Productive and Physiological Response of Organic Potato Grown under Highly Calcareous Soils to Fertilization and Mycorrhization Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site, Soil and Climate
2.2. Field Experimental Design, Plant Material and Management Practices
2.3. Crop Physiology, Measurements and Calculations
2.4. Crop Yield and Its Components
2.5. Tuber Dry Matter Determination
2.6. Soil Sampling and DNA Extraction
2.7. Real-Time Quantitative PCR Assay of Soil DNA Extracts
2.8. Statistical Analysis
3. Results
3.1. Mycorrhizal Colonization
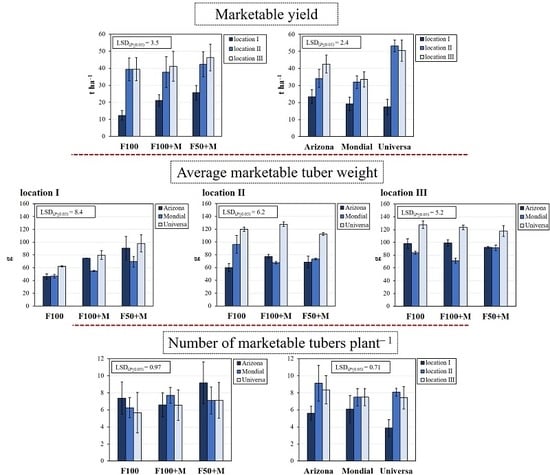
3.2. Marketable Yield and Its Components
3.3. Photosynthesis Rate (Pr)
3.4. Chl Content and Chl Fluorescence
3.5. Stomatal Conductance (g)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yiridoe, E.K.; Bonti-Ankomah, S.; Martin, R.C. Comparison of consumer perceptions and preference toward organic versus conventionally produced foods: A review and update of the literature. Renew. Agric. Food Syst. 2005, 20, 193–205. [Google Scholar] [CrossRef]
- FiBL-AMI. Statistics on Organic Agriculture. 2018. Available online: http://www.fibl.org/en/themes/organic-farmingstatistics (accessed on 20 April 2020).
- Willer, H.; Schaack, D.; Lernoud, J. Organic farming and market development in Europe and the European Union. In The World of Organic Agriculture. Statistics and Emerging Trends; Willer, H., Lernoud, J., Eds.; FiBL & IFOAM–Organics International: Nürnberg, Germany, 2019; pp. 217–254. [Google Scholar]
- Maggio, A.; Carillo, P.; Bulmetti, G.S.; Fuggi, A.; Barbieri, G.; De Pascale, S. Potato yield and metabolic profiling under conventional and organic farming. Eur. J. Agron. 2008, 28, 343–350. [Google Scholar] [CrossRef]
- Bacchi, M.A.; De Nadai Fernandes, E.A.; Tsai, S.M.; Santos, L.G.C. Conventional and organic potatoes: Assessment of elemental composition using k0-INAA. J. Radioanal. Nucl. Chem. 2004, 259, 421–424. [Google Scholar] [CrossRef]
- Warman, P.R.; Havard, K.A. Yield, vitamin and mineral contents of organically and conventionally grown potatoes and sweet corn. Agric. Ecosyst. Environ. 1998, 68, 207–216. [Google Scholar] [CrossRef]
- Lombardo, S.; Lo Monaco, A.; Pandino, G.; Parisi, B.; Mauromicale, G. The phenology, yield and tuber composition of ‘early’ crop potatoes: A comparison between organic and conventional cultivation systems. Renew. Agric. Food Syst. 2013, 28, 50–58. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The influence of growing environment on the antioxidant and mineral content of “early” crop potato. J. Food Compos. Anal. 2013, 32, 28–35. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The mineral profile in organically and conventionally grown “early” crop potato tubers. Sci. Hortic. 2014, 167, 169–173. [Google Scholar] [CrossRef]
- Buono, V.; Paradiso, A.; Serio, F.; Gonnella, M.; De Gara, L.; Santamaria, P. Tuber quality and nutritional components of early potato subjected to chemical haulm desiccation. J. Food Compos. Anal. 2009, 22, 556–562. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The effect on tuber quality of an organic versus a conventional cultivation system in the early crop potato. J. Food Compos. Anal. 2017, 62, 189–196. [Google Scholar] [CrossRef]
- Izmirlioglu, G.; Demirci, A. Enhanced bio-ethanol production from industrial potato waste by statistical medium optimization. Int. J. Mol. Sci. 2015, 16, 24490–24505. [Google Scholar] [CrossRef] [Green Version]
- Jagatee, S.; Behera, S.; Dash, P.K.; Sahoo, S.; Mohanty, R.C. Bioprospecting starchy feedstocks for bioethanol production: A future perspective. JMRR 2015, 3, 24–42. [Google Scholar]
- Ierna, A.; Mauromicale, G. Potato growth, yield and water productivity response to different irrigation and fertilization regimes. Agric. Water Manag. 2018, 201, 21–26. [Google Scholar] [CrossRef]
- Canali, S.; Ciaccia, C.; Antichi, D.; Barberi, P.; Montemurro, F.; Tittarelli, F. Interactions between green manure and amendment type and rate: Effects on organic potato and soil mineral N dynamic. J. Food Agric. Environ. 2010, 8, 537–543. [Google Scholar]
- Hepperly, P.; Lotter, D.; Ziegler, C.; Seidel, R.; Reider, C. Compost, manure and synthetic fertilizer influences crop yields, soil properties: Nitrate leaching and crop nutrient content. Compos. Sci. Util. 2009, 17, 117–126. [Google Scholar] [CrossRef]
- Rosen, C.J.; Allan, D.L. Exploring the benefits of organic nutrient sources for crop production and soil quality. HortTechnology 2007, 17, 422–430. [Google Scholar] [CrossRef]
- Caliskan, M.E.; Kilic, S.; Gunel, E.; Mert, M. Effect of farmyard manure and mineral fertilization on growth and yield of early potato (Solanum tuberosum L.) under the Mediterranean conditions in Turkey. Indian J. Agron. 2004, 49, 198–200. [Google Scholar]
- Ierna, A.; Parisi, B. Crop growth and tuber yield of “early” potato crop under organic and conventional farming. Sci. Hortic. 2014, 165, 260–265. [Google Scholar] [CrossRef]
- Clark, M.S.; Horwath, W.R.; Shennan, C.; Scow, K.M.; Lantni, W.T.; Ferris, H. Nitrogen, weeds and water as yield-limiting factors in conventional, low-input, and organic tomato systems. Agric. Ecosyst. Environ. 1999, 73, 257–270. [Google Scholar] [CrossRef]
- Lynch, D.H.; Sharifi, M.; Hammermeister, A.; Burton, D. Nitrogen management in organic potato production. In Sustainable Potato Production: Global Case Studies; He, Z., Larkin, R., Honeycutt, W., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 209–231. [Google Scholar] [CrossRef]
- Van Delden, A.; Schroder, J.J.; Kropff, M.J.; Grashoff, C.; Booij, R. Simulated potato yield and crop and soil nitrogen dynamics under different organic nitrogen management strategies in the Netherlands. Agric. Ecosyst. Environ. 2003, 96, 77–95. [Google Scholar] [CrossRef]
- Ierna, A.; Mauromicale, G. Tuber yield and irrigation water productivity in early potatoes as affected by irrigation regime. Agric. Water Manag. 2012, 115, 276–284. [Google Scholar] [CrossRef]
- Mauromicale, G.; Signorelli, P.; Ierna, A.; Foti, S. Effects of intraspecific competition on yield of early potato grown in Mediterranean environment. Am. J. Potato Res. 2003, 80, 281–288. [Google Scholar] [CrossRef]
- Taalab, A.S.; Ageeb, G.W.; Siam, H.S.; Mahmoud, S.A. Some characteristics of calcareous soils. A review. Middle East J. Agric. Res. 2019, 8, 96–105. [Google Scholar]
- Di Gregorio, A. Land Cover Classification System, Classification Concepts and User Manual, 3rd ed.; FAO: Rome, Italy, 2016. [Google Scholar]
- Koch, M.; Naumann, M.; Pawelzik, E.; Gransee, A.; Thiel, H. The importance of nutrient management for potato production Part I: Plant nutrition and yield. Potato Res. 2019, 63, 97–119. [Google Scholar] [CrossRef] [Green Version]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Optimizing nitrogen fertilization to improve qualitative performances and physiological and yield responses of potato (Solanum tuberosum L.). Agronomy 2020, 10, 352. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Mäder, P.; Edenhofer, S.; Boller, T.; Wiemken, A.; Niggli, U. Arbuscular mycorrhizae in a long-term field trial comparing low-input (organic, biological) and high-input (conventional) farming systems in a crop rotation. Biol. Fertil. Soils 2000, 31, 150–156. [Google Scholar] [CrossRef]
- Black, R.L.B.; Tinker, P.B. Interaction between effects of vesicular-arbuscular mycorrhizae and fertilizer phosphorus on yields of potatoes in the field. Nature 1977, 267, 510–511. [Google Scholar] [CrossRef]
- Wu, F.; Wang, W.; Ma, Y.; Liu, Y.; Ma, X.; An, L.; Feng, H. Prospect of beneficial microorganisms applied in potato cultivation for sustainable agriculture. Afr. J. Microbiol. Res. 2013, 7, 2150–2158. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Nagahashi, G.; Reider, C.; Hepperly, P.R. Inoculation with arbuscular mycorrhizal fungi increases the yield of potatoes in a high P soil. Biol. Agric. Hortic. 2007, 25, 67–78. [Google Scholar] [CrossRef]
- Duffy, E.M.; Cassells, A.C. The effect of inoculation of potato (Solanum tuberosum L.) microplants with arbuscular mycorrhizal fungi on tuber yield and tuber size distribution. Appl. Soil Ecol. 2000, 15, 137–144. [Google Scholar] [CrossRef]
- Hijri, M. Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 2016, 26, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Nutritional and sensory characteristics of ‘‘early’’ potato cultivars under organic and conventional cultivation systems. Food Chem. 2012, 133, 1249–1254. [Google Scholar] [CrossRef]
- Parisi, B.; Govoni, F.; Mainolfi, A.; Baschieri, T.; Ranalli, P. Nuovi cloni italiani per la pataticoltura nazionale. L’Informatore Agrar. 2002, 46, 41–45. [Google Scholar]
- Graham, S.O.; Green, N.E.; Hendrix, J.W. The influence of vesicular-arbuscular mycorrhizal fungi on growth and tuberization of potatoes. Mycologia 1976, 68, 925–929. [Google Scholar] [CrossRef]
- Johnstone, P.D.; Lowther, W.L.; Keoghan, J.M. Design and analysis of multi-site agronomic evaluation trials. N. Z. J. Agric. Res. 1993, 36, 323–326. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; US. Gov. Print. Office: Washington, DC, USA, 1999.
- Italian Society of Soil Science. Metodi Normalizzati Di Analisi Del Suolo; Edagricole: Bologna, Italy, 1985. [Google Scholar]
- Dierauer, H.; Siegrist, F.; Weidmann, G.; Commercial Organic Fertiliser as Supplementary Fertilisers in Potato Crop Production. Research Institute of Organic Agriculture‒FiBL, Practice Abstract. 2017. Available online: https://www.orgprints.org/31027/ (accessed on 20 April 2020).
- Mauromicale, G.; Ierna, A.; Marchese, M. Chlorophyll fluorescence and chlorophyll content in field-grown potato as affected by nitrogen supply, genotype, and plant age. Photosynthetica 2006, 44, 76–82. [Google Scholar] [CrossRef]
- Mauromicale, G.; Lo Monaco, A.; Longo, A.M.G. Effect of branched broomrape (Orobanche ramosa) infection on the growth and photosynthesis of tomato. Weed Sci. 2008, 56, 574–581. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Babani, F.; Lichtenthaler, H.K. Light-induced and age-dependent development of chloroplasts in etiolated barley leaves as visualized by determination of photosynthetic pigments, CO2 assimilation rates and different kinds of chlorophyll fluorescence ratios. J. Plant Physiol. 1996, 148, 555–566. [Google Scholar] [CrossRef]
- Scavo, A.; Restuccia, A.; Abbate, C.; Mauromicale, G. Seeming field allelopathic activity of Cynara cardunculus L. reduces the soil weed seed bank. Agron. Sustain. Develop. 2019, 39, 41. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Thonar, C.; Erb, A.; Jansa, J. Real-time PCR to quantify composition of arbuscular mycorrhizal fungal communities-marker design, verification, calibration and field validation. Mol. Ecol. Resour. 2012, 12, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Scavo, A.; Restuccia, A.; Lombardo, S.; Fontanazza, S.; Abbate, C.; Pandino, G.; Anastasi, U.; Onofri, A.; Mauromicale, G. Improving soil health, weed management and nitrogen dynamics by Trifolium subterraneum cover cropping. Agron. Sustain. Develop. 2020, 40, 18. [Google Scholar] [CrossRef]
- Khademi, Z.; Jones, D.L.; Malakouti, M.J.; Asadi, F. Organic acids differ in enhancing phosphorus uptake by Triticum aestivum L.—Effects of rhizosphere concentration and counterion. Plant Soil 2010, 334, 151–159. [Google Scholar] [CrossRef]
- Sylvia, D.M.; Schenck, N.C. Application of superphosphate to mycorrhizal plants stimulates sporulation of phosphorus-tolerate vesicular-arbuscular mycorrhizal fungi. New Phytol. 1983, 95, 655–661. [Google Scholar] [CrossRef]
- Alkan, N.; Gadkar, V.; Yarden, O.; Kapulnik, Y. Analysis of quantitative interactions between two species of arbuscular mycorrhizal fungi, Glomus mosseae and G. intraradices, by Real-Time PCR. Appl. Environ. Microb. 2006, 72, 4192–4199. [Google Scholar] [CrossRef] [Green Version]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agr. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Ismail, Y.; McCormick, S.; Hijri, M. A fungal symbiont of plant-roots modulates mycotoxin gene expression in the pathogen Fusarium sambucinum. PLoS ONE 2011, 6, e17990. [Google Scholar] [CrossRef] [Green Version]
- Romero-Munar, A.; Baraza, E.; Gulías, J.; Cabot, C. Arbuscular mycorrhizal fungi confer salt tolerance in giant reed (Arundo donax L.) plants grown under low phosphorus by reducing leaf Na+ concentration and improving phosphorus use efficiency. Front. Plant Sci. 2019, 10, 843. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Lozano, J.M.; Porcel, R.; Azcón, C.; Aroca, R. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants: New challenges in physiological and molecular studies. J. Exp. Bot. 2012, 63, 4033–4044. [Google Scholar] [CrossRef] [Green Version]
- Al-Karaki, G.; McMichael, B.; Zak, J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mycorrhiza 2004, 14, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Smith, F.A.; Smith, S.E. Phosphorus efficiencies and responses of barley (Hordeum vulgare L.) to arbuscular mycorrhizal fungi grown in highly calcareous soil. Mycorrhiza 2003, 13, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Eason, W.R.; Webb, K.J.; Michaelson-Yeates, T.P.T.; Abberton, M.T.; Griffith, G.W.; Culshaw, C.M.; Hooker, J.E.; Dhanoa, M.S. Effect of genotype of Trifolium repens on myconhizal symbiosis with Glomus mosseae. J. Agric. Sci. 2001, 137, 27–36. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Ruta, C.; Mauromicale, G. In vitro micropropagation and mycorrhizal treatment influences the polyphenols content profile of globe artichoke under field conditions. Food Res. Int. 2017, 99, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef]
- Sheng, M.; Tang, M.; Chen, H.; Yang, B.; Zhang, F.; Huang, Y. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 2008, 18, 287–296. [Google Scholar] [CrossRef]
- Bavaresco, L.; Poni, S. Effect of calcareous soil on photosynthesis rate, mineral nutrition, and Source-Sink ratio of table grape. J. Plant Nutr. 2007, 26, 2123–2135. [Google Scholar] [CrossRef]
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Polispitak, K.; Thongpoem, P.; Singh, H.P.; Cha-um, S. Alleviation of salt stress in upland rice (Oryza sativa L. ssp. indica cv. Leum Pua) using arbuscular mycorrhizal fungi inoculation. Front. Plant Sci. 2020, 11, 348. [Google Scholar] [CrossRef] [Green Version]
- Hajiboland, R.; Aliasgharzadeh, N.; Laiegh, S.F.; Poschenrieder, C. Colonization with arbuscular mycorrhizal fungi improves salinity tolerance of tomato (Solanum lycopersicum L.) plants. Plant Soil 2010, 331, 313–327. [Google Scholar] [CrossRef]
- Pinior, A.; Grunewaldt-Stöcker, G.; von Alten, H.; Strasser, R.T. Mycorrhizal impact on drought stress tolerance of rose plants probed by chlorophyll a fluorescence, proline content and visual scoring. Mycorrhiza 2005, 15, 596–605. [Google Scholar] [CrossRef]
- Lima, J.D.; Mosquim, P.R.; Da Matta, F.M. Leaf gas exchange and chlorophyll fluorescence parameters in Phaseolus vulgaris as affected by nitrogen and phosphorus deficiency. Photosynthetica 1999, 37, 113–121. [Google Scholar] [CrossRef]







| Soil Characteristic | Location I | Location II | Location III |
|---|---|---|---|
| Sand (%) | 42.6 | 54.1 | 51.8 |
| Silt (%) | 38.7 | 24.8 | 22.0 |
| Clay (%) | 18.7 | 21.1 | 26.2 |
| Total limestone (%) | 68.0 | 44.2 | 65.6 |
| Active limestone (%) | 27.9 | 15.5 | 18.0 |
| Organic matter (%) | 2.18 | 1.7 | 2.6 |
| Organic carbon (%) | 1.27 | 1.0 | 1.5 |
| C/N ratio | 7.0 | 7.5 | 7.5 |
| Total N (g kg−1) | 1.8 | 1.3 | 2.0 |
| Assimilable P2O5 (mg kg−1) | 28.5 | 66 | 135 |
| Exchangeable K2O (mg kg−1) | 197 | 455 | 612 |
| pH | 8 | 7.8 | 7.5 |
| Electrical conductivity (dS m−1) | 1.7 | 1.32 | 1.14 |
| Cation exchange capacity (meq 100 g−1) | 17.2 | 22.8 | 26.0 |
| Fertilization Management Treatment (F) | Phenological Stage of Application | Commercial Product | No. of Applications | Dose Rate per Application |
|---|---|---|---|---|
| F100 | At sowing | Ricin-Xed® | 1 | 1.2 t ha−1 |
| “ | Xedaneem Pel® | 1 | 1.2 t ha−1 | |
| “ | Kalisop® | 1 | 0.6 t ha−1 | |
| “ | Fosfonature 26® | 1 | 0.4 t ha−1 | |
| After emergence | Biosin® | 3 | 150 cc hL | |
| F100+M | At sowing | Ricin-Xed® | 1 | 1.2 t ha−1 |
| “ | Xedaneem Pel® | 1 | 1.2 t ha−1 | |
| “ | Kalisop® | 1 | 0.6 t ha−1 | |
| “ | Fosfonature 26® | 1 | 0.4 t ha−1 | |
| “ | Xedaopen® | 40 kg ha−1 | ||
| After emergence | Biosin® | 3 | 150 cc hL | |
| F50+M | At sowing | Ricin-Xed® | 1 | 0.6 t ha−1 |
| “ | Xedaneem Pel® | 1 | 0.6 t ha−1 | |
| “ | Kalisop® | 1 | 0.3 t ha−1 | |
| “ | Fosfonature 26® | 1 | 0.2 t ha−1 | |
| “ | Xedaopen® | 40 kg ha−1 | ||
| After emergence | Biosin® | 3 | 75 cc hL |
| Source of Variation | df | qRT-PCR Analysis | Yield and Its Components | ||||
|---|---|---|---|---|---|---|---|
| Gigaspora spp. | Glomus spp. | MY | AMTW | NMTP | TDMP | ||
| Main factors | |||||||
| Fertilization management (F) | 2 | 4641.5 *** | 3240.5 *** | 22.1 *** | 9.3 *** | 12.7 *** | 5.5 ** |
| Cultivar (C) | 2 | 573.1 *** | 342.7 *** | 53.4 *** | 199.1 *** | 11.7 *** | 19.9 *** |
| Location (L) | 2 | 39.1 *** | 122.5 *** | 219.9 *** | 141.9 *** | 80.2 *** | 13.1 *** |
| Interactions | |||||||
| (F) × (C) | 4 | 171.9 *** | 30.5 *** | 2.4 NS | 9.3 *** | 5.8 *** | 1.2 NS |
| (F) × (L) | 4 | 37.1 *** | 61.5 *** | 4.5 ** | 25.2 *** | 2.3 NS | 0.6 NS |
| (C) × (L) | 4 | 18.4 *** | 10.4 *** | 24.4 *** | 23.9 *** | 6.5 *** | 2.3 NS |
| (F) × (C) × (L) | 8 | 13.8 *** | 11.0 *** | 2.3 NS | 3.5 ** | 1.1 NS | 0.4 NS |
| Location I | |||||||
|---|---|---|---|---|---|---|---|
| Main Factors | Interactions | ||||||
| Fertilization Management (F) | Cultivar (C) | Measurement Date (M) | (F) × (C) | (F) × (M) | (C) × (M) | (F) × (C) × (M) | |
| degrees of freedom | 2 | 2 | 2 | 4 | 4 | 4 | 8 |
| Pr | 3.3 * | 8.7 *** | 208.0 *** | 8.6 *** | 3.6 ** | 14.6 *** | 6.8 *** |
| Chl content | 66.1 *** | 0.3 NS | 32.9 *** | 4.9 ** | 18.7 *** | 12.1 *** | 1.6 NS |
| Fv/Fm | 0.1 NS | 7.3 ** | 0.5 NS | 6.7 *** | 1.5 NS | 2.5 * | 1.1 NS |
| Fv/F0 | 2.9 NS | 7.4 ** | 0.9 NS | 14.8 *** | 5.7 *** | 3.3 * | 4.4 *** |
| g | 35.8 *** | 40.3 *** | 94.7 *** | 3.1 * | 3.1 * | 2.7 * | 2.3 * |
| Location II | |||||||
| Pr | 21.0 *** | 2.4 NS | 64.2 *** | 6.6 *** | 1.3 NS | 4.1 ** | 12.3 *** |
| Chl content | 11.8 *** | 44.0 *** | 144.2 *** | 29.5 *** | 2.6 * | 10.6 *** | 1.0 NS |
| Fv/Fm | 1.2 NS | 8.2 *** | 1.0 NS | 1.4 NS | 0.6 NS | 1.3 NS | 3.6 ** |
| Fv/F0 | 0.4 NS | 36.2 ** | 5.8 ** | 9.6 *** | 8.9 *** | 12.6 *** | 13.1 *** |
| g | 21.2 *** | 30.3 *** | 33.1 *** | 10.2 *** | 4.3 ** | 10.9 *** | 3.1 ** |
| Location III | |||||||
| Pr | 0.2 NS | 6.3 ** | 120.6 *** | 0.8 NS | 2.5 NS | 1.3 NS | 0.8 NS |
| Chl content | 15.1 *** | 96.6 *** | 317.5 *** | 19.9 *** | 6.0 *** | 9.7 *** | 1.7 NS |
| Fv/Fm | 0.3 NS | 2.3 NS | 4.3 * | 0.5 NS | 1.5 NS | 0.7 NS | 0.8 NS |
| Fv/F0 | 4.1 * | 5.1 ** | 9.1 *** | 3.1 * | 4.9 ** | 8.7 *** | 5.4 *** |
| g | 24.7 *** | 26.4 *** | 32.7 *** | 6.3 *** | 5.8 *** | 7.2 *** | 4.4 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, S.; Abbate, C.; Pandino, G.; Parisi, B.; Scavo, A.; Mauromicale, G. Productive and Physiological Response of Organic Potato Grown under Highly Calcareous Soils to Fertilization and Mycorrhization Management. Agronomy 2020, 10, 1200. https://doi.org/10.3390/agronomy10081200
Lombardo S, Abbate C, Pandino G, Parisi B, Scavo A, Mauromicale G. Productive and Physiological Response of Organic Potato Grown under Highly Calcareous Soils to Fertilization and Mycorrhization Management. Agronomy. 2020; 10(8):1200. https://doi.org/10.3390/agronomy10081200
Chicago/Turabian StyleLombardo, Sara, Cristina Abbate, Gaetano Pandino, Bruno Parisi, Aurelio Scavo, and Giovanni Mauromicale. 2020. "Productive and Physiological Response of Organic Potato Grown under Highly Calcareous Soils to Fertilization and Mycorrhization Management" Agronomy 10, no. 8: 1200. https://doi.org/10.3390/agronomy10081200
APA StyleLombardo, S., Abbate, C., Pandino, G., Parisi, B., Scavo, A., & Mauromicale, G. (2020). Productive and Physiological Response of Organic Potato Grown under Highly Calcareous Soils to Fertilization and Mycorrhization Management. Agronomy, 10(8), 1200. https://doi.org/10.3390/agronomy10081200