators B 2015, 208, 54–66. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Hou, L.; Shang, Z.; Dong, Z.; Jin, W. 1,4-dihydroxyanthraquinone–Cu2+ ensemble probe for selective detection of sulfide anion in aqueous solution. Anal. Methods 2013, 5, 5493. [Google Scholar] [CrossRef]
- Malik, A.H.; Hussain, S.; Tanwar, A.S.; Layek, S.; Trivedi, V.; Iyer, P.K. An anionic conjugated polymer as a multi-action sensor for the sensitive detection of Cu2+ and PPi real-time ALP assaying and cell imaging. Analyst 2015, 140, 4388–4392. [Google Scholar] [CrossRef] [PubMed]
- Hens, A.; Maity, A.; Rajak, K.K. N, N coordinating schiff base ligand acting as a fluorescence sensor for zinc(ii) and colorimetric sensor for copper(II), and zinc(II) in mixed aqueous media. Inorg. Chim. Acta 2014, 423, 408–420. [Google Scholar] [CrossRef]
- Xu, L.; Wei, S.; Diao, Q.; Ma, P.; Liu, X.; Sun, Y.; Song, D.; Wang, X. Sensitive and selective rhodamine-derived probes for fluorometric sensing of pH and colorimetric sensing of Cu2+. Sens. Actuators B 2017, 246, 395–401. [Google Scholar] [CrossRef]
- Sahoo, P.R.; Kumar, S. Photochromic spirooxazine as highly sensitive and selective probe for optical detection of Fe3+ in aqueous solution. Sens. Actuators B 2016, 226, 548–552. [Google Scholar] [CrossRef]




| Chemosensors | Target | Detection Type | Limit of Detection | Reference |
|---|---|---|---|---|
| PDBDBM | Cu2+ | Fluorescence | 5 × 10−9 M | This work |
| Poly(pentiptycene) derivatives | Cu2+ | Fluorescence | 1.65 × 10−8 M | [45] |
| Poly(fluorine) derivatives | Cu2+ | Fluorescence | 3.59 × 10−8 M | [49] |
| Poly(fluorine) derivatives | Cu2+ | Fluorescence | 2 × 10−8 M | [20] |
| Imidazole derivatives | Cu2+ | Colorimetric | 1 × 10−5 M | [50] |
| Rhodamine derivatives | Cu2+ | Fluorescence | 3.65 × 10−8 M | [51] |
| PDBDBM | Fe3+ | Fluorescence | 4 × 10−7 M | This work |
| Poly(fluorine) derivatives | Fe3+ | Fluorescence | 2.2 × 10−7 M | [4] |
| Julolidine derivatives | Fe3+ | Colorimetric | 6.8 × 10−6 M | [46] |
| Spirooxazine derivatives | Fe3+ | Fluorescence | 8.8 × 10−7 M | [52] |
| Rhodamine derivatives | Fe3+ | Fluorescence | 4 × 10−7 M | [47] |
| Quinoline derivatives | Fe3+ | Fluorescence | 1.24 × 10−7 M | [39] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Dai, C.; Hu, Y.; Liu, S.; Weng, L.; Luo, Z.; Cheng, Y.; Wang, L. A New Polymer-Based Fluorescent Chemosensor Incorporating Propane-1,3-Dione and 2,5-Diethynylbenzene Moieties for Detection of Copper(II) and Iron(III). Polymers 2017, 9, 267. https://doi.org/10.3390/polym9070267
Yang D, Dai C, Hu Y, Liu S, Weng L, Luo Z, Cheng Y, Wang L. A New Polymer-Based Fluorescent Chemosensor Incorporating Propane-1,3-Dione and 2,5-Diethynylbenzene Moieties for Detection of Copper(II) and Iron(III). Polymers. 2017; 9(7):267. https://doi.org/10.3390/polym9070267
Chicago/Turabian StyleYang, Dongliang, Chunhui Dai, Yanling Hu, Shuli Liu, Lixing Weng, Zhimin Luo, Yixiang Cheng, and Lianhui Wang. 2017. "A New Polymer-Based Fluorescent Chemosensor Incorporating Propane-1,3-Dione and 2,5-Diethynylbenzene Moieties for Detection of Copper(II) and Iron(III)" Polymers 9, no. 7: 267. https://doi.org/10.3390/polym9070267
APA StyleYang, D., Dai, C., Hu, Y., Liu, S., Weng, L., Luo, Z., Cheng, Y., & Wang, L. (2017). A New Polymer-Based Fluorescent Chemosensor Incorporating Propane-1,3-Dione and 2,5-Diethynylbenzene Moieties for Detection of Copper(II) and Iron(III). Polymers, 9(7), 267. https://doi.org/10.3390/polym9070267