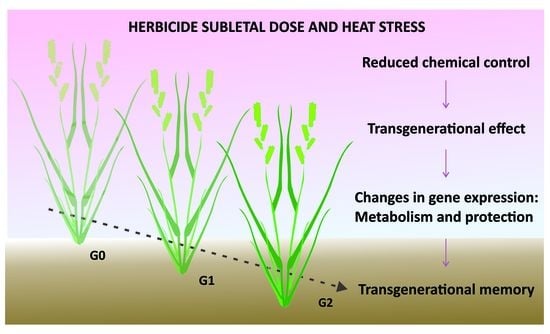
Rapid Reduction of Herbicide Susceptibility in Junglerice by Recurrent Selection with Sublethal Dose of Herbicides and Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. General Procedure for Population Generation
2.3. Determination of Susceptibility Level to Herbicides
2.4. Differential Gene Expression
2.4.1. Plant Material
2.4.2. RNA Extraction and cDNA Synthesis
2.4.3. qRT-PCR Assay
3. Results
3.1. Determination of Susceptibility Level to Herbicides
3.2. Differential Gene Expression
3.2.1. Florpyrauxifen-Benzyl
3.2.2. Glufosinate-Ammonium
3.2.3. Imazethapyr
3.2.4. Quinclorac
4. Discussion
4.1. Heat Stress Reduces Susceptibility of Junglerice to Herbicides
4.2. Gene Expression Profile Reflects the Transgenerational Reduces Susceptibility Memory
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations 2017. Available online: https://news.un.org/en/story/2017/06/560022-world-population-hit-98-billion-2050-despite-nearly-universal-lower-fertility#.WUv3anUrJnw (accessed on 30 June 2020).
- FAO Summary for Policymakers. Climate Change 2013—The Physical Science Basis; Intergovernmental Panel on Climate Change, Ed.; Cambridge University Press: Cambridge, UK, 2013; Volume 1, pp. 1–30. ISBN 9788578110796. [Google Scholar]
- Loboguerrero, A.M.; Campbell, B.M.; Cooper, P.J.M.; Hansen, J.; Sileshi, G.W.; Wollenberg, E. Food and Earth Systems: Priorities for Climate Change Adaptation and Mitigation for Agriculture and Food Systems. Sustainability 2019, 11, 1372. [Google Scholar] [CrossRef] [Green Version]
- Thornton, P.; Ericksen, P.J.; Herrero, M.; Challinor, A.J. Climate variability and vulnerability to climate change: A review. Glob. Chang. Biol. 2014, 20, 3313–3328. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, K.; Robinson, S.; Cattaneo, A. Climate Change, Agriculture and Food Security. In Sustainable Food and Agriculture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 55–74. ISBN 9780128121344. [Google Scholar] [CrossRef]
- Weed Science Society of America. 2020. Available online: http://wssa.net/wssa/weed/croploss-2/ (accessed on 1 July 2020).
- Kraehmer, H.; Jabran, K.; Mennan, H.; Chauhan, B.S. Global distribution of rice weeds—A review. Crop. Prot. 2016, 80, 73–86. [Google Scholar] [CrossRef]
- Kaya-Altop, E.; Şahin, M.; Jabran, K.; Phillippo, C.J.; Zandstra, B.H.; Mennan, H. Effect of different water management strategies on competitive ability of semi-dwarf rice cultivars with Echinochloa oryzoides. Crop. Prot. 2019, 116, 33–42. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Herbicide Resistant Weeds. 2020. Available online: www.weedscience.org (accessed on 1 July 2020).
- Zhang, Z.; Gu, T.; Zhao, B.; Yang, X.; Peng, Q.; Li, Y.; Bai, L. Effects of common Echinochloa varieties on grain yield and grain quality of rice. Field Crop. Res. 2017, 203, 163–172. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Implications of narrow crop row spacing and delayed Echinochloa colona and Echinochloa crus-galli emergence for weed growth and crop yield loss in aerobic rice. Field Crop. Res. 2010, 117, 177–182. [Google Scholar] [CrossRef]
- Burgos, N.R.; Heap, I.M.; Rouse, C.E.; Lawton-Rauh, A.L. Evolution of Herbicide-Resistant Weeds. In Weed Control Sustainability, Hazards and Risks in Cropping Systems Worldwide; CRC Press: Boca Raton, FL, USA, 2019; pp. 92–132. ISBN 9781498719087. [Google Scholar]
- Bagavathiannan, M.; Davis, A. An ecological perspective on managing weeds during the great selection for herbicide resistance. Pest Manag. Sci. 2018, 74, 2277–2286. [Google Scholar] [CrossRef]
- Rouse, C.E.; Roma-Burgos, N.; Norsworthy, J.K.; Tseng, T.-M.; Starkey, C.E.; Scott, R.C. Echinochloa Resistance to Herbicides Continues to Increase in Arkansas Rice Fields. Weed Technol. 2017, 32, 34–44. [Google Scholar] [CrossRef]
- Neve, P.; Powles, S. High survival frequencies at low herbicide use rates in populations of Lolium rigidum result in rapid evolution of herbicide resistance. Heredity 2005, 95, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Busi, R.; Neve, P.; Powles, S. Evolved polygenic herbicide resistance in Lolium rigidum by low-dose herbicide selection within standing genetic variation. Evol. Appl. 2012, 6, 231–242. [Google Scholar] [CrossRef]
- Busi, R.; Powles, S.B. Evolution of glyphosate resistance in a Lolium rigidum population by glyphosate selection at sublethal doses. Heredity 2009, 103, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, M.B.; Walsh, M.J.; Flower, K.C.; Powles, S.B. Recurrent selection with reduced 2,4-D amine doses results in the rapid evolution of 2,4-D herbicide resistance in wild radish (Raphanus raphanistrum L). Pest Manag. Sci. 2016, 72, 2091–2098. [Google Scholar] [CrossRef] [PubMed]
- Tehranchian, P.; Norsworthy, J.K.; Powles, S.; Bararpour, M.T.; Bagavathiannan, M.V.; Barber, T.; Scott, R.C. Recurrent Sublethal-Dose Selection for Reduced Susceptibility of Palmer Amaranth (Amaranthus palmeri) to Dicamba. Weed Sci. 2017, 65, 206–212. [Google Scholar] [CrossRef]
- Manalil, S.; Busi, R.; Renton, M.; Powles, S.B. Rapid Evolution of Herbicide Resistance by Low Herbicide Dosages. Weed Sci. 2011, 59, 210–217. [Google Scholar] [CrossRef]
- Varanasi, A.; Prasad, P.V.; Jugulam, M. Impact of Climate Change Factors on Weeds and Herbicide Efficacy. In Advances in Agronomy; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 135, pp. 107–146. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; Volume 9781107025, p. 151. ISBN 9781139177245. [Google Scholar]
- Ziska, L.H. The role of climate change and increasing atmospheric carbon dioxide on weed management: Herbicide efficacy. Agric. Ecosyst. Environ. 2016, 231, 304–309. [Google Scholar] [CrossRef] [Green Version]
- Refatti, J.P.; De Avila, L.A.; Camargo, E.R.; Ziska, L.H.; Oliveira, C.; Salas-Perez, R.; Rouse, C.E.; Roma-Burgos, N. High [CO2] and Temperature Increase Resistance to Cyhalofop-Butyl in Multiple-Resistant Echinochloa colona. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Matzrafi, M.; Brunharo, C.A.C.G.; Tehranchian, P.; Hanson, B.D.; Jasieniuk, M. Increased temperatures and elevated CO2 levels reduce the sensitivity of Conyza canadensis and Chenopodium album to glyphosate. Sci. Rep. 2019, 9, 2228. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Malone, J.M.; Boutsalis, P.; Shirley, N.J.; Preston, C. Temperature influences the level of glyphosate resistance in barnyardgrass (Echinochloa colona). Pest Manag. Sci. 2015, 72, 1031–1039. [Google Scholar] [CrossRef]
- Matzrafi, M.; Seiwert, B.; Reemtsma, T.; Rubin, B.; Peleg, Z. Climate change increases the risk of herbicide-resistant weeds due to enhanced detoxification. Planta 2016, 244, 1217–1227. [Google Scholar] [CrossRef]
- Ritz, C.; Streibig, J. Analysis of Dose-Response Curve Data. Package ‘drc’. Available online: http://cran.r-project.org/web/packages/drc/drc.pdf (accessed on 24 June 2020).
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-Logistic Analysis of Herbicide Dose-Response Relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.; Hellemans, J.; Huggett, J.F.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldana, C.; Scheible, W.-R.; Mueller-Roeber, B.; Ruzicic, S. A quantitative RT-PCR platform for high-throughput expression profiling of 2500 rice transcription factors. Plant Methods 2007, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, M.; Nijhawan, A.; Tyagi, A.K.; Khurana, J.P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006, 345, 646–651. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Wu, J.; Zheng, X.; Zheng, S.; Sun, X.; Qiu, Q.; Lu, T. Gene Knockout Study Reveals That Cytosolic Ascorbate Peroxidase 2 (OsAPX2) Plays a Critical Role in Growth and Reproduction in Rice under Drought, Salt and Cold Stresses. PLoS ONE 2013, 8, e57472. [Google Scholar] [CrossRef] [PubMed]
- Rouse, C.E. Characterization of Multiple-Herbicide-Resistant Echinochloa colona from Arkansas. Ph.D. Thesis, University of Arkansas, Fayetteville, NC, USA, 2017. [Google Scholar]
- Ye, S.; Yu, S.; Shu, L.; Wu, J.; Wu, A.; Luo, L. Expression profile analysis of 9 heat shock protein genes throughout the life cycle and under abiotic stress in rice. Chin. Sci. Bull. 2011, 57, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Saeed, A.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A Free, Open-Source System for Microarray Data Management and Analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef] [Green Version]
- Ganie, Z.A.; Jugulam, M.; Jhala, A.J. Temperature Influences Efficacy, Absorption, and Translocation of 2,4-D or Glyphosate in Glyphosate-Resistant and Glyphosate-Susceptible Common Ragweed (Ambrosia artemisiifolia) and Giant Ragweed (Ambrosia trifida). Weed Sci. 2017, 65, 588–602. [Google Scholar] [CrossRef]
- Matzrafi, M. Climate change exacerbates pest damage through reduced pesticide efficacy. Pest Manag. Sci. 2019, 75, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Lv, M.; Islam, F.; Gill, R.A.; Yang, C.; Ali, B.; Yan, G.; Zhou, W. Salicylic acid mediates antioxidant defense system and ABA pathway related gene expression in Oryza sativa against quinclorac toxicity. Ecotoxicol. Environ. Saf. 2016, 133, 146–156. [Google Scholar] [CrossRef]
- Holsinger, K.E. Reproductive systems and evolution in vascular plants. Proc. Natl. Acad. Sci. USA 2000, 97, 7037–7042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neve, P.; Powles, S. Recurrent selection with reduced herbicide rates results in the rapid evolution of herbicide resistance in Lolium rigidum. Theor. Appl. Genet. 2005, 110, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Debono, M.-W.; Souza, G.M. Plants as electromic plastic interfaces: A mesological approach. Prog. Biophys. Mol. Biol. 2019, 146, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.-G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef]
- Simontacchi, M.; Egalatro, A.; Artuso, F.E.; Santa-María, G.E. Plant Survival in a Changing Environment: The Role of Nitric Oxide in Plant Responses to Abiotic Stress. Front. Plant Sci. 2015, 6, 977. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of Antioxidant Defense System Is Associated with Combined Drought and Heat Stress Tolerance in Citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.B.; Rathnayake, K.N.; Yim, W.C.; Garcia, T.M.; Wone, B.; Cushman, J.C.; Wone, B.W.M. Crassulacean Acid Metabolism Abiotic Stress-Responsive Transcription Factors: A Potential Genetic Engineering Approach for Improving Crop Tolerance to Abiotic Stress. Front. Plant Sci. 2019, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Balfagón, D.; Zandalinas, S.I.; Gómez-Cadenas, A. High temperatures change the perspective: Integrating hormonal responses in citrus plants under co-occurring abiotic stress conditions. Physiol. Plant. 2018, 165, 183–197. [Google Scholar] [CrossRef]
- Ehong, Y.; Ezhang, H.; Ehuang, L.; Eli, D.; Song, F. Overexpression of a Stress-Responsive NAC Transcription Factor Gene ONAC022 Improves Drought and Salt Tolerance in Rice. Front. Plant Sci. 2016, 7, 4. [Google Scholar] [CrossRef]
- Walter, J.; Jentsch, A.; Beierkuhnlein, C.; Kreyling, J. Ecological stress memory and cross stress tolerance in plants in the face of climate extremes. Environ. Exp. Bot. 2013, 94, 3–8. [Google Scholar] [CrossRef]
- Délye, C.; Gardin, J.A.C.; Boucansaud, K.; Chauvel, B.; Petit, C. Non-target-site-based resistance should be the centre of attention for herbicide resistance research: Alopecurus myosuroides as an illustration. Weed Res. 2011, 51, 433–437. [Google Scholar] [CrossRef]
- Gressel, J. Evolving understanding of the evolution of herbicide resistance. Pest Manag. Sci. 2009, 65, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Radwan, D.E.M. Salicylic acid induced alleviation of oxidative stress caused by clethodim in maize (Zea mays L.) leaves. Pestic. Biochem. Physiol. 2012, 102, 182–188. [Google Scholar] [CrossRef]
- Cummins, I.; Dixon, D.P.; Freitag-Pohl, S.; Skipsey, M.; Edwards, R. Multiple roles for plant glutathione transferases in xenobiotic detoxification. Drug Metab. Rev. 2011, 43, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Délye, C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest Manag. Sci. 2012, 69, 176–187. [Google Scholar] [CrossRef]
- Nandula, V.K.; Riechers, D.E.; Ferhatoglu, Y.; Barrett, M.; Duke, S.O.; Dayan, F.E.; Goldberg-Cavalleri, A.; Tétard-Jones, C.; Wortley, D.J.; Onkokesung, N.; et al. Herbicide Metabolism: Crop Selectivity, Bioactivation, Weed Resistance, and Regulation. Weed Sci. 2019, 67, 149–175. [Google Scholar] [CrossRef] [Green Version]
- Powles, S.B.; Yu, Q. Evolution in Action: Plants Resistant to Herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, K.; Matloob, A.; Aslam, F.; Florentine, S.K.; Chauhan, B.S. Weeds in a Changing Climate: Vulnerabilities, Consequences, and Implications for Future Weed Management. Front. Plant Sci. 2017, 8, 95. [Google Scholar] [CrossRef]
- Wright, A.A.; Rodriguez-Carres, M.; Sasidharan, R.; Koski, L.; Peterson, D.G.; Nandula, V.K.; Ray, J.D.; Bond, J.A.; Shaw, D.R. Multiple Herbicide–Resistant Junglerice (Echinochloa colona): Identification of Genes Potentially Involved in Resistance through Differential Gene Expression Analysis. Weed Sci. 2018, 66, 347–354. [Google Scholar] [CrossRef]
- D’Urso, A.; Brickner, J.H. Mechanisms of epigenetic memory. Trends Genet. 2014, 30, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Mozgova, I.; Mikulski, P.; Pecinka, A.; Farrona, S. Epigenetic Mechanisms of Abiotic Stress Response and Memory in Plants. In Epigenetics in Plants of Agronomic Importance: Fundamentals and Applications; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–64. [Google Scholar]
- Caverzan, A.; Piasecki, C.; Chavarria, G.; Stewart, C.N.; Vargas, L. Defenses Against ROS in Crops and Weeds: The Effects of Interference and Herbicides. Int. J. Mol. Sci. 2019, 20, 1086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostinetto, D.; Benemann, D.P.; Cechin, J.; Nohatto, M.A.; Langaro, A.C.; Piasecki, C.; Vargas, L. Gene Expression Related to Oxidative Stress Induced by Herbicides in Rice. Agron. J. 2019, 111, 1239–1246. [Google Scholar] [CrossRef]
- Friedrich, T.; Faivre, L.; Bäurle, I.; Schubert, D. Chromatin-based mechanisms of temperature memory in plants. Plant Cell Environ. 2018, 42, 762–770. [Google Scholar] [CrossRef]
- Takano, H.K.; Beffa, R.; Preston, C.; Westra, P.; Dayan, F. Reactive oxygen species trigger the fast action of glufosinate. Planta 2019, 249, 1837–1849. [Google Scholar] [CrossRef]
- Sunohara, Y.; Matsumoto, H. Oxidative injury induced by the herbicide quinclorac on Echinochloa oryzicola Vasing. and the involvement of antioxidative ability in its highly selective action in grass species. Plant Sci. 2004, 167, 597–606. [Google Scholar] [CrossRef]
- Baucom, R.S. Evolutionary and ecological insights from herbicide-resistant weeds: What have we learned about plant adaptation, and what is left to uncover? New Phytol. 2019, 223, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Duhoux, A.; Carrère, S.; Gouzy, J.; Bonin, L.; Délye, C. RNA-Seq analysis of rye-grass transcriptomic response to an herbicide inhibiting acetolactate-synthase identifies transcripts linked to non-target-site-based resistance. Plant Mol. Biol. 2015, 87, 473–487. [Google Scholar] [CrossRef]
- Guo, F.; Iwakami, S.; Yamaguchi, T.; Uchino, A.; Sunohara, Y.; Matsumoto, H. Role of CYP81A cytochrome P450s in clomazone metabolism in Echinochloa phyllopogon. Plant Sci. 2019, 283, 321–328. [Google Scholar] [CrossRef]
- Salas-Perez, R.A.; Saski, C.A.; Noorai, R.E.; Srivastava, S.K.; Lawton-Rauh, A.L.; Nichols, R.L.; Roma-Burgos, N. RNA-Seq transcriptome analysis of Amaranthus palmeri with differential tolerance to glufosinate herbicide. PLoS ONE 2018, 13, 0195488. [Google Scholar] [CrossRef]
- Varanasi, V.K.; Brabham, C.; Korres, N.E.; Norsworthy, J.K. Nontarget site resistance in Palmer amaranth [Amaranthus palmeri (S.) Wats.] confers cross-resistance to protoporphyrinogen oxidase-inhibiting herbicides. Weed Technol. 2019, 33, 349–354. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S.B. Resistance to AHAS inhibitor herbicides: Current understanding. Pest Manag. Sci. 2014, 70, 1340–1350. [Google Scholar] [CrossRef]
- Iwakami, S.; Kamidate, Y.; Yamaguchi, T.; Ishizaka, M.; Endo, M.; Suda, H.; Nagai, K.; Sunohara, Y.; Toki, S.; Uchino, A.; et al. CYP 81A P450s are involved in concomitant cross-resistance to acetolactate synthase and acetyl-CoA carboxylase herbicides in Echinochloa phyllopogon. New Phytol. 2018, 221, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Khanom, S.; Jang, J.; Lee, O.R. Overexpression of ginseng cytochrome P450 CYP736A12 alters plant growth and confers phenylurea herbicide tolerance in Arabidopsis. J. Ginseng Res. 2019, 43, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Powles, S.B. Metabolism-Based Herbicide Resistance and Cross-Resistance in Crop Weeds: A Threat to Herbicide Sustainability and Global Crop Production. Plant Physiol. 2014, 166, 1106–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, N.; Yan, Y.; Ge, L.; Zhu, B.; Liu, W.; Wang, J. Target site mutations and cytochrome P450s confer resistance to fenoxaprop-P-ethyl and mesosulfuron-methyl in Alopecurus aequalis. Pest Manag. Sci. 2019, 75, 204–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, D.R. Cytochrome P450 diversity in the tree of life. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Yasuor, H.; Milan, M.; Eckert, J.W.; Fischer, A.J. Quinclorac resistance: A concerted hormonal and enzymatic effort in Echinochloa phyllopogon. Pest Manag. Sci. 2011, 68, 108–115. [Google Scholar] [CrossRef]
- Dalazen, G.; Markus, C.; Merotto, A., Jr. Differential Expression of Genes Associated with Degradation Enhancement of Imazethapyr in Barnyardgrass (Echinochloa crus-galli). J. Agric. Sci. 2018, 10, 389. [Google Scholar] [CrossRef]
- Chayapakdee, P.; Sunohara, Y.; Endo, M.; Yamaguchi, T.; Fan, L.; Uchino, A.; Matsumoto, H.; Iwakami, S. Quinclorac resistance in Echinochloa phyllopogon is associated with reduced ethylene synthesis rather than enhanced cyanide detoxification by β-cyanoalanine synthase. Pest Manag. Sci. 2019, 76, 1195–1204. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, L.; Sun, Y.; Zhang, T.; Dong, L.; Li, J. Resistance to quinclorac caused by the enhanced ability to detoxify cyanide and its molecular mechanism in Echinochloa crus-galli var. zelayensis. Pestic. Biochem. Physiol. 2017, 143, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Baluska, F.; Gagliano, M.; Witzany, G. Memory and Learning in Plants. In Signaling and Communication in Plants; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-75595-3. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef] [PubMed]
- Charng, Y.-Y.; Liu, H.-C.; Liu, N.-Y.; Hsu, F.-C.; Ko, S.-S. Arabidopsis Hsa32, a Novel Heat Shock Protein, Is Essential for Acquired Thermotolerance during Long Recovery after Acclimation. Plant Physiol. 2006, 140, 1297–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Tielas, C.; Graña, E.; Sánchez-Moreiras, A.M.; Reigosa, M.J.; Vaughn, J.N.; Pan, Z.; Bajsa-Hirschel, J.; Duke, M.V.; Duke, S.O.; Bajsa-Hirshel, J. Transcriptome responses to the natural phytotoxin t-chalcone in Arabidopsis thaliana L. Pest Manag. Sci. 2019, 75, 2490–2504. [Google Scholar] [CrossRef]
- Dyer, W.E. Stress-induced evolution of herbicide resistance and related pleiotropic effects. Pest Manag. Sci. 2018, 74, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Simões, T.; Teixeira, M.C.; Fernandes, A.R.; Sá-Correia, I. Adaptation of Saccharomyces cerevisiae to the Herbicide 2,4-Dichlorophenoxyacetic Acid, Mediated by Msn2p- and Msn4p-Regulated Genes: Important Role of SPI1. Appl. Environ. Microbiol. 2003, 69, 4019–4028. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Haslbeck, M.; Vierling, E. A First Line of Stress Defense: Small Heat Shock Proteins and Their Function in Protein Homeostasis. J. Mol. Biol. 2015, 427, 1537–1548. [Google Scholar] [CrossRef] [Green Version]
- Kitichantaropas, Y.; Boonchird, C.; Sugiyama, M.; Kaneko, Y.; Harashima, S.; Auesukaree, C. Cellular mechanisms contributing to multiple stress tolerance in Saccharomyces cerevisiae strains with potential use in high-temperature ethanol fermentation. AMB Express 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément, C. Trehalose and plant stress responses: Friend or foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Ilhan, S.; Ozdemir, F.; Türkan, I. Contribution of trehalose biosynthetic pathway to drought stress tolerance of Capparis ovata Desf. Plant Biol. 2014, 17, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Agreda-Laguna, K.-A.; Cabrera-Ponce, J.-L.; Ruiz Medrano, R.; Antonio Garzon-Tiznado, J.; Xoconostle-Cazares, B. Trehalose Accumulation Provides Drought Tolerance to Genetically-Modified Maize in Open Field Trials. Pak. J. Agric. Sci. 2018, 55, 1009–1020. [Google Scholar] [CrossRef]
- Chen, J.; Gao, T.; Wan, S.; Zhang, Y.; Yang, J.; Yu, Y.; Wang, W. Genome-Wide Identification, Classification and Expression Analysis of the HSP Gene Superfamily in Tea Plant (Camellia sinensis). Int. J. Mol. Sci. 2018, 19, 2633. [Google Scholar] [CrossRef] [Green Version]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Yang, J.; Wang, Q.; Zhu, H.; Chen, Z.; Dao, Y.; Wang, K. Overexpression of the trehalose-6-phosphate phosphatase family gene AtTPPF improves the drought tolerance of Arabidopsis thaliana. BMC Plant Biol. 2019, 19, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Fu, L.; Qin, P.; Sun, Y.; Liu, J.; Wang, X. Overexpression of the wheat trehalose 6-phosphate synthase 11 gene enhances cold tolerance in Arabidopsis thaliana. Gene 2019, 710, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Mishra, D.; Shekhar, S.; Singh, D.; Chakraborty, S.; Chakraborty, N. Heat Shock Proteins and Abiotic Stress Tolerance in Plants. In Heat Shock Protein Responses, Heat Shock Protein; Springer: Cham, Switzerland, 2018; Volume 13. [Google Scholar] [CrossRef]
- Oliveira, C.; Agostinetto, D.; Langaro, A.; Garcia, J.; Lamego, F. Physiological and Molecular Responses in Rice, Weedy Rice and Barnyardgrass Exposed to Supra-Optimal Temperatures. Planta Daninha 2019, 37, 1–11. [Google Scholar] [CrossRef]
- Tian, L.; Xie, Z.; Lu, C.; Hao, X.; Wu, S.; Huang, Y.; Li, D.; Chen, L. The trehalose-6-phosphate synthase TPS5 negatively regulates ABA signaling in Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 869–882. [Google Scholar] [CrossRef]
- Baek, Y.S.; Goodrich, L.V.; Brown, P.J.; James, B.T.; Moose, S.P.; Lambert, K.N.; Riechers, D.E. Transcriptome Profiling and Genome-Wide Association Studies Reveal GSTs and Other Defense Genes Involved in Multiple Signaling Pathways Induced by Herbicide Safener in Grain Sorghum. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef]
- Dong, N.-Q.; Sun, Y.; Guo, T.; Shi, C.-L.; Zhang, Y.-M.; Kan, Y.; Xiang, Y.-H.; Zhang, H.; Yang, Y.-B.; Li, Y.-C.; et al. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef]
- Benedetti, L.; Rangani, G.; Viana, V.E.; Carvalho-Moore, P.; Camargo, E.R.; De Avila, L.A.; Roma-Burgos, N. Recurrent Selection by Herbicide Sublethal Dose and Drought Stress Results in Rapid Reduction of Herbicide Sensitivity in Junglerice. Agronomy 2020, 10, 1619. [Google Scholar] [CrossRef]




| Active Ingredient | Trade Name | WSSA Group | MOA a | Chemical Family | Labeled Rate (g ai ha–1) | Application Rate b (g ai ha–1) |
|---|---|---|---|---|---|---|
| Florpyrauxifen-benzyl | Loyant™ | 4 | Synthetic auxins | Arylpicolinate | 30 | 3.75 |
| Glufosinate-ammonium | Liberty™ 280 SL | 10 | Inhibition of glutamine synthetase | Phosphinic acid | 448 | 56 |
| Imazethapyr | Newpath™ | 2 | Inhibition of acetolactate synthase | Imidazolinone | 211 | 26.38 |
| Quinclorac | Facet L™ | 4 | Synthetic auxins | Quinoline carboxylic acid | 432 | 54 |
| Gene | Family/Name | Oligonucleotide Sequences (5′→3′) | Reference |
|---|---|---|---|
| Act1 (Reference gene) | Actin 1 | F: ATCCTTGTATGCTAGCGGTCGA | [31] |
| R: ATCCAACCGGAGGATAGCATG | |||
| EF1-α (Reference gene) | Elongation factor 1α | F: GTCATTGGCCACGTCGACTC | [31] |
| R: TGTTCATCTCAGCGGCTTCC | |||
| UBQ5 (Reference gene) | Ubiquitin 5 | F: ACCACTTCGACCGCCACTACT | [32] |
| R: ACGCCTAAGCCTGCTGGTT | |||
| APX2 | Ascorbate peroxidase | F: CATCCTCTCCTACGCCGAC | [33] |
| R: CCTTCAGGAGGAGGCTCAG | |||
| CYP709B1 | Cytochrome P450 709B1 | F: GTCGTCAAGCAGGTGCTCTT | [34] |
| R: CAGTGAGGACGAGACCCTTG | |||
| CYP709B2 | Cytochrome P450 709B2 | F: GCCTGAGAGGTTCGAGTACG | [34] |
| R: CGATCATCGCAAAGTTCTGA | |||
| CYP72A14 | Cytochrome P450 72A14 | F: TCGGTGGCATCAAATATCCT | [34] |
| R: GAACTTGCCTGCGTCTTTTC | |||
| CYP72A15 | Cytochrome P450 72A15 | F: CCAGTGAGCTGATACGCAGA | [34] |
| R: GACGTCGCCTGTGAGATTTT | |||
| UGT75D | Glycosyltransferase | F: GCTCACTTTCCCGTTCCAG | [34] |
| R: GTGGTGGAGAATGTGACGAG | |||
| HSP10 | Heat shock protein 71.10 | F: CCGTGTGCTTCGACATTGAC | [35] |
| R: CGTTGGTGATGGTGHTCTTGTT | |||
| HSP15 | Heat shock protein 24.15 | F: GATCAAGGCGGAGATGAAGAAC | [35] |
| R: ACTCGACGTTGACCTGGAAGA | |||
| TPP | Trehalose phosphate phosphatase | F: TTGAAGGTGCGAGTGTTGAG | [34] |
| R: AACCACTCCCCAGTCCTTCT | |||
| TPS | Trehalose phosphate synthase | F: ACAGAGGGGCTACATTGCAC | [34] |
| R: CTGCAACTGCTCCAAGTGAA |
| Log-Logistic Regression Estimates a | |||||
|---|---|---|---|---|---|
| Treatments b | b | d | ED50 | p Value c | SI d |
| Florpyrauxifen-benzyl | (g ae ha−1) | ||||
| G0∙30 °C | −1.99 (0.08) | 100.79 (0.67) | 3.34 (0.07) | - | |
| G0∙45 °C | −1.36 (0.06) | 103.93 (0.95) | 3.91 (0.12) | 0.00053 | 1.17 |
| G1∙30 °C | −2.23 (0.10) | 100.28 (0.61) | 3.46 (0.07) | 1.04 | |
| G1∙45 °C | −1.69 (0.07) | 101.34 (0.81) | 4.52 (0.11) | 2.6685 × 10−11 | 1.35 |
| G2∙30 °C | −2.23 (0.08) | 100.28 (0.49) | 3.46 (0.06) | 1.04 | |
| G2∙45 °C | −1.81 (0.06) | 102.08 (0.66) | 5.69 (0.11) | 2.5105 × 10−24 | 1.70 |
| Glufosinate-ammonium | (g ae ha−1) | ||||
| G0∙30 °C | −1.67 (0.09) | 101.89 (0.98) | 53.65 (1.73) | - | |
| G0∙45 °C | −1.47 (0.08) | 102.65 (1.13) | 56.95 (2.07) | 0.214212 | 1.06 |
| G1∙30 °C | −1.85 (0.11) | 101.58 (1.11) | 58.93 (2.11) | 1.10 | |
| G1∙45 °C | −1.43 (0.08) | 104.00 (1.58) | 81.00 (3.76) | 2.7443 × 10−7 | 1.51 |
| G2∙30 °C | −1.85 (0.16) | 101.58 (1.52) | 58.93 (2.89) | 1.10 | |
| G2∙45 °C | −1.30 (0.10) | 106.35 (2.72) | 109.31 (7.93) | 2.1293 × 10−11 | 2.04 |
| Imazethapyr | (g ai ha−1) | ||||
| G0∙30 °C | −2.83 (0.32) | 98.20 (1.31) | 38.60 (1.37) | - | |
| G0∙45 °C | −1.83 (0.14) | 102.23 (1.60) | 39.98 (1.83) | 0.54070 | 1.04 |
| G1∙30 °C | −3.46 (0.49) | 97.01 (1.40) | 39.53 (1.44) | 1.02 | |
| G1∙45 °C | −1.95 (0.16) | 102.55 (1.95) | 51.98 (2.65) | 1.6142 × 10−5 | 1.35 |
| G2∙30 °C | −3.47 (0.54) | 96.57 (1.49) | 39.40 (1.54) | 1.02 | |
| G2∙45 °C | −2.03 (0.17) | 102.61 (2.17) | 61.18 (3.29) | 1.0319 × 10−9 | 1.58 |
| Quinclorac | (g ae ha−1) | ||||
| G0∙30 °C | −1.62 (0.13) | 104.90 (2.25) | 100.44 (6.07) | - | |
| G0∙45 °C | −1.86 (0.16) | 104.69 (2.12) | 116.99 (6.24) | 0.04857 | 1.16 |
| G1∙30 °C | −1.60 (0.12) | 104.88 (2.01) | 100.94 (5.44) | 1.01 | |
| G1∙45 °C | −2.01 (0.16) | 104.20 (1.89) | 133.34 (5.93) | 5.7162 × 10−5 | 1.33 |
| G2∙30 °C | −1.60 (0.10) | 104.88 (1.69) | 100.94 (4.56) | 1.01 | |
| G2∙45 °C | −2.24 (0.15) | 102.71 (1.54) | 139.53 (4.95) | 1.2312 × 10−7 | 1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, L.; Rangani, G.; Ebeling Viana, V.; Carvalho-Moore, P.; Merotto, A., Jr.; Rabaioli Camargo, E.; Antonio de Avila, L.; Roma-Burgos, N. Rapid Reduction of Herbicide Susceptibility in Junglerice by Recurrent Selection with Sublethal Dose of Herbicides and Heat Stress. Agronomy 2020, 10, 1761. https://doi.org/10.3390/agronomy10111761
Benedetti L, Rangani G, Ebeling Viana V, Carvalho-Moore P, Merotto A Jr., Rabaioli Camargo E, Antonio de Avila L, Roma-Burgos N. Rapid Reduction of Herbicide Susceptibility in Junglerice by Recurrent Selection with Sublethal Dose of Herbicides and Heat Stress. Agronomy. 2020; 10(11):1761. https://doi.org/10.3390/agronomy10111761
Chicago/Turabian StyleBenedetti, Lariza, Gulab Rangani, Vívian Ebeling Viana, Pâmela Carvalho-Moore, Aldo Merotto, Jr., Edinalvo Rabaioli Camargo, Luis Antonio de Avila, and Nilda Roma-Burgos. 2020. "Rapid Reduction of Herbicide Susceptibility in Junglerice by Recurrent Selection with Sublethal Dose of Herbicides and Heat Stress" Agronomy 10, no. 11: 1761. https://doi.org/10.3390/agronomy10111761
APA StyleBenedetti, L., Rangani, G., Ebeling Viana, V., Carvalho-Moore, P., Merotto, A., Jr., Rabaioli Camargo, E., Antonio de Avila, L., & Roma-Burgos, N. (2020). Rapid Reduction of Herbicide Susceptibility in Junglerice by Recurrent Selection with Sublethal Dose of Herbicides and Heat Stress. Agronomy, 10(11), 1761. https://doi.org/10.3390/agronomy10111761