Comparison of the Agricultural Use of Products from Organic Waste Processing with Conventional Mineral Fertilizer: Potential Effects on Mineral Nitrogen Leaching and Soil Quality
Abstract
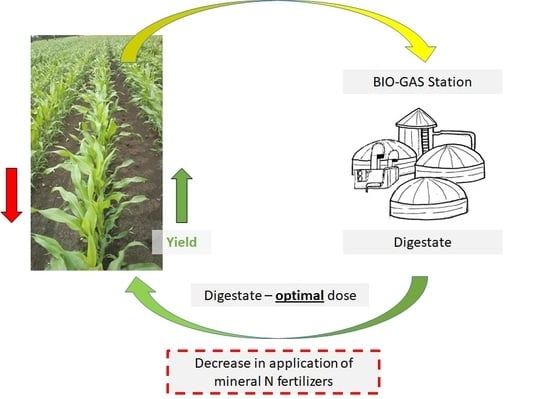
:1. Introduction
2. Materials and Methods
2.1. Laboratory Experiment
2.2. Soil Supplements
2.3. Basal and Substrate-Induced Respiration
2.4. Analysis of Arable Soil Chemical Properties
2.5. Leaching of Mineral Nitrogen
2.6. Statistical Analysis
3. Results and Discussion
3.1. Basal and Substrate-Induced Respiration
3.2. Plant Available Nutrient Content in Soil at the End of the Experiment
3.3. Leaching of Mineral Nitrogen
3.4. Plant Biomas Production of Lactuca sativa L.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
Design of the Experiment


Appendix B
Additional Statistically Processed Data


References
- Altieri, M.A.; Nicholls, C.I. Agroecology and the Search for a Truly Sustainable Agriculture, 1st ed.; United Nations Environment Programme: Mexico City, Mexico, 2005; p. 291. [Google Scholar]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Jensen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Policy and technological constraints to implementation of greenhouse gas mitigation options in agriculture. Agric. Ecosyst. Environ. 2007, 118, 6–28. [Google Scholar] [CrossRef]
- Sadet-Bourgeteau, S.; Hout, S.; Karimi, B.; Mathieu, O.; Mercier, V.; Montenach, D.; Morvan, T.; Sappin-Didier, V.; Watteau, V.; Nowak, V.; et al. Microbial communities from different soil types respond differently to organic waste input. Appl. Soil Ecol. 2019, 143, 70–79. [Google Scholar] [CrossRef]
- Scaglia, B.; Pognani, M.; Adani, F. Evaluation of hormone-like activity of the dissolved organic matter fraction (DOM) of compost and digestate. Sci. Total Environ. 2015, 514, 314–321. [Google Scholar] [CrossRef]
- Harrison-Kirk, T.; Beare, M.H.; Meenken, E.D.; Condron, L.M. Soil organic matter and texture affect responses to dry/wet cycles: Changes in soil organic matter fractions and relationships with C and N mineralisation. Soil Biol. Biochem. 2014, 74, 50–60. [Google Scholar] [CrossRef]
- Wolf, B.; Snyder, G.H. Sustainable Soils: The Place of Organic Matter in Sustaining Soils and Their Productivity, 1st ed.; CRC Press: Binghamton, NY, USA, 2003; p. 380. [Google Scholar]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.; Erisman, J.; Billen, G.; Bleeker, A.; Grennfelt, P.; van Grinsven, H.; Grizzetti, B. Assessing our nitrogen inheritance and the challenge to integrate nitrogen science and policies: The European Nitrogen Assessment approach. In The European Nitrogen Assessment: Sources, Effects and Policy Perspectives, 1st ed.; Binghamton Sutton, M.A., Howard, C., Erisman, J., Billen, G., Bleeker, A., Grennfelt, P., van Grinsven, H., Grizzetti, B., Eds.; Cambridge University Press: New York, NY, USA, 2011; pp. 1–6 and pp. 82–96. [Google Scholar]
- Caracciolo, A.B.; Bustamante, M.A.; Nogues, I.; Di Lenola, M.; Luprano, M.L.; Grenni, P. Changes in microbial community structure and functioning of a semiarid soil due to the use of anaerobic digestate derived composts and rosemary plants. Geoderma 2015, 245–246, 89–97. [Google Scholar] [CrossRef]
- Diaz, L.F.; De Bertoldi, M.; Bidlingmaier, W. Compost Science and Technology, 1st ed.; Elsevier Science: Boston, MA, USA, 2007; p. 364. [Google Scholar]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. organic amendments: Microbial community structure, activity and abundance of agriculturally relevant microbes are driven by long-term fertilization strategies. Front Microbiol. 2016, 14, 1446. [Google Scholar] [CrossRef] [Green Version]
- Farina, R.; Testani, E.; Campanelli, G.; Leteo, F.; Napoli, R.; Canali, S.; Tittarelli, F. Potential carbon sequestration in a Mediterranean organic vegetable cropping system. A model approach for evaluating the effects of compost and Agro-ecological Service Crops (ASCs). Agric. Syst. 2018, 162, 239–248. [Google Scholar] [CrossRef]
- Chalhoub, M.; Garnier, P.; Coquet, Y.; Mary, B.; Lafolie, F.; Houot, S. Increased nitrogen availability in soil after repeated compost applications: Use of the PASTIS model to separate short and long-term effects. Soil Biol. Biochem. 2013, 65, 144–157. [Google Scholar] [CrossRef] [Green Version]
- Claassen, V.P.; Carey, J.L. Comparison of slow-release nitrogen yield from organic soil amendments and chemical fertilizers and implications for regeneration of disturbed sites. Land Degrad. Dev. 2007, 132, 119–132. [Google Scholar] [CrossRef]
- Tiwary, A.; Williams, I.D.; Pant, D.C.; Kishore, V.V.N. Assessment and mitigation of the environmental burdens to air from land applied food-based digestate. Environ. Pollution. 2015, 203, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Massaccesi, L.; Sordi, A.; Micale, C.; Cucina, M.; Zadra, C.; Di Maria, F.; Gigliotti, G. Chemical characterization of percolate and digestate during the hybrid solid anaerobic digestion batch process. Process Biochem. 2013, 48, 1361–1367. [Google Scholar] [CrossRef]
- Wang, J.; Snah, D.; Zhang, G.; Wang, Y.; Wang, C.; Teng, Y.; Christie, P. Nitrogen and phosphorus leaching losses from intensively manager paddy fields with straw retention. Agric. Water Manag. 2014, 141, 66–73. [Google Scholar] [CrossRef]
- Valkama, E.; Salo, T.; Esala, M.; Turtola, E. Nitrogen balances and yields of spring cereals as affected by nitrogen fertilization in northern conditions: A meta-analysis. Agric. Ecosyst. Environ. 2013, 164, 1–13. [Google Scholar] [CrossRef]
- D´antuono, L.F.; Neri, R. The evaluation of nitrogen effect on lettuce quality by means of descriptive sensory profiling. Acta Hortic. 2001, 563, 217–223. [Google Scholar] [CrossRef]
- Šimek, M.; Virtanen, S.; Krištůfek, V.; Simojoki, A.; Yli-Halla, M. Evidence of rich microbial communities in the subsoil of boreal acid sulphate soil conductive to greenhouse gas emissions. Agric. Ecosyst. Environ. 2011, 140, 113–122. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Zbíral, J. Analysis of Soils I. Unified Techniques, 2nd ed.; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2002. (In Czech) [Google Scholar]
- Maňásek, J.; Lošák, T.; Prokeš, K.; Hlušek, J.; Vítězová, M.; Škarpa, P.; Filipčík, R. Effect of nitrogen and Potassium fertilization on micronutrient content in grain maize (Zea mays L.). Acta Univ. Agric. Silvic. Mendel. Brun. 2013, 61, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Novosadová, I.; Záhora, J.; Ruiz-Sinoga, J.D. The availability of mineral nitrogen in Mediterranean open steppe dominated by Stipa tenacissima L. Acta Univ. Agric. Silvic. Mendel. Brun. 2011, 59, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. Review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Ananyeva, N.D.; Susyan, E.A.; Chernova, O.V.; Wirth, S. Microbial respiration activities of soils from different climatic regions of European Russia. Eur. J. Soil Biol. 2008, 44, 147–157. [Google Scholar] [CrossRef]
- Bloem, J.; Hopkins, D.W.; Benedetti, A. Microbiological Methods for Assessing Soil Quality, 1st ed.; CABI: Wallingford, UK, 2006; p. 320. [Google Scholar]
- Geisseler, D.; Horwath, W.R.; Doane, T.A. Significance of organic nitrogen uptake from plant residues by soil microorganisms as affected by carbon and nitrogen availability. Soil Biol. Biochem. 2009, 41, 1281–1288. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage 2019, 187, 85–91. [Google Scholar] [CrossRef]
- Peng, S.Z.; Yang, S.H.; Xu, J.Z.; Luo, Y.F.; Hou, H.J. Nitrogen and phosphorus leaching losses from paddy fields with different water and nitrogen managements. Paddy Water Environ. 2011, 9, 333–342. [Google Scholar] [CrossRef]
- Courtney, R.G.; Mullen, G.J. Soil quality and barley growth as influenced by the land application of two compost types. Biores. Technol. 2007, 99, 2913–2918. [Google Scholar] [CrossRef]
- Gil, M.V.; Carballo, M.T.; Calvo, L.F. Fertilization of maize with compost from cattle manure supple-mented with additional mineral nutrients. Waste Manag. 2007, 28, 1432–1440. [Google Scholar] [CrossRef]
- Radziemska, M.; Vaverková, M.D.; Adamcová, D.; Brtnický, M.; Mazur, Z. Valorization of fish waste compost as a fertilizer for agricultural use. Waste Biomass Valori. 2016, 10, 2537–2545. [Google Scholar] [CrossRef] [Green Version]
- Baldi, E.; Toselli, M.; Marcolini, G.; Quartieri, M.; Cirillo, E.; Innocenti, A.; Marangoni, B. Compost can successfully replace mineral fertilizers in the nutrient management of commercial peach orchard. Soil Use Manag. 2010, 26, 346–353. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.H.; Zhang, Z.; Wu, Y.; Mwiya, R. Response of vertical migration and leaching of nitrogen in percolation water of paddy fields under water-saving irrigation and straw return conditions. Water 2019, 11, 868. [Google Scholar] [CrossRef] [Green Version]
- Obire, O.; Ogan, A.; Okigbo, R.N. Impact of fertilizer plant effluent on water quality. Int. J. Environ. Sci. Technol. 2008, 5, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Namdev, G.R.; Bajpai, A.; Malik, S. Effect of chemical fertilizers on water quality of irrigation reservoir (Kaliasote Reservoir) of Bhopal (M.P.). Curr. World. Environ. 2011, 6, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Makádi, M.; Tomócsik, A.; Orosz, V. Digestate: A new nutrient source—Review. In Biogas, 1st ed.; Kumar, S., Ed.; IntechOpen: Rijeka, Croatia, 2012; pp. 295–310. [Google Scholar]
- Lošák, T.; Hlušek, J.; Válka, T.; Elbl, J.; Vítěz, T.; Bělíková, H.; Von Bennewitz, E. The effect of fertilisation with digestate on kohlrabi yields and quality. Plant Soil Environ. 2016, 62, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Bazzoffi, P.; Pellegrini, S.; Rocchini, A.; Morandi, M.; Grasselli, O. The effect of urban refuse compost and different tractors tyres on soil physical properties, soil erosion and maize yield. Soil Tillage Res. 1998, 48, 275–286. [Google Scholar] [CrossRef]
- Nevens, F.; Reheul, D. The application of vegetable, fruit and garden waste compost in addition to cattle slurry in a silage maize monoculture: Nitrogen availability and use. Eur. J. Agron. 2003, 19, 189–203. [Google Scholar] [CrossRef]
- Huang, T.; Ju, X.; Yang, H. Nitrate leaching in a winter wheat-summer maize rotation on a calcareous soil as affected by nitrogen and straw management. Sci. Rep. 2017, 7, 42247. [Google Scholar] [CrossRef] [Green Version]
- Shaheen, S.M.; Tsadilas, C.D.; Rinklebe, J. A review of the distribution coefficients of trace elements in soils: Influence of sorption system, element characteristics, and soil colloidal properties. Adv. Colloid Interface Sci. 2013, 201–202, 43–56. [Google Scholar] [CrossRef]
- Wang, Y.; Chikamatsu, S.; Gegen, T.; Sawada, K.; Toyota, K.; Riya, S.; Hosomi, M. Application of biogas digestate with rice straw mitigates nitrate leaching potential and suppresses root-knot nematode (Meloidogyne incognita). Agronomy 2019, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Tambone, F. Adani, Nitrogen mineralization from digestate in comparison to sewage sludge, compost and urea in a laboratory incubated soil experiment. J. Plant Nutr. Soil Sci. 2017, 180, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar]
- Schievano, A.; Adani, F.; Tambone, F.; D´imporzano, G.; Scaglia, B.; Genevini, P.L. What Is the Digestate? 1st ed.; Faculty of Agricultural Sciences, University of Milano: Milano, Italy, 2009. [Google Scholar]
- Johansen, A.; Carter, M.S.; Jensen, E.S.; Hauggard-Nielsen, H.; Ambus, P. Effects of digestate from anaerobically digested cattle slurry and plant materials on soil microbial community and emission of CO2 and N2O. Appl. Soil Ecol. 2013, 63, 36–44. [Google Scholar] [CrossRef]
- Lošák, T.; Hlušek, J.; Filipčík, R.; Pospíšilová, L.; Maňásek, J.; Prokeš, K.; Buňka, F.; Kráčmar, S.; Martensson, A.; Orosz, F. Effect of nitrogen fertilization on metabolisms of essential and non-essential amino acids in field-grown grain maize (Zea mays L.). Plant Soil Environ. 2010, 56, 574–579. [Google Scholar] [CrossRef]
- Al-Bataina, B.; Young, T.M.; Ranieri, E. Effects of compost age on the release of nutrients. ISWCR 2016, 4, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Gutser, R.; Ebertseder, T.; Weber, A.; Schraml, M.; Schmidhalter, U. Short-term and residual availability of nitrogen after long-term application of organic fertilizers on arable land. J. Plant Nutr. Soil Sci. 2005, 168, 439–446. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.A.; Cayuela, M.L.; Sánhez-Garcia, M.; Vandecasteele, B.; D’Hose, T.; López, G.; Martínez-Gaitán, C.; Kuikman, P.J.; Sinicco, T.; Mondini, C. Agronomic evaluation of biochar, compost and biochar-blended compost across different cropping systems: Perspective from the European project FERTIPLUS. Agronomy 2019, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Blanco, J.; Lazcano, C.; Christensen, T.H.; Muñoz, P.; Rieradevall, J.; Møller, J.; Antón, A.; Boldrin, A. Compost benefits for agriculture evaluated by life cycleassessment. A review. Agron. Sustain. Dev. 2013, 33, 721–732. [Google Scholar] [CrossRef] [Green Version]
- Hernández, T.; Chocano, C.; Moreno, J.L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Till. Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]



| Treatment | Abb. | Dose of N (kg N ha−1) | Dose of Fertilizer (kg ha−1) | Nutrient Input (kg ha−1) | |||
|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | ||||
| Control | - | 0 | - | 0 | 0 | 0 | 0 |
| Digestate | DG 80 | 80 | 25,806 | 21 | 126 | 49 | 21 |
| Digestate | DG 150 | 150 | 48,387 | 39 | 237 | 92 | 39 |
| Digestate | DG 220 | 220 | 70,968 | 57 | 348 | 135 | 57 |
| Mineral fertilizer | NPK | 150 | 1500 | 150 | 150 | - | - |
| Compost | CP | 150 | 9416 | 5 | 60 | 106 | 12 |
| Fertilizers | Parameter (mg kg−1) | pH CaCl2 | C:N | ||
|---|---|---|---|---|---|
| Dry matter | Ctot | Ntotal | |||
| Digestate | 92 | 4 | 3.1 | 8.17 | 1.29 |
| Compost | 550 | 182 | 13.1 | 7.41 | 13.9 |
| Sample | Parameter (mg kg−1) | K:Mg | |||
|---|---|---|---|---|---|
| P | K | Ca | Mg | ||
| Topsoil | 39 | 278 | 735.00 | 176 | 1.57 |
| low | good | low | good | suitable | |
| Digestate | 800 | 4900 | 1900 | 800 | 6.1 |
| Compost | 565 | 6422 | 11,235 | 1255 | 5.1 |
| Soil Sample | pH (CaCl2) | pH (H2O) | EC (dS cm−1) | TDS (mg L−1) |
|---|---|---|---|---|
| Topsoil | 5.77 | 6.4 | 0.11 | 72.3 |
| strongly acidic | weakly acidic |
| Variant | Dose of N (kg ha−1) | Differences | |
|---|---|---|---|
| BAS | SIR | ||
| Control | 0 | 100% | 100% |
| DG 80 | 80 | 88% | 160% |
| DG 150 | 150 | 112% | 236% |
| DG 220 | 220 | 95% | 177% |
| NPK | 150 | 62% | 150% |
| Compost | 150 | 115% | 172% |
| Variant | Dose of N (kg ha−1) | Differences | |||
|---|---|---|---|---|---|
| P | K | Ca | Mg | ||
| Soil—default value | 0 | 100% | 100% | 100% | 100% |
| Control | 0 | +213% | −2% | +23% | +4% |
| DG 80 | 80 | +211% | +7% | +19% | +1% |
| DG 150 | 150 | +211% | +31% | +18% | +3% |
| DG 220 | 220 | +222% | +105% | +16% | +5% |
| NPK | 150 | +361% | +32% | +34% | −2% |
| Compost | 150 | +242% | +24% | +42% | +9% |
| Variant/ Dose of N | mg kg−1 | K:Mg | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | ||||||
| Mean | ±SE | Mean | ±SE | Mean | ±SE | Mean | ±SE | ||
| Soil—default value | 39d | 0.38 | 278b | 5.32 | 735e | 15.60 | 176b,c | 1.46 | 1.58 |
| Control | 122c | 0.49 | 273b | 3.60 | 904c | 7.05 | 183a,b,c | 0.65 | 0.14 |
| DG 80 80 kg N/ha | 120c | 0.65 | 296b | 24.14 | 871c,e | 10.08 | 177b | 1.81 | 1.67 |
| DG 150 150 kg N/ha | 121c | 1.82 | 364b | 32.98 | 866c,e | 2.33 | 181b | 1.80 | 2.01 |
| DG 220 220 kg N/ha | 124c | 0.86 | 569a | 41.49 | 853e | 8.66 | 184b | 2.93 | 3.09 |
| NPK 150 kg N/ha | 179a | 2.54 | 365b | 15.80 | 987b | 8.76 | 172c | 3.78 | 2.12 |
| Compost 150 kg N/ha | 132b | 1.50 | 344b | 19.51 | 1044a | 8.14 | 192a | 1.20 | 1.79 |
| Variant | Dose of N (kg ha−1) | Differences |
|---|---|---|
| Leaching of Nmin | ||
| Control | 0 | 100% |
| DG 80 | 80 | 103% |
| DG 150 | 150 | 136% |
| DG 220 | 220 | 239% |
| NPK | 150 | 694% |
| Compost | 150 | 98% |
| Variant | Yields of Plant Biomass (g 0.01 m−2) | R:S | |||||
|---|---|---|---|---|---|---|---|
| Aboveground | Underground | Total | |||||
| Mean | ±SD | mean | ±SD | mean | ±SD | ||
| Control | 1.89 b | 0.19 | 0.29 b | 0.01 | 2.18 b | 0.20 | 0.15 |
| DG 80 | 3.28 a,b | 0.39 | 0.74 b | 0.09 | 4.02 a | 0.47 | 0.22 |
| DG 150 | 3.57 a | 0.23 | 1.19 a | 0.11 | 4.76 a | 0.34 | 0.33 |
| DG 220 | 2.90 b | 0.47 | 0.65 b | 0.05 | 3.55 a,b | 0.48 | 0.22 |
| NPK | 3.04 a,b | 0.43 | 0.75 b | 0.02 | 3.79 a | 0.42 | 0.24 |
| Compost | 1.79 b | 0.19 | 0.21 b | 0.03 | 2.00b | 0.22 | 0.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbl, J.; Šimečková, J.; Škarpa, P.; Kintl, A.; Brtnický, M.; Vaverková, M.D. Comparison of the Agricultural Use of Products from Organic Waste Processing with Conventional Mineral Fertilizer: Potential Effects on Mineral Nitrogen Leaching and Soil Quality. Agronomy 2020, 10, 226. https://doi.org/10.3390/agronomy10020226
Elbl J, Šimečková J, Škarpa P, Kintl A, Brtnický M, Vaverková MD. Comparison of the Agricultural Use of Products from Organic Waste Processing with Conventional Mineral Fertilizer: Potential Effects on Mineral Nitrogen Leaching and Soil Quality. Agronomy. 2020; 10(2):226. https://doi.org/10.3390/agronomy10020226
Chicago/Turabian StyleElbl, Jakub, Jana Šimečková, Petr Škarpa, Antonín Kintl, Martin Brtnický, and Magdalena Daria Vaverková. 2020. "Comparison of the Agricultural Use of Products from Organic Waste Processing with Conventional Mineral Fertilizer: Potential Effects on Mineral Nitrogen Leaching and Soil Quality" Agronomy 10, no. 2: 226. https://doi.org/10.3390/agronomy10020226
APA StyleElbl, J., Šimečková, J., Škarpa, P., Kintl, A., Brtnický, M., & Vaverková, M. D. (2020). Comparison of the Agricultural Use of Products from Organic Waste Processing with Conventional Mineral Fertilizer: Potential Effects on Mineral Nitrogen Leaching and Soil Quality. Agronomy, 10(2), 226. https://doi.org/10.3390/agronomy10020226