Morpho-Physiological Characterization of Diverse Rice Genotypes for Seedling Stage High- and Low-Temperature Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crop Husbandry
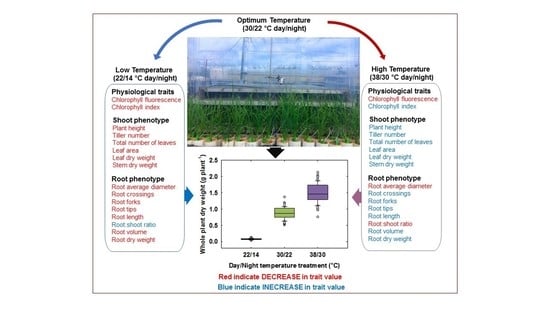
2.2. Temperature Treatments
2.3. Data Collection
2.3.1. Physiology
2.3.2. Phenotyping of Agronomic Traits
2.3.3. Root Phenotypes
2.4. Statistical Analysis
(SHWTl/SHWTo) + (StWTl/StWTo) + (TdWTl/TdWTo) + (RLl/RLo) + (RSAl/RSAo) + (AvgRDl/AvgRDo) +
(RVl/RVo) + (RNl/RNo) + (RTl/RTo) + (RFl/RFo) + (RCl/RCo) + (R/Sl/R/So) + (SPADl/SPADo) + (Fol/Foo) +
(Fml/Fmo) + (Fvl/Fvo) + (Fv/Fml/Fv/Fmo)
(SHWTh/SHWTo) + (StWTh/StWTo) + (TdWTh/TdWTo) + (RLh/RLo) + (RSAh/RSAo) +
(AvgRDh/AvgRDo) + (RVh/RVo) + (RNh/RNo) + (RTh/RTo) + (RFh/RFo) + (RCh/RCo) + (R/Sh/R/So) +
(SPADh/SPADo) + (Foh/Foo) + (Fmh/Fmo) + (Fvh/Fvo) + (Fv/Fmh/Fv/Fmo)
3. Results and Discussion
3.1. Shoot Growth and Development
3.2. Dry Weight Traits
3.3. Root Growth and Development
3.4. Physiological Traits
3.5. Phenotypic Trait Correlations and Contributions of Component Traits to Seedling Vigor
3.6. Cumulative Low and High-Temperature Response Index
3.7. Distinct Rice Genotypes Associated with Low- and/or High-Temperature Tolerance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CHTRI | Cumulative high-temperature response index |
| CLTRI | Cumulative low-temperature response index |
| CTRI | Cumulative temperature response index |
| DAS | Days after sowing |
| Fm | maximal fluorescence intensity |
| Fo | minimal Fluorescence Intensity |
| Fv | maximal variable fluorescence |
| Fv/Fm | Chlorophyll fluorescence |
| HTS | High-temperature sensitive |
| HTT | High-temperature tolerant |
| IHSRI | Individual heat-stress response index |
| ILSRI | Individual low-stress response index |
| LA | Leaf area |
| LN | Total number of leaves |
| LTS | Low-temperature sensitive |
| LTT | Low-temperature tolerant |
| LWT | Leaf dry weight |
| PCA | Principal Component Analysis |
| PH | Plant height |
| AvgRD | Root average diameter |
| RC | Root crossings |
| RF | Root forks |
| RI | Relative injury |
| RL | Root length |
| RN | Root number |
| R/S | Root:shoot ratio |
| RSA | Root surface area |
| RT | Root tips |
| RV | Root volume |
| RWT | Root dry weight |
| SPAD | Chlorophyll index |
| SPAR | Soil –plant–atmosphere research |
| StWT | Stem dry weight |
| SHWT | Above-ground dry weight |
| TdWT | Total dry weight |
| TN | Tiller number |
References
- Manzanilla, D.O.; Paris, T.R.; Vergara, G.V.; Ismail, A.M.; Pandey, S.; Labios, R.V.; Tatlonghari, G.T.; Acda, R.D.; Chi, T.T.N.; Duoangsila, K.; et al. Submergence risks and farmers’ preferences: Implications for breeding Sub1 rice in southeast Asia. Agric. Syst. 2011, 104, 335–347. [Google Scholar] [CrossRef]
- Lone, J.; Khan, M.N.; Bhat, M.A.; Shikari, A.B.; Wani, S.H.; Sofi, R.N.; Imran Khan, M.; Lone, R.A. Cold tolerance at the germination stage of rice: Methods of evaluation and characterization of genotypes. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1103–1109. [Google Scholar] [CrossRef]
- Ali, J.; Xu, J.-L.; Gao, Y.-M.; Ma, X.-F.; Meng, L.-J.; Wang, Y.; Pang, Y.-L.; Guan, Y.-S.; Xu, M.-R.; Revilleza, J.E.; et al. Harnessing the hidden genetic diversity for improving multiple abiotic stress tolerance in rice (Oryza sativa L.). PLoS ONE 2017, 12, e0172515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Tung, C.-W.; Eizenga, G.C.; Wright, M.H.; Ali, M.L.; Price, A.H.; Norton, G.J.; Islam, M.R.; Reynolds, A.; Mezey, J.; et al. Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza Sativa. Nat. Commun. 2011, 2, 467. [Google Scholar] [CrossRef]
- Singhal, P.; Jan, A.T.; Azam, M.; Haq, Q.M.R. Plant abiotic stress: A prospective strategy of exploiting promoters as alternative to overcome the escalating burden. Front. Life Sci. 2016, 9, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Wassmann, R.; Jagadish, S.V.K.; Sumfleth, K.; Pathak, H.; Howell, G.; Ismail, A.; Serraj, R.; Redona, E.; Singh, R.K.; Heuer, S. Regional Vulnerability of Climate Change Impacts on Asian Rice Production and Scope for Adaptation. Adv. Agron. 2009, 102, 91–133. [Google Scholar]
- Wani, S.H.; Sah, S.K.; Hossain, M.A.; Kumar, V.; Balachandran, S.M. Transgenic Approaches for Abiotic Stress Tolerance in Crop Plants. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 345–396. ISBN 978-3-319-22517-3. [Google Scholar]
- Sandhu, N.; Subedi, S.R.; Singh, V.K.; Sinha, P.; Kumar, S.; Singh, S.P.; Ghimire, S.K.; Pandey, M.; Yadaw, R.B.; Varshney, R.K.; et al. Deciphering the genetic basis of root morphology, nutrient uptake, yield, and yield-related traits in rice under dry direct-seeded cultivation systems. Sci. Rep. 2019, 9, 9334. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, N.; Torres, R.O.; Sta Cruz, M.T.; Maturan, P.C.; Jain, R.; Kumar, A.; Henry, A. Traits and QTLs for development of dry direct-seeded rainfed rice varieties. J. Exp. Bot. 2015, 66, 225–244. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Q.; Wang, S.; Hong, Y.; Wang, Z. Rice and cold stress: Methods for its evaluation and summary of cold tolerance-related quantitative trait loci. Rice 2014, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Najeeb, S.; Ali, J.; Mahender, A.; Pang, Y.L.; Zilhas, J.; Murugaiyan, V.; Vemireddy, L.R.; Li, Z. Identification of main-effect quantitative trait loci (QTLs) for low-temperature stress tolerance germination- and early seedling vigor-related traits in rice (Oryza sativa L.). Mol. Breed. 2020, 40, 10. [Google Scholar] [CrossRef] [Green Version]
- Cong Dien, D.; Yamakawa, T. Phenotypic variation and selection for cold-tolerant rice (Oryza sativa L.) at germination and seedling stages. Agriculture 2019, 9, 162. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Zhu, T.; Breisinger, C.; Hai, N.M. Impacts of Climate Change on Agriculture and Policy Options for Adaptation: The Case of Vietnam; IFPRI Discussion Papers; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2010. [Google Scholar]
- van Oort, P.A.J. Mapping abiotic stresses for rice in Africa: Drought, cold, iron toxicity, salinity and sodicity. Field Crops Res. 2018, 219, 55–75. [Google Scholar] [CrossRef]
- Jiang, L.; Xun, M.; Wang, J.; Wan, J. QTL analysis of cold tolerance at seedling stage in rice (Oryza sativa L.) using recombination inbred lines. J. Cereal Sci. 2008, 48, 173–179. [Google Scholar] [CrossRef]
- Kilasi, N.L.; Singh, J.; Vallejos, C.E.; Ye, C.; Jagadish, S.V.K.; Kusolwa, P.; Rathinasabapathi, B. Heat stress tolerance in rice (oryza sativa L.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Front. Plant. Sci. 2018, 9, 1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.G.; Naylor, R.E.L.; Matthews, S. Distinguishing the effects of genotype and seed physiological age on low temperature tolerance of rice (Oryza sativa L.). Exp. Agric. 2006, 42, 337–349. [Google Scholar] [CrossRef]
- Shimono, H.; Hasegawa, T.; Iwama, K. Response of growth and grain yield in paddy rice to cool water at different growth stages. Field Crops Res. 2002, 73, 67–79. [Google Scholar] [CrossRef]
- Baruah, A.R.; Ishigo-Oka, N.; Adachi, M.; Oguma, Y.; Tokizono, Y.; Onishi, K.; Sano, Y. Cold tolerance at the early growth stage in wild and cultivated rice. Euphytica 2009, 165, 459–470. [Google Scholar] [CrossRef]
- da Cruz, R.P.; Sperotto, R.A.; Cargnelutti, D.; Adamski, J.M.; FreitasTerra, T.; Fett, J.P. Avoiding damage and achieving cold tolerance in rice plants. Food Energy Secur. 2013, 2, 96–119. [Google Scholar] [CrossRef]
- Aghamolki, M.T.K.; Yusop, M.K.; Oad, F.C.; Zakikhani, H.; Jaafar, H.; Kharidah, S.; Musa, M. Heat Stress Effects on Yield Parameters of Selected Rice Cultivars at Reproductive Growth Stages. Available online: https://www.semanticscholar.org/paper/Heat-stress-effects-on-yield-parameters-of-selected-Aghamolki-Yusop/257f66ff020bf2f3bd32a5d94247a0239473ec74 (accessed on 16 December 2020).
- Krishnan, P.; Ramakrishnan, B.; Reddy, K.R.; Reddy, V.R. High-temperature Effects on Rice Growth, Yield, and Grain Quality. Adv. Agron. 2011, 111, 87–206. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant. Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajanayake, B.; Reddy, K.R.; Shankle, M.W.; Arancibia, R.A.; Villordon, A.O. Quantifying storage root initiation, growth, and developmental responses of sweetpotato to early season temperature. Agron. J. 2014, 106, 1795–1804. [Google Scholar] [CrossRef]
- Raju, B.R.; Mohankumar, M.V.; Sumanth, K.K.; Rajanna, M.P.; Udayakumar, M.; Prasad, T.G.; Sheshshayee, M.S. Discovery of QTLs for water mining and water use efficiency traits in rice under water-limited condition through association mapping. Mol. Breed. 2016, 36, 35. [Google Scholar] [CrossRef]
- Raju, B.R.; Narayanaswamy, B.R.; Mohankumar, M.V.; Sumanth, K.K.; Rajanna, M.P.; Mohanraju, B.; Udayakumar, M.; Sheshshayee, M.S. Root traits and cellular level tolerance hold the key in maintaining higher spikelet fertility of rice under water limited conditions. Funct. Plant. Biol. 2014, 41, 930. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, S.V.K.; Septiningsih, E.M.; Kohli, A.; Thomson, M.J.; Ye, C.; Redoña, E.; Kumar, A.; Gregorio, G.B.; Wassmann, R.; Ismail, A.M.; et al. Genetic advances in adapting rice to a rapidly changing climate. J. Agron. Crop. Sci. 2012, 198, 360–373. [Google Scholar] [CrossRef]
- Reddy, K.R.; Hodges, H.; Read, J.; Mckinion, J.; Baker, J.T.; Tarpley, L.; Tarpley, L.; Reddy, V.R. Soil-Plant-Atmosphere-Research (Spar) Facility: A Tool for Plant Research and Modeling. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.617.9409&rep=rep1&type=pdf (accessed on 16 December 2020).
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Read, J.J.; Sullivan, J.H. Growth and physiological responses of cotton (Gossypium hirsutum L.) to elevated carbon dioxide and ultraviolet-B radiation under controlled-environmental conditions. Plant. Cell Environ. 2003, 26, 771–782. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, E.J. Sand and Water Culture Methods Used in the Study of Plant Nutrition; Technical Communication No. 22, Commonwealth Bureau of Horticulture and Plantation Crops; Commonwealth Agricultural Bureaux: Farnham Royal, UK, 1952. [Google Scholar]
- Arai-Sanoh, Y.; Ishimaru, T.; Ohsumi, A.; Kondo, M. Effects of soil temperature on growth and root function in rice. Plant. Prod. Sci. 2010, 13, 235–242. [Google Scholar] [CrossRef]
- Wijewardana, C.; Hock, M.; Henry, B.; Reddy, K.R. Screening corn hybrids for cold tolerance using morphological traits for early-season seeding. Crop. Sci. 2015, 55, 851–867. [Google Scholar] [CrossRef] [Green Version]
- Boonjung, H.; Fukai, S. Effects of soil water deficit at different growth stages on rice growth and yield under upland conditions. 1. Growth during drought. Field Crops Res. 1996, 48, 37–45. [Google Scholar] [CrossRef]
- Makino, A.; Sato, T.; Nakano, H.; Mae, T. Leaf photosynthesis, plant growth and nitrogen allocation in rice under different irradiances. Planta 1997, 203, 390–398. [Google Scholar] [CrossRef]
- Ben-Haj-Salah, H.; Tardieu, F. Temperature affects expansion rate of maize leaves without change in spatial distribution of cell length (analysis of the coordination between cell division and cell expansion). Plant. Physiol. 1995, 109, 861–870. [Google Scholar] [CrossRef] [Green Version]
- de Freitas, G.M.; Thomas, J.; Liyanage, R.; Lay, J.O.; Basu, S.; Ramegowda, V.; do Amaral, M.N.; Benitez, L.C.; Braga, E.J.B.; Pereira, A. Cold tolerance response mechanisms revealed through comparative analysis of gene and protein expression in multiple rice genotypes. PLoS ONE 2019, 14, e0218019. [Google Scholar] [CrossRef]
- Tang, L.; Zhu, X.-C.; Cao, M.-Y.; Cao, W.-X.; Zhu, Y. Relationships of rice canopy par interception and light use efficiency to grain yield. Ying Yong Sheng Tai Xue Bao J. Appl. Ecol. 2012, 23, 1269–1276. [Google Scholar]
- Raja Reddy, K.; Hodges, H.F.; McKinion, J.M. Crop Modeling and Applications: A Cotton Example. Adv. Agron. 1997, 59, 225–290. [Google Scholar]
- Hnilickova, H.; Dufek, J.; Hnilicka, F.; Ceska, Z.U. Effects of low temperatures on photosynthesis and growth in selected tomato varieties (Lycopersicon esculentum). Sci. Agric. Bohem. Czech. Repub. 2002, 33, 101–105. [Google Scholar]
- Singh, B.; Norvell, E.; Wijewardana, C.; Wallace, T.; Chastain, D.; Reddy, K.R. Assessing morphological characteristics of elite cotton lines from different breeding programmes for low temperature and drought tolerance. J. Agron. Crop. Sci. 2018, 204, 467–476. [Google Scholar] [CrossRef]
- Alsajri, F.A.; Singh, B.; Wijewardana, C.; Irby, J.T.; Gao, W.; Reddy, K.R. Evaluating soybean cultivars for low- and high-temperature tolerance during the seedling growth stage. Agronomy 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.P.; Schneider, H.M.; Perkins, A.C.; Brown, K.M.; Lynch, J.P. Multiple integrated root phenotypes are associated with improved drought tolerance. Plant. Physiol. 2020, 183, 1011–1025. [Google Scholar] [CrossRef] [Green Version]
- Paez-Garcia, A.; Motes, C.; Scheible, W.-R.; Chen, R.; Blancaflor, E.; Monteros, M. Root traits and phenotyping strategies for plant improvement. Plants 2015, 4, 334–355. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Hechanova, S.; Kshirod, J.K.; Krishna Jagadish, S.V. Root anatomical traits of wild-rices reveal links between flooded rice and dryland sorghum. Plant. Physiol. Rep. 2019, 24, 155–167. [Google Scholar] [CrossRef]
- Costa, C.; Dwyer, L.M.; Zhou, X.; Dutilleul, P.; Hamel, C.; Reid, L.M.; Smith, D.L. Root morphology of contrasting maize genotypes. Agron. J. 2002, 94, 96. [Google Scholar] [CrossRef]
- Hammer, G.L.; Dong, Z.; McLean, G.; Doherty, A.; Messina, C.; Schussler, J.; Zinselmeier, C.; Paszkiewicz, S.; Cooper, M. Can changes in canopy and/or root system architecture explain historical maize yield trends in the US Cornbelt? Crop. Sci. 2009, 49, 299–312. [Google Scholar] [CrossRef]
- Munyon, J.W.; Bheemanahalli, R.; Walne, C.H.; Reddy, K.R. Developing functional relationships between temperature and cover crop species vegetative growth and development. Agron. J. 2020. [Google Scholar] [CrossRef]
- Setter, T.L.; Greenway, H. Growth reductions of rice at low root temperature: Decreases in nutrient uptake and development of chlorosis. J. Exp. Bot. 1988, 39, 811–829. [Google Scholar] [CrossRef]
- Venuprasad, R.; Bool, M.E.; Quiatchon, L.; Sta Cruz, M.T.; Amante, M.; Atlin, G.N. A Large-effect QTL for rice grain yield under upland drought stress on chromosome 1. Mol. Breed. 2012, 30, 535–547. [Google Scholar] [CrossRef]
- Nagasuga, K.; Murai-Hatano, M.; Kuwagata, T. Effects of low root temperature on dry matter production and root water uptake in rice plant. Plant. Prod. Sci. 2011, 14, 22–29. [Google Scholar] [CrossRef]
- Sattelmacher, B.; Marschner, H.; Kühne, R. Effects of root zone temperature on root activity of two potato (Solanum tuberosum L.) clones with different adaptation to high temperature. J. Agron. Crop. Sci. 1990, 165, 131–137. [Google Scholar] [CrossRef]
- Xu, Q.; Huang, B. Effects of differential air and soil temperature on carbohydrate metabolism in creeping bentgrass. Crop. Sci. 2000, 40, 1368–1374. [Google Scholar] [CrossRef]
- Du, Y.C.; Tachibana, S. Effect of supraoptimal root temperature on the growth, root respiration and sugar content of cucumber plants. Sci. Hortic. 1994, 58, 289–301. [Google Scholar] [CrossRef]
- Aghaee, A.; Moradi, F.; Zare-Maivan, H.; Zarinkamar, F.; Irandoost, H.P.; Sharifi, P. Physiological responses of two rice (Oryza sativa L.) genotypes to chilling stress at seedling stage. Afr. J. Biotechnol. 2011, 10, 7617–7621. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Pan, X.; Chen, X.; Zhang, Z.; Lu, X.; Huang, R. Overexpression of ethylene response factor TERF2 confers cold tolerance in rice seedlings. Transgenic Res. 2011, 20, 857–866. [Google Scholar] [CrossRef]
- Kuk, Y.I.; Shin, J.S.; Burgos, N.R.; Hwang, T.E.; Han, O.; Cho, B.H.; Jung, S.; Guh, J.O. Antioxidative enzymes offer protection from chilling damage in rice plants. Crop. Sci. 2003, 43, 2109–2117. [Google Scholar] [CrossRef]
- Ortiz, D.; Hu, J.; Salas Fernandez, M.G. Genetic architecture of photosynthesis in Sorghum bicolor under non-stress and cold stress conditions. J. Exp. Bot. 2017, 68, 4545–4557. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Reddy, K.R.; Redoña, E.D.; Walker, T. Screening of rice cultivars for morpho-physiological responses to early-season soil moisture stress. Rice Sci. 2017, 24, 322–335. [Google Scholar] [CrossRef]
- Lamers, J.; van der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant. Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [Green Version]
- Xiao, N.; Gao, Y.; Qian, H.; Gao, Q.; Wu, Y.; Zhang, D.; Zhang, X.; Yu, L.; Li, Y.; Pan, C.; et al. Identification of genes related to cold tolerance and a functional allele that confers cold tolerance. Plant. Physiol. 2018, 177, 1108–1123. [Google Scholar] [CrossRef] [Green Version]
- Rang, Z.W.; Jagadish, S.V.K.; Zhou, Q.M.; Craufurd, P.Q.; Heuer, S. Effect of high temperature and water stress on pollen germination and spikelet fertility in rice. Environ. Exp. Bot. 2011, 70, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Zheng, J.; Liu, B.; Peng, S.; Leung, H.; Zhao, J.; Wang, X.; Yang, T.; Huang, Z. Identification of QTLs for cold tolerance at seedling stage in rice (Oryza sativa L.) using two distinct methods of cold treatment. Euphytica 2014, 195, 95–104. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, S.; Ram, T.; Yadaw, R.B.; Mishra, K.K.; Mandal, N.P. Breeding high-yielding drought-tolerant rice: Genetic variations and conventional and molecular approaches. J. Exp. Bot. 2014, 65, 6265–6278. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Low Cold Tolerance | Moderately Cold Tolerance | High Cold Tolerance | Very High Cold Tolerance |
|---|---|---|---|
| 6.6343–7.6464 | 7.6465–8.6585 | 8.6585–9.6706 | >9.6707 |
| CT6510-24-1-2 (6.6343) | MILYANG 240 (7.6545) | CL163 (8.6878) | RU1504197 (9.7162) |
| CT18237-13-11-3-3-5-1 (6.7392) | CT6946-9-1-2-M-1P (7.6995) | Rex (8.7552 | NIPONBARE (9.7499) |
| CT18372-8-1-6-3-1-5 (6.7477) | CT18244-9-4-4-2-1-2 (7.7703) | Sabine (8.7711) | N-22 (9.8631) |
| 12DS-GMET-15 (6.9824) | IR78049-25-2-2-2 (7.7949) | RoyJ (8.7959) | LAKAST (10.2485) |
| FEDEARROZ 473 (7.0562) | CT18245-4-7-1-1-2-1 (7.7961) | El Paso 144 (8.8085) | CL111 (10.2936) |
| IRRI 123 (7.1203) | IR65600-81-5-2-3 (7.8207) | Bowman (8.8909) | RU1404156 (10.5430) |
| CT18615-1-5-1-2-1 (7.2403) | CT19561-3-57-2P-2-1-2-M (7.8536) | RU1504122 (8.9239) | CL271 (10.5537) |
| FEDEARROZ 2000 (7.4287) | HHZ 1-Y4-Y1 (8.0026) | CL151 (8.9251) | RU1504114 (11.0994) |
| RU1504083 (7.4676) | IR86635-2-3-3-3 (8.0417) | RU1402174 (8.9802) | |
| CT18247-12-8-1-4-2-2 (7.4685) | CT18614-4-1-2-3-2 (8.0502) | IR09L179 (9.0320) | |
| COLOMBIA XXI (7.5123) | CT18233-15-6-6-4-8-1 (8.1615) | JES (9.0341) | |
| WAB 56-125 (7.5625) | IR86052-32-3-2 (8.1997) | RU1204156 (9.0628) | |
| RU1504154 (7.6098) | RU1404196 (8.2025) | Taggart (9.1040) | |
| 12DS-GMET-25 (8.2734) | RU0603075 (9.1826) | ||
| IR64-EMF NIL (8.3062) | RU1304154 (9.1826) | ||
| FEDEARROZ 21 (8.4975) | CT18593-1-7-2-2-5 (9.1930) | ||
| Thad (8.5053) | MERMENTAU (9.2998) | ||
| Cheniere (8.51322) | IrGA 409 (9.3959) | ||
| Apo (8.6027) | FEDEARROZ MOCARE (9.4066) | ||
| RU1204197 (9.4702) | |||
| RU1303138 (9.5439) | |||
| IR6 (PAKISTAN) (9.5753) | |||
| INIA Tacuari (9.5826) | |||
| RU1504198 (9.6234) |
| Low Heat Tolerance | Moderate Heat Tolerance | High Heat Tolerance | Very High Heat Tolerance |
|---|---|---|---|
| 24.0647–27.8029 | 27.8030–31.5410 | 31.5411–35.2792 | 35.2793–39.0174 |
| IR09L179 (24.0647) | NIPONBARE (27.8056) | CT18247-12-8-1-4-2-2 (31.7148) | INIA Tacuari (35.9532) |
| JES (25.1469) | CT19561-3-57-2P-2-1-2-M (28.0089) | IrGA 409 (31.7274) | RU1204197 (36.8627) |
| IR86052-32-3-2 (25.4620) | CT18233-15-6-6-4-8-1 (28.0844) | CT18237-13-11-3-3-5-1 (31.7754) | CT18593-1-7-2-2-5 (36.8784) |
| RU1504197 (25.7155) | Thad (28.3560) | Rex (32.4221 | IRRI 123 (36.9060) |
| 12DS-GMET-15 (25.7315) | IR86635-2-3-3-3 (28.5095) | Cheniere (32.5563) | Bowman (36.9310) |
| CT6946-9-1-2-M-1P (26.6467) | CT6510-24-1-2 (28.6993) | CL111 (33.3937) | RU1504122 (37.1084) |
| FEDEARROZ 2000 (27.3866) | FEDEARROZ MOCARE (29.1116) | CT18372-8-1-6-3-1-5 (34.0439) | IR78049-25-2-2-2 (37.2010) |
| CT18615-1-5-1-2-1 (27.4010) | Sabine (29.2603) | IR6 (PAKISTAN) (34.0496) | HHZ 1-Y4-Y1 (37.3457) |
| RU1303138 (27.4013) | CT18245-4-7-1-1-2-1 (29.4525) | RU0603075 (34.6069) | RU1504114 (37.7292) |
| Taggart (27.5529) | RU1504154 (29.6024) | LAKAST (34.8213) | MILYANG 240 (37.8333) |
| Apo (29.6167) | El Paso 144 (35.0925) | IR65600-81-5-2-3 (42.0246) | |
| RU1402174 (29.7321) | N-22 (35.1243) | ||
| COLOMBIA XXI (30.0382) | |||
| CL271 (30.1294) | |||
| FEDEARROZ 21 (30.2457) | |||
| CL163 (30.3132) | |||
| CL151 (30.3852) | |||
| RU1204156 (30.4613) | |||
| WAB 56-125 (30.5225) | |||
| RU1304154 (30.6095) | |||
| RoyJ (30.6671) | |||
| RU1404156 (30.7194) | |||
| RU1504198 (30.8046) | |||
| MERMENTAU (30.8833) | |||
| RU1404196 (30.8856) | |||
| 12DS-GMET-25 (30.8925) | |||
| IR64-EMF NIL (30.9995) | |||
| FEDEARROZ 473 (31.0337) | |||
| CT18614-4-1-2-3-2 (31.4380) | |||
| RU1504083 (31.4866) | |||
| CT18244-9-4-4-2-1-2 (31.4997) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, K.R.; Seghal, A.; Jumaa, S.; Bheemanahalli, R.; Kakar, N.; Redoña, E.D.; Wijewardana, C.; Alsajri, F.A.; Chastain, D.; Gao, W.; et al. Morpho-Physiological Characterization of Diverse Rice Genotypes for Seedling Stage High- and Low-Temperature Tolerance. Agronomy 2021, 11, 112. https://doi.org/10.3390/agronomy11010112
Reddy KR, Seghal A, Jumaa S, Bheemanahalli R, Kakar N, Redoña ED, Wijewardana C, Alsajri FA, Chastain D, Gao W, et al. Morpho-Physiological Characterization of Diverse Rice Genotypes for Seedling Stage High- and Low-Temperature Tolerance. Agronomy. 2021; 11(1):112. https://doi.org/10.3390/agronomy11010112
Chicago/Turabian StyleReddy, Kambham Raja, Akanksha Seghal, Salah Jumaa, Raju Bheemanahalli, Naqeebullah Kakar, Edilberto D. Redoña, Chathurika Wijewardana, Firas Ahmed Alsajri, Daryl Chastain, Wei Gao, and et al. 2021. "Morpho-Physiological Characterization of Diverse Rice Genotypes for Seedling Stage High- and Low-Temperature Tolerance" Agronomy 11, no. 1: 112. https://doi.org/10.3390/agronomy11010112
APA StyleReddy, K. R., Seghal, A., Jumaa, S., Bheemanahalli, R., Kakar, N., Redoña, E. D., Wijewardana, C., Alsajri, F. A., Chastain, D., Gao, W., Taduri, S., & Lone, A. A. (2021). Morpho-Physiological Characterization of Diverse Rice Genotypes for Seedling Stage High- and Low-Temperature Tolerance. Agronomy, 11(1), 112. https://doi.org/10.3390/agronomy11010112