Enzymatic Activity as New Moorsh-Forming Process Indicators of Peatlands
Abstract
:1. Introduction
- Ash content, specific and bulk density increases;
- Porosity, moisture, shrinkage, compressibility, the content of bitumen, cellulose, and lignin is reduced;
- The hydrophilic abilities of moorsh decreases, hydrophobic increases;
- The ratio of fulvic to humic acids, the content of mineral substances and greenhouse gas emissions, in particular CH4, CO2, and N2O increases.
2. Materials and Methods
2.1. Study Area
2.1.1. Undrained Peatlands
Mukhrino
Tagan Mire 1
Stążka Mire
2.1.2. Drained Peatlands
Wrześnica River Valley
General Dezydery Chłapowski Landscape Park
Tagan Mire 2
The Sudetes Peatlands
2.2. Soil Physicochemical and Biochemical Analyses
2.3. Statistical Analysis
3. Results
3.1. Soil Physicochemical Properties
3.2. Soil Biochemical Properties
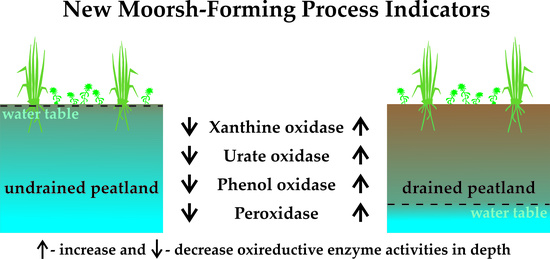
3.2.1. Xanthine Oxidase Activity
3.2.2. Urate Oxidase Activity
3.2.3. Phenol Oxidase Activity
3.2.4. Peroxidase Activity
4. Discussion
4.1. Soil Physicochemical Properties
4.2. Soil Biochemical Properties
4.2.1. Xanthine Oxidase Activity
4.2.2. Urate Oxidase Activity
4.2.3. Phenol Oxidase Activity
4.2.4. Peroxidase Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grzybowski, M.; Glińska-Lewczuk, K. The principal threats to the peatlands habitats, in the continental bioregion of Central Europe—A case study of peatland conservation in Poland. J. Nat. Conserv. 2020, 53, 125778. [Google Scholar] [CrossRef]
- Joosten, H. Peatlands, Climate Change Mitigation and Biodiversity Conservation; Nordic Council of Ministers: Copenhagen, Denmark, 2015; pp. 1–15. [Google Scholar]
- Schumann, M.; Joosten, H. Global Peatland Restoration Manual; Institute of Botany and Landscape Ecology, Greifswald University: Greifswald, Germany, 2008; pp. 1–64. [Google Scholar]
- Joosten, H. The global peatland CO2 picture. In Peatland Status and Emissions in All Countries of the World; Greifswald University Wetlands International, Ede: Greifswald, Germany, 2010; pp. 1–10. [Google Scholar]
- Hooijer, A.; Page, S.; Jauhiainen, J.; Lee, W.A.; Lu, X.X.; Idris, A.; Anshari, G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 2012, 9, 1053–1071. [Google Scholar] [CrossRef] [Green Version]
- Pfadenhauer, J.; Klötzli, F. Restoration experiments in middle European wet terrestrial ecosystems: An overview. Vegetatio 1996, 126, 101–115. [Google Scholar] [CrossRef]
- Gawlik, J. An attempt to evaluate changes in the water retain ability of peat soils in the context of their advancing degradation. Pol. J. Soil Sci. 1993, 26, 81–86. [Google Scholar]
- Sokołowska, Z.; Szajdak, L.; Matyka-Sarzyńska, D. Impact of the degree of secondary transformation on acid–base properties of organic compounds in mucks. Geoderma 2005, 127, 80–90. [Google Scholar] [CrossRef]
- Hooghoudt, S.B.; Woerdt, D.; Dennema, J.; van Dijk, H. Irreversibly Drying Peat Soils in the West of Netherlands; Pudoc: Wageningen, The Netherlands, 1960; pp. 1–308. [Google Scholar]
- Okruszko, H. Muck soils of valley peatlands, and their chemical and physical properties. Rocz. Nauk Roln. Seria F 1960, 74, 5–89. [Google Scholar]
- Okruszko, H. Transformation of fen-peat soils under the impact of draining. Zesz. Probl. Post. Nauk Roln. 1993, 406, 3–73. [Google Scholar]
- Schmidt, W. Zur Bestimmung der Einheitswasserzahl von Torfen. Arch. Acker-Pflanzenbau Bodenk. 1986, 30, 251–257. [Google Scholar]
- Gawlik, J. Division of differently silted peat formation into classes according to their state of secondary transformations. Acta Agrophys. 2000, 26, 17–24. [Google Scholar]
- Dick, R.P.; Breakwell, D.P.; Turco, R.F. Soil Enzyme activities and biodiversity measurements as integrative microbiological indicators. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1997; pp. 247–271. [Google Scholar]
- Datta, R.; Anand, S.; Moulick, A.; Baraniya, D.; Pathan, S.I.; Rejsek, K.; Vranova, V.; Sharma, M.; Sharma, D.; Kelkar, A.; et al. How enzymes are adsorbed on soil solid phase and factors limiting its activity: A Review. Int. Agrophysics 2017, 31, 287–302. [Google Scholar] [CrossRef]
- Inisheva, L.I.; Golubina, O.A.; Zaplatnikova, Y.D.; Dubrovskaya, L.I. Eutrophic Mire, Its Characteristics and Modern Conditions of Peat Genesis; EGU General Assembly: Vienna, Austria, 2009; EGUGA.11.3770I. [Google Scholar]
- Lamentowicz, M.; Tobolski, K.; Mitchell, E.A.D. Palaeoecological evidence for anthropogenic acidification of a kettle-hole peatland in northern Poland. Holocene 2007, 17, 1185–1196. [Google Scholar] [CrossRef]
- Marcinek, J.; Komisarek, J. Polish Soil Taxonomy. Rocz. Glebozn. 2011, 62, 1–193. (In Polish) [Google Scholar]
- Bogacz, A.; Glina, B. Distribution, age and transformation of organic soils in the Sudety Mountains. In Soils of Lower Silesia: Origin, Diversity and Protection; Kabała, C., Ed.; PTG, PTSH: Wrocław, Poland, 2015; pp. 225–234. [Google Scholar]
- Glina, B.; Bogacz, A.; Pikus, H.; Pawluczuk, J. The impact of anthropopreassure and weather Conditions on the mineral nitrogen content in the organic soils from fen peatlands (Stołowe Mountains, Poland). Pol. J. Soil Sci. 2017, 49, 1–13. [Google Scholar] [CrossRef] [Green Version]
- von Post, L. Sveriges geologiska undersöknings torvinventering och några av dess hitills vunna resultat. Sven. Mosskulturföreningens Tidskr. 1922, 36, 1–27. [Google Scholar]
- PN-76/G-02501. Peat and peat varies. In Determination of Classes, Types and Species of Peat; Polish Normalization Committee: Warsaw, Poland, 1977; pp. 1–11. (In Polish) [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis. Part 1 Physical and Mineralogical Methods. Agronomy Monograph 9; Klute, A., Ed.; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Borren, W.; Bleuten, W.; Lapshina, E.D. Holocene peat and carbon accumulation rates in the southern taiga of western Siberia. Quat. Res. 2004, 61, 42–51. [Google Scholar] [CrossRef]
- Smolander, A.; Kitunen, V. Soil microbial activities and characteristics of dissolved organic C and N in relation to tree species. Soil Biol. Biochem. 2002, 34, 651–660. [Google Scholar] [CrossRef]
- Szajdak, L.W.; Gaca, W. Nitrate reductase activity in soil under shelterbelt and an adjoining cultivated field. Chem. Ecol. 2010, 26, 123–134. [Google Scholar] [CrossRef]
- Krawczyński, J. Diagnostyka Enzymologiczna W Medycynie Praktycznej (Enzymology Diagnosis in Practical Medicine); PZWL: Warszawa, Poland, 1972; pp. 182–184. (In Polish) [Google Scholar]
- Martin-Smith, M. Uricolytic enzymes in soil. Nature 1963, 197, 361–362. [Google Scholar] [CrossRef]
- Perucci, P.; Casucci, C.; Dumontet, S. An improved method to evaluate the o-diphenol oxidase activity of soil. Soil Biol. Biochem. 2000, 32, 1927–1933. [Google Scholar] [CrossRef]
- Bartha, R.; Bordeleau, L. Cell-free peroxidases in soil. Soil Biol. Biochem. 1969, 1, 139–143. [Google Scholar] [CrossRef]
- Zeng, N.; Zhang, G.; Hu, X.; Pan, J.; Gong, D. Mechanism of fisetin suppressing superoxide anion and xanthine oxidase activity. J. Funct. Foods 2019, 58, 1–10. [Google Scholar] [CrossRef]
- Ravichandran, R.; Hemaasri, S.; Cameotra, S.S.; Jayaprakash, N.S. Purification and characterization of an extracellular uricase from a new isolate of Sphingobacterium thalpophilum (VITPCB5). Protein Expr. Purif. 2015, 114, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Saraswati, S.; Parsons, C.T.; Strack, M. Access roads impact enzyme activities in boreal forested peatlands. Sci. Total Environ. 2019, 651, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kwon, M.J.; Kim, S.; Lee, S.; Jones, T.G.; Johncock, A.C.; Haraguchi, A.; Freeman, C. Author Correction: Biologically driven DOC release from peatlands during recovery from acidification. Nat. Commun. 2018, 9, 4617. [Google Scholar] [CrossRef] [PubMed]
- Jassey, V.E.J.; Chiapusio, G.; Gilbert, D.; Toussaint, M.-L.; Binet, P. Phenoloxidase and peroxidase activities in Sphagnum-dominated peatland in a warming climate. Soil Biol. Biochem. 2012, 46, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Brandyk, T.; Szatyłowicz, J.; Oleszczuk, R.; Gnatowski, T. Water-related physical attributes of organic soils. In Organic Soils and Peat Materials for Sustainable Agriculture; Parent, L., Ilnicki, P., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 35–70. [Google Scholar]
- Perdana, L.R.; Ratnasari, N.G.; Ramadhan, M.L.; Palamba, P.; Nugroho, Y.S. Hydrophilic and hydrophobic characteristics of dry peat. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012083. [Google Scholar] [CrossRef]
- Potila, H.; Sarjala, T. Seasonal fluctuation in microbial biomass and activity along a naturalnitrogen gradient in a drained peatland. Soil Biol. Biochem. 2004, 36, 1047–1055. [Google Scholar] [CrossRef]
- Xu, J.; Morris, P.J.; Liu, J.; Ledesma, J.L.J.; Holden, J. Increased Dissolved Organic Carbon Concentrations in Peat-Fed UK Water Supplies Under Future Climate and Sulfate Deposition Scenarios. Water Resour. Res. 2020, 56, e2019WR025592. [Google Scholar] [CrossRef] [Green Version]
- Jaszczyński, J.; Sapek, A.; Chrzanowski, S. Rozpuszczalny węgiel organiczny w wodzie z siedlisk pobagiennych na tle temperatury gleby. Water Environ. Rural Areas 2008, 8, 117–126. [Google Scholar]
- Szajdak, L.; Szatyłowicz, J. Impact of drainage on hydrophobicity of fen peat-moorsh soils. In Mires and Peat; Klavins, M., Ed.; University of Latvia Press: Riga, Latvia, 2010; pp. 158–174. [Google Scholar]
- Woche, S.K.; Goebel, M.-O.; Mikutta, R.; Schurig, C.; Kaestner, M.; Guggenberger, G.; Bachmann, J. Soil wettability can be explained by the chemical composition of particle interface-An XPS study. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Klingenfuß, C.; Roßkopf, N.; Walter, J.; Heller, C.; Zeitz, J. Soil organic matter to soil organic carbon ratios of peatland soil substrates. Geoderma 2014, 235–236, 410–417. [Google Scholar] [CrossRef]
- Becher, M.; Kalembasa, D.; Pakuła, K.; Malinowska, E. Carbon and nitrogen fractions in drained organic soils. Environ. Prot. Nat. Resour. 2013, 24, 1–5. [Google Scholar] [CrossRef]
- Pawluczuk, J. Mineralizacja azotu w glebach torfowo-murszowych strefy morenowej Pojezierza Mazurskiego. Ann. UMCS 2004, 59, 559–567. (In Polish) [Google Scholar]
- Tripathi, N.; Singh, R.S. Influence of different land uses on soil nitrogen transformations after conversion from an Indian dry tropical forest. CATENA 2009, 77, 216–223. [Google Scholar] [CrossRef]
- Laine, M.P.P.; Strömmer, R.; Arvola, L. Nitrogen release in pristine and drained peat profiles in response to water table fluctuations: A mesocosm experiment. Appl. Environ. Soil Sci. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Krueger, J.P.; Leifeld, J.; Glatzel, S.; Szidat, S.; Alewell, C. Biogeochemical indicators of peatland degradation—A case study of a temperate bog in northern Germany. Biogeosciences 2015, 12, 2861–2871. [Google Scholar] [CrossRef] [Green Version]
- Szajdak, L.W.; Jezierski, A.; Wegner, K.; Meysner, T.; Szczepański, M. Influence of drainage on peat organic matter: Implications for development, stability, and transformation. Molecules 2020, 25, 2587. [Google Scholar] [CrossRef]
- Szajdak, L.W.; Gaca, W.; Augustin, J.; Meysner, T. Impact of Shelterbelts on Oxidation-Reduction Properties and Greenhouse Gases Emission from Soils. Ecol. Chem. Eng. S 2018, 25, 643–658. [Google Scholar] [CrossRef]
- Szajdak, L.; Maryganova, V.; Meysner, T.; Tychinskaja, L. Effect of shelterbelt on two kinds of soils on the transformation of organic matter. Environ. Int. 2002, 28, 383–392. [Google Scholar] [CrossRef]
- Szajdak, L.W.; Lapshina, E.D.; Gaca, W.; Styła, K.; Meysner, T.; Szczepański, M.; Zarov, E.A. Physical, chemical and biochemical properties of Western Siberia Sphagnum and Carex peat soils. Environ. Dyn. Glob. Clim. Chang. 2016, 7, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okruszko, H.; Ilnicki, P. The moorsh horizons as quality indicators of reclaimed organic soils. In Organic Soils and Peat Materials for Sustainable Agriculture; Parent, L.-E., Ilnicki, P., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2003; pp. 12–25. [Google Scholar]
- Sheoran, I.S.; Luthra, Y.P.; Kuhad, M.S.; Singh, R. Cluster bean-a ureide- or amide-producing legume. Plant Physiol. 1982, 70, 917–918. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.; Montalbini, P.; Pineda, M. Urate oxidase from the rust Puccinia recondite is a heterotetramer with two different-sized monomers. Cur. Microbiol. 2002, 44, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Bobuľská, L.; Fazekašová, D.; Angelovičová, L. Vertical profiles of soil properties and microbial activities in peatbog soils in Slovakia. Environ. Process. 2015, 2, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Niu, W.; Wang, J.; Liu, L.; Zhang, M.; Xu, J. Effects of artificial soil aeration volume and frequency on soil enzyme activity and microbial abundance when cultivating greenhouse Tomato. Soil Sci. Soc. Am. J. 2016, 80, 1208–1221. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, X.; Sun, G.; Li, X.; Guo, K. Isolation of a thermostable uricase-producing bacterium and study on its enzyme production conditions. Process. Biochem. 2005, 40, 3749–3753. [Google Scholar] [CrossRef]
- Urbanová, Z.; Bárta, J. Effects of long-term drainage on microbial community composition vary between peatland types. Soil Biol. Biochem. 2016, 92, 16–26. [Google Scholar] [CrossRef]
- Fenner, N.; Freeman, C.; Reynolds, B. Hydrological effects on the diversity of phenolic degrading bacteria in a peatland: Implications for carbon cycling. Soil Biol. Biochem. 2005, 37, 1277–1287. [Google Scholar] [CrossRef]
- Rezanezhad, F.; Price, J.S.; Quinton, W.L.; Lennartz, B.; Milojevic, T.; Van Cappellen, P. Structure of peat soils and implications for water storage, flow and solutetransport: A review update for geochemists. Chem. Geol. 2016, 429, 75–84. [Google Scholar] [CrossRef]
- Brouns, K. The Effects of Climate Change on Decomposition in Dutch Peatlands: An Exploration of Peat Origin and Land Use Effects. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2016. [Google Scholar]
- Efremova, T.T.; Ovchinnikova, T.M. Oxidoreductase activity of peat soils as an indicator of the degree of biochemical transformation of drained and forested bogs in west Siberia. Biol. Bull. 2007, 34/3, 297–302. [Google Scholar] [CrossRef]
- Tian, L.; Dell, E.; Shi, W. Chemical composition of dissolved organic matter in agroecosystems: Correlations with soil enzyme activity and carbon and nitrogen mineralization. Appl. Soil Ecol. 2010, 46, 426–435. [Google Scholar] [CrossRef]
- Dec, J.; Haider, K.; Bollag, J.M. Decarboxylation and demethoxylation of naturally occurring phenols during coupling reactions and polymerization. Soil Sci. 2001, 166, 660–671. [Google Scholar] [CrossRef]
- Zavarzina, A.G. Heterophase synthesis of humic acids in soils by immobilized phenol oxidases. In Soil Enzymology. Soil Biology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 22, pp. 187–205. [Google Scholar]
- Szajdak, L.W.; Meysner, T.; Inisheva, L.I.; Lapshina, E.; Szczepański, M.; Gaca, W. Dynamics of organic matter and mineral components in Sphagnum- and Carex-dominated organic soils. Mires Peat 2019, 24, 1–15. [Google Scholar] [CrossRef]















| Place of Sampling | Botanical Composition of Vegetation Cover of Investigated Places | Layers (cm) | Types of Peat Based on Macrofossil Analysis | Degree of Decomp. (von Post) | |
|---|---|---|---|---|---|
| Mukhrino | |||||
| Bog peat | KM2 | Betula nana L., Chamaedaphne calyculata (L.) Moench, Ledum palustre L., Pinus sibirica Du Tour, P. sylvestris L., Rubus chamaemorus L., Sphagnum angustifolium (Warnst.) C.E.O. Jensen, S. fuscum (Schimp.) Klinggr., S. magellanicum Brid. | 0–50 | Sphagnum | H1 |
| 50–100 | Sphagnum | H2 | |||
| KM3 | Betula nana, Chamaedaphne calyculata, Cladonia sp. P. Browne, Ledum palustre, Pinus sylvestris, Rubus chamaemorus, Sphagnum fuscum | 0–50 | Sphagnum | H1 | |
| 50–100 | Sphagnum | H2 | |||
| KM4 | Chamaedaphne calyculata, Ledum palustre, Oxycoccus palustris L., Pinus sylvestris, Rubus chamaemorus, Sphagnum fuscum | 0–50 | Sphagnum | H1 | |
| 50–100 | Sphagnum | H1 | |||
| KM10 | Betula nana, Chamaedaphne calyculata, Ledum palustre, Oxycoccus microcarpus (Turcz. ex Rupr.) Schmalh., Pinus sibirica, P. sylvestris, Sphagnum capillifolium (Ehrh.) Hedw., S. angustifolium, S. fuscum, Vaccinium uliginosum L., Vaccinium vitis-idaea L., Rubus chamaemorus | 0–50 | Sphagnum | H1 | |
| 50–100 | Sphagnum | H2 | |||
| KM18 | Andromeda polifolia L., Carex limosa L., Drosera anglica Huds., Eriophorum russeolum L., Menyanthes trifoliata L., Oxycoccus microcarpus (Turcz. ex Rupr.) Schmalh., Rhynchospora alba (L.) Vahl., Scheuchzeria palustris L., Sphagnum papillosum Lindb. | 0–50 | Sphagnum | H1 | |
| 50–100 | Sphagnum | H1 | |||
| Mukhrino | |||||
| Fen peat | KM1 | Aulacomnium palustre (Hedw.) Schwägr., Carex globularis L., Chamaedaphne calyculata, Dicranum polysetum Sw., Ledum palustre, Oxycoccus microcarpus, Pinus sylvestris, P. sibirica, Pleurozium schreberi (Willd. ex Brid.) Mitt., Polytrichum strictum Brid., Rubus chamaemorus L., Sphagnum fuscum, S. capillifolium, S. magellanicum, S. angustifolium, Vaccinium myrtillus, Vacinium vitis-idaea | 0–50 | sedge woody | H2 |
| 50–100 | woody-cotton grass | H3/H4 | |||
| KM15 | Carex juncea, Comarum palustre L., Phalaris arundinacea L., Lactuca sibirica (L.) Benth. ex Maxim., Calamagrostis stricta (Timm) Koeler (C. neglecta), C. phragmitoides Hartm., Lythrum salicaria L., Lysimachia thyrsiflora L., L. vulgaris L., Rumex aquatilis, Galium ruprechtii Pobed., Lathyrus palustris L., Anemone dichotoma L., Betula pubescens Ehrh., Salix pentandra L., Salix cinerea L. | 0–50 | sedge woody | H5 | |
| 50–100 | sedge woody | H6 | |||
| KM16 | Betula pendula, B. pubescens, Carex rostrata Stokes, C. lasiocarpa Ehrh., C. limosa L., Eriophorum vaginatum, Lysimachia thyrsiflora, Menyanthes trifoliata, S. riparium L., | 0–50 | sedge-Sphagnum | H2 | |
| 50–100 | herbaceous-Equisetum | H2 | |||
| Tagan Mire 1 | |||||
| Fen peat | Betula pendula, Calamagrostis stricte (Timm.) Koeler, Carex lasiocarpa Ehrh., Carex sp., Hieracium sp. L., Larix sibirica Ledeb., Picea obovata Ledeb., Pinus cembra var. sibirica Du Tour, Pinus sylvestris, Prunus avium L., Sorbus aucuparia L., Viburnum opulus L. | 0–50 | grasses | H4 | |
| 50–100 | grasses | H4 | |||
| Stążka Mire | |||||
| Fen peat | Alnus glutinosa Gaertn., Andromeda polifolia, Calluna vulgaris (L.) Hull, Carex limosa, Drosera rotundifolia L., Empetrum nigrum L., Eriophorum vaginatum, Juncus effusus L., Ledum palustre, Pinus sylvestris, Pleurozium schreberi (Willd. ex Brid.) Mitt., Rhynchospora alba (L.) Vahl., Scheuchzeria palustris (L.) Dulac., Sphagnum cuspidatum Ehrh. ex Hoffm., S. fallax (Klinggr.) Klinggr., S. fuscum, S. magellanicum, Vaccinium oxycoccos L. | 0–50 | sedge-Hypnum | H3 | |
| 50–100 | sedge, fragments of wood | H4/H5 | |||
| Place of Sampling | Botanical Composition of Vegetation Cover of Investigated Places | Layers (cm) | Types of Peat Based on Macrofossil Analysis | Degree of Decomp. (von Post) | |
|---|---|---|---|---|---|
| Wrześnica River valley | |||||
| Fen peat | 1 | Achillea millefolium L., Alopecurus geniculatus L., Caltha palustris L., Carex gracilis L., C. hirta L., Cirsium arvense (L.) Scop., C. oleraceum (L.) Scop., Crepis paludosa L., Daucus carota L., Eleocharis palustris L., Equisetum fluviatile L., Erodium cicutarium (L.) L‘Her., Filipendula ulmaria L.) Maxim., Galium palustre L., Heracleum sphondylium L., Iris pseudacorus L., Juncus articulatus L., J. conglomeratus L., J. effusus L., Lychnis flos-cuculi (L.) Greuter & Burdet, Myosotis palustris L., Phragmites australis (Cav.)Trin. ex Steud, Plantago lanceolata L., P. major L., Polygonum hydropiper L., Potentilla anserina L., Prunella vulgaris L., Ranunculus acris L., R. repens L., Rorippa palustris L., Rumex acetosa L., R. obtusifolius L., Scirpus sylvaticus L., Stellaria media (L.) Vill., Taraxacum officinale F.H. Wigg., Trifolium pratense L., T. repens, Vicia cracca L., Viola palustris L. | 0–50 | Alneti | H5 |
| 50–100 | Alneti | H2 | |||
| 2 | Acorus calamus L., Carex acutiformis L., C. pseudocyperus L., C. rostrata, Cirsium palustre (L.) Scop., Epilobium hirsutum L., Equisetum palustre L., Fallopia convolvulus (L.) Á. Löve, Filipendula ulmaria, Galium palustre, Iris pseudacorus L., Lycopus europaeus L., Lysimachia vulgaris L., Lythrum salicaria, Myosoton aquaticum (L.) Moench, Ranunculus sceleratus L., Rorippa palustris, Rumex hydrolapathum Huds., Salix cinerea, S. fragilis L., Scirpus sylvaticus L., Solanum dulcamara L., Symphytum officinale L., Urtica dioica L. | 0–50 | Alneti | H5 | |
| 50–100 | Alneti | H2 | |||
| General Dezydery Chłapowski Landscape Park | |||||
| Fen peat | 1 | Achillea millefolium L., Acorus calamus L., Alnus glutinosa (L.) Gaertn., Bidens frondosa L., Carex acutiformis, Cerastium holosteoides L., Cirsium arvense, Conyza canadensis (L.) Cronquist, Epilobium hirsutum L., Galium mollugo L., G. palustre, G. uliginosum L., Glechoma hederacea L., Holcus lanatus L., Iris pseudacorus L., Lathyrus palustris L., Lemna minor L., Lycopus europaeus, Lythrum salicaria, Matricaria maritima (L.) W. D. J. Koch, Mentha aquatica L., Phalaris arundinacea L., Phleum pratense L., Phragmites australis, Plantago lanceolata, P. major, Polygonum amphibium (L.) Delarbre, Potentilla reptans L., Ranunculus repens, Rumex crispus L., Sonchus asper (L.) Hill., Stachys palustris L., Taraxacum officinale, Trifolium hybridum L., T. repens, Urtica dioica | 0–50 | moorsh soil, alder swamp | H8 |
| 50–100 | wooden sedge, sedge-reed | H8 | |||
| 2 | Achillea millefolia L., Agrostis canina L., Arrhenatherum elatior (L.) P. Beauv. ex J. & C. Presl, Carex acutiformis, C. gracilis, Ceratophyllum demersum L., Cirsium arvense, C. oleraceum, Deschampsia caespitosa (L.) P.B., Epilobium hirsutum L., Galium mollugo, Glechoma hederacea L., Heracleum sphondylum L., Holcus lanatus, Hydrocharis morsus–ranae L., Lemna trisulca L., Leucanthemum vulgare Lam., Lolium multiflorum Lam., Lysimachia vulgaris, Lythrum salicaria, Phragmites australis, Plantago lanceolata, P. major, Ranunculus repens, Rumex acetosa, R. crispus, R. hydrolapathum, Salix alba L., S. cinerea L., Serratula tinctoria L., Solanum dulcamara, Taraxacum officinale, Trifolium pratense L., T. repens, Typha angustifolia L., Urtica dioica | 0–50 | moorsh soil, sedge | H7 | |
| 50–100 | sedge-reed | H8 | |||
| 3 | Achillea millefolium L., Agrostis canina, Betula pendula, Calystegia sepium (L.) R.Br, Cardaminopsis arenosa (L.) Hayek, Carex gracilis, C. hirta, Centaurea jacea L., Cerastium holosteoides Fr. em. Hyl., Cirsium arvense, C. oleraceum, Dactylis glomerata L., Daucus carota, Deschampsia caespitosa, Eupatorium cannabinum L., Festuca arundinacea Schreb., Frangula alnus Mill., Galium album Mill., G. uliginosum L., Holcus lanatus, Hypericum tetrapterum Fr., Lycopus europaeus, Lysimachia vulgaris, Mentha aquatica, Molinia caerulea (L.) Moench, Nymphaea alba L., Phleum pratense L., Plantago lanceolata, P. major, Poa pratensis L., P. trivialis L., Potentilla anserine L., Ranunculus repens, Rhamnus catharticus L., Rubus plicatus W. et N., Salix cinerea, Solanum dulcamara, Sonchus arvensis L., Sparganium ramosum L., Taraxacum officinale, Typha latifolia, Viburnum opulus | 0–50 | moorsh soil, sedge with wooden | H8 | |
| 50–100 | sedge | H7 | |||
| 4 | Achillea millefolium, Alnus glutinosa, Angelica sylvestris L., Caltha palustris, Carex acutiformis, C. gracilis, Centaurea jacea, Cerastium holosteoides, Dactylis glomerata, Deschampsia casespitosa, Echinochloa crus–galli (L.) P.Beauv., Eupatorium cannabinum, Filipendula ulmaria, Frangula alnus, Galium mollugo, Glechoma hederacea, Heracleum sphondylium, Holcus lanatus, Humulus lupulus L., Lolium multiflorum, Lycopus europaeus, Lysimachia vulgaris, Lythrum salicaria, Mentha verticillata L., Phleum pratense L., Plantago lanceolata, P. major, Polygonum amphibium, P. persicaria L., Ranunculus repens, Rorippa palustris, Rumex acetosa, R. obtusifolius, Sonchus arvensis, Stellaria media, Trifolium pratense, T. repens, Vicia cracca | 0–50 | moorsh soil, alder swamp | H8 | |
| 50–100 | sedge with wooden | H8 | |||
| Tagan Mire 2 | |||||
| Peat- moorsh | Betula pendula, Calamagrostis stricta, Carex lasiocarpa, Carex sp., Hieracium sp., Larix sibirica, Picea obovata, Pinus cembra var. sibirica, P. sylvestris, Prunus avium, Sorbus aucuparia, Thelypteris palustris Schott, Viburnum opulus | 0–50 | wooden | H4 | |
| 50–100 | wooden-grasses | H4 | |||
| Great Batorowskie | |||||
| Bog peat | Betula pubescens, Calamagrostis villosa, Deshampsia flexuosa, Picea abies, Pinus sylvestris, Polytrichum commune Hedw., P. formosum, Sphagnum gigensoni, S. magelanicum, S. palustris., Vaccinium myrtylis | 0–50 | Eriophoro-Sphagneti | H6 | |
| 50–100 | Eriophoro-Sphagneti | H5 | |||
| Zieleniec Mire | |||||
| Fen peat | Caluna sp., Betula pubescens, Pinus sylvestris, Sphagnum palustris, Vaccinium myrtylius, V. uliginosum | 0–50 | Eriophoro-Sphagneti | H6 | |
| 50–100 | Eriophoro-Sphagneti | H4 | |||
| Sampling Sites | Age (AD/BC) | Thickness of the Peat Deposit (m) | Mean Annual Air Temperature (°C) | Mean Annual Precipitation (mm) | pH (1M KCl) | ||
|---|---|---|---|---|---|---|---|
| Layers (cm) | |||||||
| 0–50 | 50–100 | ||||||
| Undrained peatlands | |||||||
| Mukhrino | Bog peat | AD 712–780 | 2.0–4.5 | −1.1 | 531 | 2.40–3.28 | 2.41–2.77 |
| Fen peat | 3.72–4.43 | 3.75–4.33 | |||||
| Tagan Mire 1 | BC 4594–3979 | 9.3 | 0.8 | 532 | 4.83–5.21 | 4.71–5.32 | |
| Stążka | AD 655–779 | 1.4 | 7.2 | 589 | 5.61–5.79 | 7.04–7.26 | |
| Drained peatlands | |||||||
| Wrześnica River valley | 1 | BC549–398 | 1.4 | 8.9 | 530 | 5.54–6.44 | 5.64–6.14 |
| 2 | 5.52–6.31 | 5.49–6.02 | |||||
| General Dezydery Chłapowski Landscape Park | 1 | - | 1.5–2.8 | 7.0 | 650 | 5.13–5.30 | 5.69–5.91 |
| 2 | 5.78–5.91 | 5.81–5.83 | |||||
| 3 | 6.28–6.64 | 5.71–6.30 | |||||
| 4 | 4.63–5.29 | 5.86–6.09 | |||||
| Tagan Mire 2 | BC 4594–3979 | 9.3 | 0.8 | 532 | 5.14–5.42 | 5.81–5.95 | |
| Great Batorowskie | BC 4225–3961 | 1.2 | 4.8 | 750–920 | 2.50–2.68 | 2.64–2.85 | |
| Zieleniec Mire | BC 166–AD 20 | 6.0 | 6.4 | 665 | 2.75–2.92 | 2.75–2.92 | |
| Parameters | Moisture | Ash | Bulk Density | Porosity | CHWE | TOC | TN | N-NH4+ | N-NO3− | C/N | Xanthine Oxidase | Urate Oxidase | Phenol Oxidase | Peroxidase |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | - | |||||||||||||
| Ash | −0.174 | - | ||||||||||||
| Bulk density | −0.248 | 0.924 | - | |||||||||||
| Porosity | 0.049 | −0.238 | −0.217 | - | ||||||||||
| CHWE | −0.236 | −0.239 | −0.269 | 0.041 | - | |||||||||
| TOC | 0.266 | −0.323 | −0.161 | 0.050 | −0.588 | - | ||||||||
| TN | −0.012 | −0.071 | −0.097 | 0.089 | −0.580 | 0.092 | - | |||||||
| N-NH4+ | −0.269 | 0.250 | 0.138 | 0.001 | −0.023 | −0.603 | 0.657 | - | ||||||
| N-NO3− | −0.261 | 0.316 | 0.139 | 0.011 | 0.210 | −0.749 | 0.252 | 0.809 | - | |||||
| C/N | 0.055 | 0.001 | 0.055 | −0.075 | 0.518 | 0.040 | −0.960 | −0.744 | −0.420 | - | ||||
| Xanthine oxidase | 0.174 | −0.272 | −0.349 | 0.200 | −0.259 | −0.090 | 0.828 | 0.600 | 0.190 | −0.788 | - | |||
| Urate oxidase | −0.330 | −0.966 | −0.873 | 0.204 | 0.964 | −0.364 | 0.789 | 0.114 | −0.887 | −0.814 | 0.954 | - | ||
| Phenol oxidase | 0.147 | −0.750 | −0.338 | 0.007 | −0.127 | 0.791 | −0.249 | −0.754 | −0.772 | 0.345 | −0.344 | 0.932 | - | |
| Peroxidase | −0.482 | 0.118 | 0.168 | −0.092 | 0.754 | −0.561 | −0.456 | 0.175 | 0.388 | 0.347 | −0.447 | 0.963 | −0.120 | - |
| Parameters | Moisture | Ash | Bulk Density | Porosity | CHWE | TOC | TN | N-NH4+ | N-NO3− | C/N | Xanthine Oxidase | Urate Oxidase | Phenol Oxidase | Peroxidase |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | - | |||||||||||||
| Ash | −0.878 | - | ||||||||||||
| Bulk density | −0.868 | 0.990 | - | |||||||||||
| Porosity | 0.534 | −0.663 | −0.667 | - | ||||||||||
| CHWE | 0.428 | −0.500 | −0.499 | 0.285 | - | |||||||||
| TOC | 0.850 | −0.831 | −0.817 | 0.446 | 0.503 | - | ||||||||
| TN | 0.091 | 0.076 | 0.100 | −0.179 | −0.378 | 0.181 | - | |||||||
| N-NH4+ | 0.700 | −0.705 | −0.696 | 0.453 | 0.331 | 0.508 | 0.039 | - | ||||||
| N-NO3− | 0.652 | −0.671 | −0.668 | 0.428 | 0.654 | 0.618 | −0.427 | 0.688 | - | |||||
| C/N | 0.587 | −0.689 | −0.699 | 0.506 | 0.690 | 0.539 | −0.681 | 0.462 | 0.860 | - | ||||
| Xanthine oxidase | 0.531 | −0.554 | −0.553 | 0.423 | −0.016 | 0.208 | 0.098 | 0.833 | 0.309 | 0.210 | - | |||
| Urate oxidase | −0.006 | −0.066 | −0.068 | 0.015 | −0.105 | 0.157 | 0.459 | −0.432 | −0.473 | −0.323 | −0.374 | - | ||
| Phenol oxidase | 0.587 | −0.694 | −0.702 | 0.710 | 0.559 | 0.742 | −0.622 | 0.450 | 0.839 | 0.949 | 0.222 | −0.314 | - | |
| Peroxidase | 0.656 | −0.755 | −0.766 | 0.739 | −0.725 | 0.580 | −0.486 | 0.589 | 0.787 | 0.912 | 0.389 | −0.182 | 0.874 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szajdak, L.W.; Meysner, T.; Szczepański, M. Enzymatic Activity as New Moorsh-Forming Process Indicators of Peatlands. Agronomy 2021, 11, 113. https://doi.org/10.3390/agronomy11010113
Szajdak LW, Meysner T, Szczepański M. Enzymatic Activity as New Moorsh-Forming Process Indicators of Peatlands. Agronomy. 2021; 11(1):113. https://doi.org/10.3390/agronomy11010113
Chicago/Turabian StyleSzajdak, Lech W., Teresa Meysner, and Marek Szczepański. 2021. "Enzymatic Activity as New Moorsh-Forming Process Indicators of Peatlands" Agronomy 11, no. 1: 113. https://doi.org/10.3390/agronomy11010113
APA StyleSzajdak, L. W., Meysner, T., & Szczepański, M. (2021). Enzymatic Activity as New Moorsh-Forming Process Indicators of Peatlands. Agronomy, 11(1), 113. https://doi.org/10.3390/agronomy11010113