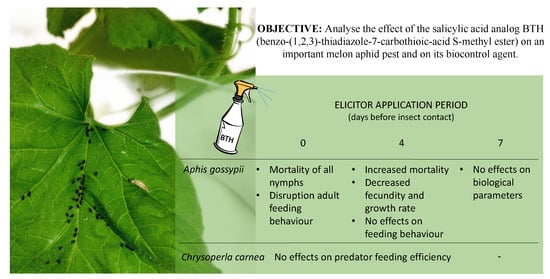
Effects of a Salicylic Acid Analog on Aphis gossypii and Its Predator Chrysoperla carnea on Melon Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Treatments
2.2. Plants and Insects: General Aspects
2.3. Aphis gossypii Individual Fitness
2.4. Aphis gossypii Feeding Behavior
2.5. Feeding Efficiency of Chrysoperla carnea
2.6. Statistical Analysis
3. Results
3.1. Aphis gossypii Individual Fitness
3.2. Aphis gossypii Feeding Behavior
3.3. Feeding Efficiency of Chrysoperla carnea
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Sequeira, L. Mechanisms of Induced Resistance in Plants. Annu. Rev. Microbiol. 1983, 37, 51–79. [Google Scholar] [CrossRef]
- Santamaria, M.E.; Martínez, M.; Cambra, I.; Grbic, V.; Diaz, I. Understanding plant defence responses against herbivore attacks: An essential first step towards the development of sustainable resistance against pests. Transgenic Res. 2013, 22, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Arnaiz, A.; Gonzalez-Melendi, P.; Martinez, M.; Diaz, I. Plant perception and short-term responses to phytophagous insects and mites. Int. J. Mol. Sci. 2018, 19, 1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, H.-S.; Yang, J.W.; Ryu, C.-M. ISR meets SAR outside: Additive action of the endophyte Bacillus pumilus INR7 and the chemical inducer, benzothiadiazole, on induced resistance against bacterial spot in field-grown pepper. Front. Plant Sci. 2013, 4, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical elicitors of systemic acquired resistance—Salicylic acid and its functional analogs. Curr. Plant Biol. 2019, 17, 48–59. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic Acquired Resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Hammerschmidt, R. Systemic Acquired Resistance. Adv. Bot. Res. 2009, 51, 173–222. [Google Scholar]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef]
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeshita, M.; Okuda, M.; Okuda, S.; Hyodo, A.; Hamano, K.; Furuya, N.; Tsuchiya, K. Induction of Antiviral Responses by Acibenzolar-S-Methyl Against Cucurbit chlorotic yellows virus in Melon. Phytopathology 2013, 103, 960–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anfoka, G.H. Benzo-(1,2,3)-thiadiazole-7-carbothioic acid S-methyl ester induces systemic resistance in tomato (Lycopersicon esculentum Mill cv. Vollendung) to Cucumber mosaic virus. Crop Prot. 2000, 19, 401–405. [Google Scholar] [CrossRef]
- Faize, L.; Faize, M. Functional analogues of salicylic acid and their use in crop protection. Agronomy 2018, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Goggin, F.L. Plant-aphid interactions: Molecular and ecological perspectives. Curr. Opin. Plant Biol. 2007, 10, 399–408. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2006, 120, 175–188. [Google Scholar] [CrossRef]
- Cooper, W.C.; Jia, L.; Goggin, F.L. Acquired and r-gene-mediated resistance against the potato aphid in tomato. J. Chem. Ecol. 2004, 30, 2527–2542. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Erb, M.; Lou, Y.; Turlings, T.C.J. The prospect of applying chemical elicitors and plant strengtheners to enhance the biological control of crop pests. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20120283. [Google Scholar] [CrossRef] [Green Version]
- Sobhy, I.S.; Erb, M.; Sarhan, A.A.; El-Husseini, M.M.; Mandour, N.S.; Turlings, T.C.J. Less is more: Treatment with BTH and Laminarin Reduces herbivore-induced volatile emissions in maize but increases parasitoid attraction. J. Chem. Ecol. 2012, 38, 348–360. [Google Scholar] [CrossRef] [Green Version]
- Sobhy, I.S.; Erb, M.; Turlings, T.C.J. Plant strengtheners enhance parasitoid attraction to herbivore-damaged cotton via qualitative and quantitative changes in induced volatiles. Pest Manag. Sci. 2015, 71, 686–693. [Google Scholar] [CrossRef]
- Rostás, M.; Turlings, T.C.J. Induction of systemic acquired resistance in Zea mays also enhances the plant’s attractiveness to parasitoids. Biol. Control 2008, 46, 178–186. [Google Scholar] [CrossRef]
- Cipollini, D.; Heil, M. Costs and benefits of induced resistance to herbivores and pathogens in plants. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2010, 5. [Google Scholar] [CrossRef]
- FAO. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 18 July 2020).
- Gondim, D.M.F.; Terao, D.; Martins-Miranda, A.S.; Vasconcelos, I.M.; Oliveira, J.T.A. Benzo-thiadiazole-7-carbothioic acid S-methyl ester does not protect melon fruits against Fusarium pallidoroseum infection but induces defence responses in melon seedlings. J. Phytopathol. 2008, 156, 607–614. [Google Scholar] [CrossRef]
- Buzi, A.; Chilosi, G.; De Sillo, D.; Magro, P. Induction of Resistance in Melon to Didymella bryoniae and Sclerotinia sclerotiorum by Seed Treatments with Acibenzolar-S-methyl and Methyl Jasmonate but not with Salicylic Acid. J. Phytopathol. 2004, 152, 34–42. [Google Scholar] [CrossRef]
- Li, X.; Bi, Y.; Wang, J.; Dong, B.; Li, H.; Gong, D.; Zhao, Y.; Tang, Y.; Yu, X.; Shang, Q. BTH treatment caused physiological, biochemical and proteomic changes of muskmelon (Cucumis melo L.) fruit during ripening. J. Proteom. 2015, 120, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.L.; Eastop, V.F. Taxonomic Issues. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CAB International: Oxfordshire, UK, 2017; pp. 1–36. [Google Scholar]
- CABI. Aphis Gossypii. Available online: http://www.cabi.org/isc/datasheet/6204 (accessed on 11 August 2020).
- Biobest Chrysopa. Available online: https://www.biobestgroup.com/es/biobest/productos/control-biologico-4462/insectos-y-acaros-depredadores-4477/chrysopa-system-4784/ (accessed on 26 June 2020).
- Koppert Chrysopa. Available online: https://www.koppert.com/chrysopa/ (accessed on 26 June 2020).
- Vogt, H.; Bigler, F.; Brown, K.; Candolfi, M.P.; Kemmeter, F.; Kühner, C.; Moll, M.; Travis, A.; Ufer, A.; Viñuela, E.; et al. Laboratory method to test effects of plant protection products on larvae of Chrysoperla carnea (Neuroptera: Chrysopidae). In Guidelines to Evaluate Side-Effects of Plant Protection Products to Non-Target Arthropods; Candolfi, M.P., Blümel, S., Forster, R., Bakker, F.M., Grimm, C., Hassan, S.A., Heimbach, U., Mead-Briggs, M.A., Raber, B., Schmuck, R., et al., Eds.; IOBC/WPRS: Gent, Belgium, 2000; pp. 27–44. [Google Scholar]
- Feller, V.C.; Bleiholder, H.; Buhr, L.; Hack, H.; Heẞ, M.; Klose, R.; Meier, U.; Stauẞ, R.; Boom, T.; van den Webe, E. Phanologische Entwicklungsstadien von Gemusepflanzen II. Fruchtgemuse und Hulsenfruchte (Phenological growth stages of vegetable crops II. Fruit vegetables and pulses). Nachrichtenbl. Deut. Pflanzenschutzd. 1995, 47, 217–232. [Google Scholar]
- Carmo-Sousa, M.; Moreno, A.; Garzo, E.; Fereres, A. A non-persistently transmitted-virus induces a pull-push strategy in its aphid vector to optimize transmission and spread. Virus Res. 2014, 186, 38–46. [Google Scholar] [CrossRef]
- Fereres, A.; Lister, R.M.; Araya, J.E.; Foster, J.E. Development and reproduction of the English grain aphid (Homoptera: Aphididae) on wheat cultivars infected with Barley yellow dwarf virus. Environ. Entomol. 1989, 18, 388–393. [Google Scholar] [CrossRef]
- Wyatt, I.J.; White, P.F. Simple estimation of intrinsic increase rates for aphids and tetranychid mites. J. Appl. Ecol. 1977, 14, 757–766. [Google Scholar] [CrossRef]
- Birch, L.C. The Intrinsic Rate of Natural Increase of an Insect Population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Parthenogenetic reproduction and the rate of increase in aphids. In Aphids: Their Biology, Natural Enemies and Control; Minks, A.K., Harrewinjn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 269–287. [Google Scholar]
- Tjallingii, W.F. Electrical recording of stylet penetration activities. In Aphids, Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1988; pp. 95–108. [Google Scholar]
- Moreno-Delafuente, A.; Garzo, E.; Moreno, A.; Fereres, A. A Plant Virus Manipulates the Behavior of Its Whitefly Vector to Enhance Its Transmission Efficiency and Spread. PLoS ONE 2013, 8, e61543. [Google Scholar] [CrossRef] [PubMed]
- Sarria, E.; Cid, M.; Garzo, E.; Fereres, A. Workbook for automatic parameter calculation of EPG data. Comput. Electron. Agric. 2009, 67, 35–42. [Google Scholar] [CrossRef]
- Backus, E.A.; Cline, A.R.; Ellerseick, M.R.; Serrano, M.S. Ligus hesperus (Hemiptera: Miridae) Feeding on Cotton: New Methods and Parameters for Analysis of Nonsequential Electrical Penetration Graph Data. Ann. Entomol. Soc. Am. 2007, 100, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Wanumen, A.C.; Sánchez-Ramos, I.; Viñuela, E.; Medina, P.; Adán, Á. Impact of feeding on contaminated prey on the life parameters of Nesidiocoris tenuis (Hemiptera: Miridae) adults. J. Insect Sci. 2016, 16, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Goel, M.; Khanna, P.; Kishore, J. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274. [Google Scholar] [CrossRef] [Green Version]
- Prado, E.; Tjallingii, W.F. Aphid activities during sieve element punctures. Entomol. Exp. Appl. 1994, 72, 157–165. [Google Scholar] [CrossRef]
- Foster, S.E.; Devine, G.; Devonshire, A. Insecticide Resistance. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CAB International: Oxfordshire, UK, 2017; pp. 426–447. [Google Scholar]
- Choh, Y.; Ozawa, R.; Takabayashi, J. Effects of exogenous Jasmonic acid and benzo (1,2,3) thiadiazole-7-carbothioic acid S-methyl ester (BTH), a functional analogue of salicylic acid, on the egg production of a herbivorous mite Tetranychus urticae (Acari: Tetranychidae). Appl. Entomol. Zool. 2004, 39, 311–314. [Google Scholar] [CrossRef]
- Sarwar, N.; Zahid, C.; Haq, I.; Jamil, F.F. Induction of systemic resistance in chickpea against Fusarium wilt by seed treatment with salicylic acid and Bion. Pak. J. Bot. 2005, 37, 989–995. [Google Scholar]
- Inbar, M.; Doostdar, H.; Gerling, D.; Mayer, R.T. Induction of systemic acquired resistance in cotton by BTH has a negligible effect on phytophagous insects. Entomol. Exp. Appl. 2001, 99, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Hodge, S.; Ward, J.L.; Galster, A.M.; Beale, M.H.; Powell, G. The effects of a plant defence priming compound, β-aminobutyric acid, on multitrophic interactions with an insect herbivore and a hymenopterous parasitoid. BioControl 2011, 56, 699–711. [Google Scholar] [CrossRef]
- Rodríguez-López, M.J.; Garzo, E.; Bonani, J.P.; Fereres, A.; Fernández-Muñoz, R.; Moriones, E. Whitefly resistance traits derived from the wild tomato Solanum pimpinellifolium affect the preference and feeding behavior of Bemisia tabaci and reduce the spread of Tomato yellow leaf curl virus. Phytopathology 2011, 101, 1191–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garzo, E.; Moreno, A.; Hernando, S.; Mariño, V.; Torne, M.; Santamaria, E.; Díaz, I.; Fereres, A. Electrical penetration graph technique as a tool to monitor the early stages of aphid resistance to insecticides. Pest Manag. Sci. 2015, 72, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Garzo, E.; Soria, C.; Gomez-Guillamon, M.L.; Fereres, A. Feeding behavior of Aphis gossypii on resistant accessions of different melon genotypes (Cucumis melo). Phytoparasitica 2002, 30, 129–140. [Google Scholar] [CrossRef]
- Powell, G.; Hodge, S. Effects of β-aminobutyric acid on aphid stylet activities. Induc. Resist. Plants Against Insects Dis. IOBC-WPRS Bull. 2018, 135, 124–126. [Google Scholar]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, W.R.; Goggin, F.L. Effects of jasmonate-induced defenses in tomato on the potato aphid, Macrosiphum euphorbiae. Entomol. Exp. Appl. 2005, 115, 107–115. [Google Scholar] [CrossRef]
- Strapasson, P.; Pinto-Zevallos, D.M.; Paudel, S.; Rajotte, E.G.; Felton, G.W.; Zarbin, P.H.G. Enhancing Plant Resistance at the Seed Stage: Low Concentrations of Methyl Jasmonate Reduce the Performance of the Leaf Miner Tuta absoluta but do not Alter the Behavior of its Predator Chrysoperla externa. J. Chem. Ecol. 2014, 40, 1090–1098. [Google Scholar] [CrossRef]
- Karatolos, N.; Hatcher, P.E. The effect of acetylsalicylic acid and oxalic acid on Myzus persicae and Aphidius colemani. Entomol. Exp. Appl. 2009, 130, 98–105. [Google Scholar] [CrossRef]
- Garzo, E.; Moreno, A.; Plaza, M.; Fereres, A. Feeding Behavior and Virus-transmission Ability of Insect Vectors Exposed to Systemic Insecticides. Plants 2020, 9, 895. [Google Scholar] [CrossRef]
- Stevens, M.; Lacomme, C. Transmission of Plant Viruses. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CAB International: Wallingford, UK, 2017; pp. 323–361. ISBN 978-1-78064-709-8. [Google Scholar]


| Aphid Parameter | Treatment | Mean ± SE | Statistic Test | p-Value |
|---|---|---|---|---|
| N1 | Control | 1.13 ± 0.09 | H = 2.413 | 0.299 |
| B4 | 1.36 ± 0.15 | |||
| B7 | 1.16 ± 0.09 | |||
| N2 | Control | 1.00 ± 0.00 | H = 3.091 | 0.213 |
| B4 | 1.09 ± 0.09 | |||
| B7 | 1.00 ± 0.00 | |||
| N3 | Control | 1.07 ± 0.07 | H = 4.452 | 0.108 |
| B4 | 1.18 ± 0.12 | |||
| B7 | 1.37 ± 0.11 | |||
| N4 | Control | 1.60 ± 0.13 | H = 2.914 | 0.233 |
| B4 | 1.36 ± 0.15 | |||
| B7 | 1.32 ± 0.11 | |||
| d | Control | 5.80 ± 0.11 | H = 2.316 | 0.314 |
| B4 | 6.00 ± 0.00 | |||
| B7 | 5.84 ± 0.09 | |||
| Md | Control | 59.867 ± 1.693 a | H = 7.565 | 0.023 * |
| B4 | 46.000 ± 4.665 b | |||
| B7 | 52.158 ± 2.367 ab | |||
| rm | Control | 0.522 ± 0.008 a | F2,42 = 8.371 | 0.001 *** |
| B4 | 0.463 ± 0.015 b | |||
| B7 | 0.498 ± 0.007 a | |||
| Td | Control | 7.859 ± 0.145 | H = 2.316 | 0.314 |
| B4 | 8.130 ± 0.000 | |||
| B7 | 7.916 ± 0.116 | |||
| RGR | Control | 0.607 ± 0.010 a | F2,42 = 8.371 | 0.001 *** |
| B4 | 0.538 ± 0.017 b | |||
| B7 | 0.580 ± 0.008 a |
| Variables | Treatment | PPW | NWEI | Test Statistic | p-Value | WDI | Test Statistic | p-Value | WDE | Test Statistic | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-probe | Control | 20/20 | 16.15 ± 1.62 (5.00–32.00) ab | F2,65 = 4.725 | 0.012 * | 26.90 ± 3.68 (6.23–63.04) ab | H = 10.461 | 0.005 ** | 1.68 ± 0.10 (0.10–16.82) ab | H = 6.876 | 0.032 * |
| B0 | 24/24 | 22.38 ± 2.22 (3.00–41.00) a | 43.44 ± 5.04 (2.83–98.84) a | 1.95 ± 0.10 (0.03–28.34) a | |||||||
| B4 | 24/24 | 13.96 ± 1.72 (4.00–33.00) b | 23.58 ± 4.27 (1.87–96.76) b | 1.70 ± 0.10 (0.06–13.66) b | |||||||
| Probe | Control | 20/20 | 16.15 ± 1.62 (5.00–32.00) ab | H = 8.526 | 0.014 * | 333.10 ± 3.68 (296.96–353.77) ab | H = 10.918 | 0.004 ** | 20.63 ± 3.03 (0.19–331.00) | H = 5.883 | 0.053 |
| B0 | 24/24 | 22.29 ± 2.21 (3.00–40.00) a | 316.56 ± 5.04 (261.16–357.17) b | 14.20 ± 1.72 (0.17–340.44) | |||||||
| B4 | 24/24 | 13.96 ± 1.72 (4.00–33.00) b | 337.89 ± 4.65 (263.24–381.28) a | 24.14 ± 3.44 (0.17–351.50) | |||||||
| C | Control | 20/20 | 17.55 ± 1.81 (5.00–35.00) ab | H = 8.061 | 0.018 * | 157.68 ± 18.36 (44.61–313.89) ab | H = 9.413 | 0.009 ** | 8.98 ± 0.65 (0.19–65.52) | F2,1328 = 0.100 | 0.905 |
| B0 | 24/24 | 25.13 ± 2.65 (3.00–51.00) a | 221.99 ± 17.94 (58.63–321.45) a | 8.84 ± 0.67 (0.17–161.44) | |||||||
| B4 | 24/24 | 15.67 ± 1.92 (5.00–39.00) b | 145.58 ± 18.81 (16.38–336.68) b | 9.27 ± 0.70 (0.14–99.43) | |||||||
| short probes (C < 3 min) | Control | 20/20 | 7.95 ± 0.93 (2.00–20.00) ab | H = 7.983 | 0.018 * | ||||||
| B0 | 24/24 | 12.71 ± 1.61 (1.00–30.00) a | |||||||||
| B4 | 24/24 | 7.17 ± 1.12 (1.00–24.00) b | |||||||||
| E1 | Control | 20/20 | 2.25 ± 0.31 (1.00–5.00) | H = 0.105 | 0.949 | 1.38 ± 0.32 (0.42–5.67) | H = 2.205 | 0.332 | 0.61 ± 0.13 (0.11–5.00) | H = 3.535 | 0.171 |
| B0 | 23/24 | 3.17 ± 0.90 (0.00–17.00) | 3.45 ± 1.71 (0.00–41.60) | 1.09 ± 0.38 (0.08–27.82) | |||||||
| B4 | 23/24 | 2.29 ± 0.38 (0.00–8.00) | 5.06 ± 1.58 (0.00–28.16) | 2.21 ± 0.73 (0.06–28.16) | |||||||
| single E1 | Control | 4/20 | 0.25 ± 0.12 (0.00–2.00) | H = 3.542 | 0.170 | 0.33 ± 0.23 (0.00–4.51) | H = 3.753 | 0.153 | |||
| B0 | 8/24 | 1.46 ± 0.78 (0.00–15.00) | 2.77 ± 1.70 (0.00–40.75) | ||||||||
| B4 | 3/24 | 0.13 ± 0.07 (0.00–1.00) | 0.15 ± 0.08 (0.00–1.38) | ||||||||
| E2 | Control | 20/20 | 1.95 ± 0.29 (1.00–5.00) | H = 1.390 | 0.499 | 170.65 ± 22.28 (5.90–308.33) a | H = 10.081 | 0.006 ** | 87.51 ± 17.72 (0.14–308.33) | H = 2.860 | 0.239 |
| B0 | 20/24 | 1.71 ± 0.30 (0.00–5.00) | 88.31 ± 20.79 (0.00–282.87) b | 51.69 ± 13.60 (0.24–278.36) | |||||||
| B4 | 23/24 | 2.13 ± 0.34 (0.00–7.00) | 174.76 ± 23.17 (0.00–334.86) a | 82.24 ± 15.70 (0.15–318.71) | |||||||
| sE2 | Control | 18/20 | 0.95 ± 0.09 (0.00–2.00) ab | H = 7.930 | 0.019 * | 177.81 ± 21.84 (14.07–308.33) | H = 1.050 | 0.592 | |||
| B0 | 14/24 | 0.63 ± 0.12 (0.00–2.00) b | 139.13 ± 24.16 (19.54–278.36) | ||||||||
| B4 | 21/24 | 1.04 ± 0.11 (0.00–2.00) a | 165.62 ± 21.90 (10.56–318.71) |
| Sequential EPG Variables | Treatment | PPW | Mean ± SE (Range) | Test Statistic | p-Value | |
|---|---|---|---|---|---|---|
| WDI | Time to 1st probe from start of EPG | Control | 20/20 | 2.19 ± 0.86 (0.00–16.82) | H = 0.821 | 0.663 |
| B0 | 24/24 | 1.59 ± 0.61 (0.00–14.32) | ||||
| B4 | 24/24 | 0.85 ± 0.20 (0.00–3.44) | ||||
| Time from 1st probe to 1st E | Control | 20/20 | 110.98 ± 16.14 (20.53–343.71) b | H = 9.586 | 0.008 ** | |
| B0 | 23/24 | 190.26 ± 19.76 (43.85–345.68) a | ||||
| B4 | 23/24 | 119.60 ± 17.58 (18.99–359.94) b | ||||
| Time from the beginning of that probe to 1st E | Control | 20/20 | 33.75 ± 3.90 (13.59–65.52) | H = 1.267 | 0.531 | |
| B0 | 23/24 | 46.03 ± 7.91 (8.98–161.44) | ||||
| B4 | 23/24 | 38.89 ± 8.52 (10.21–206.26) | ||||
| Time from 1st probe to 1st sE2 (>10 min) | Control | 18/20 | 172.16 ± 20.36 (51.45–358.11) b | H = 8.426 | 0.015 * | |
| B0 | 14/24 | 257.93 ± 22.03 (73.44–360.00) a | ||||
| B4 | 21/24 | 170.96 ± 22.74 (19.72–359.94) b | ||||
| Time from the beginning of that probe to 1st sE2 (>10 min) | Control | 18/20 | 32.41 ± 3.51 (12.87–64.21) | H = 3.630 | 0.163 | |
| B0 | 14/24 | 58.39 ± 11.39 (14.62–162.19) | ||||
| B4 | 21/24 | 47.50 ± 9.47 (11.31–206.95) | ||||
| Time from 1st probe to 1st E2 | Control | 20/20 | 125.03 ± 17.68 (20.73–344.86) b | F2,65 = 7.157 | 0.002 ** | |
| B0 | 20/24 | 214.66 ± 21.48 (57.96–360.00) a | ||||
| B4 | 23/24 | 123.28 ± 17.75 (19.72–359.94) b | ||||
| Time from the beginning of that probe to 1st E2 | Control | 20/20 | 33.97 ± 3.93 (14.08–66.68) | H = 0.728 | 0.695 | |
| B0 | 20/24 | 46.83 ± 8.78 (14.35–162.19) | ||||
| B4 | 23/24 | 42.73 ± 8.43 (10.42–206.95) | ||||
| Total duration of non-probe before the 1st E | Control | 20/20 | 18.19 ± 3.06 (0.20–55.34) ab | H = 8.425 | 0.015 * | |
| B0 | 23/24 | 30.89 ± 4.12 (2.83–83.58) a | ||||
| B4 | 23/24 | 16.54 ± 2.47 (1.51–48.80) b | ||||
| NWEI | Number of probes to the 1st E1 | Control | 20/20 | 10.95 ± 1.23 (1.00–22.00) b | F2,65 = 4.88 | 0.011 * |
| B0 | 23/24 | 16.88 ± 1.73 (3.00–32.00) a | ||||
| B4 | 23/24 | 10.79 ± 1.25 (3.00–22.00) b |
| Chrysoperla carnea Parameter | Treatment | Mean ± SE | Test Statistic | p-Value a | |
|---|---|---|---|---|---|
| Development time (d) | L3 | Control | 6.53 ± 0.46 | H = 0.040 | 0.980 |
| B0 | 6.88 ± 0.76 | ||||
| B4 | 6.70 ± 0.50 | ||||
| Pupae | Control | 10.83 ± 0.17 | H = 3.123 | 0.210 | |
| B0 | 10.41 ± 0.15 | ||||
| B4 | 10.65 ± 0.11 | ||||
| Consumption (%) b | 24 h | Control | 42.51 ± 4.61 | F2,55 = 0.159 | 0.854 |
| B0 | 43.88 ± 4.64 | ||||
| B4 | 40.49 ± 3.64 | ||||
| 48 h | Control | 46.42 ± 5.05 | F2,55 = 1.222 | 0.303 | |
| B0 | 53.61 ± 5.26 | ||||
| B4 | 42.75 ± 4.71 | ||||
| 72 h | Control | 39.37 ± 3.63 | H = 1.038 | 0.595 | |
| B0 | 46.29 ± 5.02 | ||||
| B4 | 42.20 ± 6.40 | ||||
| Total | control | 42.73 ± 3.29 | F2,53 = 0.562 | 0.573 | |
| B0 | 47.13 ± 4.07 | ||||
| B4 | 41.53 ± 4.28 | ||||
| Weight (mg) c | 0 h | Control | 3.72 ± 0.29 | F2,30 = 0.364 | 0.698 |
| B0 | 3.83 ± 0.35 | ||||
| B4 | 4.07 ± 0.21 | ||||
| 72 h | Control | 11.65 ± 0.48 | F2,47 = 0.210 | 0.811 | |
| B0 | 11.99 ± 0.50 | ||||
| B4 | 12.16 ± 0.69 | ||||
| Total increase d | Control | 7.17 ± 0.29 | F2,30 = 3.049 | 0.062 | |
| B0 | 8.10 ± 0.51 | ||||
| B4 | 8.91 ± 0.69 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Delafuente, A.; Garzo, E.; Fereres, A.; Viñuela, E.; Medina, P. Effects of a Salicylic Acid Analog on Aphis gossypii and Its Predator Chrysoperla carnea on Melon Plants. Agronomy 2020, 10, 1830. https://doi.org/10.3390/agronomy10111830
Moreno-Delafuente A, Garzo E, Fereres A, Viñuela E, Medina P. Effects of a Salicylic Acid Analog on Aphis gossypii and Its Predator Chrysoperla carnea on Melon Plants. Agronomy. 2020; 10(11):1830. https://doi.org/10.3390/agronomy10111830
Chicago/Turabian StyleMoreno-Delafuente, Ana, Elisa Garzo, Alberto Fereres, Elisa Viñuela, and Pilar Medina. 2020. "Effects of a Salicylic Acid Analog on Aphis gossypii and Its Predator Chrysoperla carnea on Melon Plants" Agronomy 10, no. 11: 1830. https://doi.org/10.3390/agronomy10111830
APA StyleMoreno-Delafuente, A., Garzo, E., Fereres, A., Viñuela, E., & Medina, P. (2020). Effects of a Salicylic Acid Analog on Aphis gossypii and Its Predator Chrysoperla carnea on Melon Plants. Agronomy, 10(11), 1830. https://doi.org/10.3390/agronomy10111830