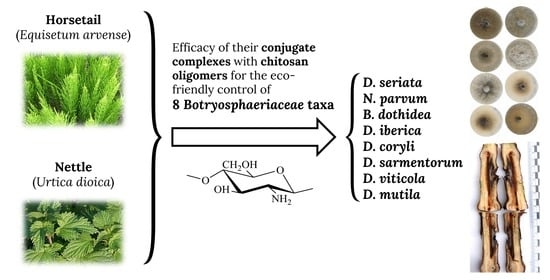
Assessment of Conjugate Complexes of Chitosan and Urtica dioica or Equisetum arvense Extracts for the Control of Grapevine Trunk Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Reagents and Preparation of Chitosan Oligomers and Bioactive Formulations
2.3. Horsetail and Nettle Extracts Characterization
2.4. In Vitro Tests of Mycelial Growth Inhibition
2.5. Greenhouse Bioassays in Grafted Plants
2.6. Statistical Analyses
3. Results
3.1. Horsetail and Nettle Extracts
3.2. In Vitro Efficacy
3.3. In Planta Assays
4. Discussion
4.1. Efficacy Comparisons
4.2. Mechanism of Action
4.3. Significance of the Reported Findings, Limitations of the Study and Further Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damalas, C.A.; Koutroubas, S.D. Botanical Pesticides for Eco-Friendly Pest Management. In Pesticides in Crop Production; Srivastava, P.K., Singh, V.P., Singh, A., Tripathi, D.K.S., Samiksha, Prasad,, S.M., Chauhan, D.K., Eds.; Wiley: Chichester, UK, 2020; pp. 181–193. [Google Scholar]
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest Manag. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Marchand, P.A. Basic Substances under EU Pesticide Regulation: An Opportunity for Organic Production? Org. Farming 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and Chitosan Fragments Responsible for Plant Elicitor and Growth Stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro- and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Patova, O.A.; Smirnov, V.V.; Golovchenko, V.V.; Vityazev, F.V.; Shashkov, A.S.; Popov, S.V. Structural, rheological and antioxidant properties of pectins from Equisetum arvense L. and Equisetum sylvaticum L. Carbohydr. Polym. 2019, 209, 239–249. [Google Scholar] [CrossRef]
- Francescato, L.N.; Debenedetti, S.L.; Schwanz, T.G.; Bassani, V.L.; Henriques, A.T. Identification of phenolic compounds in Equisetum giganteum by LC–ESI-MS/MS and a new approach to total flavonoid quantification. Talanta 2013, 105, 192–203. [Google Scholar] [CrossRef] [Green Version]
- Stajner, D.; Popović, B.M.; Čanadanovicć-Brunet, J.; Anacčkov, G. Exploring Equisetum arvense L., Equisetum ramosissimum L. and Equisetum telmateia L. as sources of natural antioxidants. Phytother. Res. 2009, 23, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Committee on Herbal Medicinal Products. European Union Herbal Monograph on Equisetum arvense L., Herba. EMA/HMPC/278091/2015. Available online: https://www.ema.europa.eu/en/medicines/herbal/equiseti-herba (accessed on 12 May 2021).
- Milovanović, V.; Radulović, N.; Todorović, Z.; Stanković, M.; Stojanović, G. Antioxidant, Antimicrobial and Genotoxicity Screening of Hydro-alcoholic Extracts of Five Serbian Equisetum Species. Plant Foods Hum. Nutr. 2007, 62, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Mimica-Dukic, N.; Simin, N.; Cvejic, J.; Jovin, E.; Orcic, D.; Bozin, B. Phenolic Compounds in Field Horsetail (Equisetum arvense L.) as Natural Antioxidants. Molecules 2008, 13, 1455–1464. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). Technical report on the outcome of the consultation with Member States and EFSA on the basic substance application for approval of Equisetum arvense L. for the extension of use in plant protection against fungal diseases on horticulture and vegetable crops. Efsa Support. Publ. 2020, 17, 42. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Technical report on the outcome of the consultation with Member States and EFSA on the basic substance applications for Urtica spp. for use in plant protection as insecticide, acaricide and fungicide. Efsa Support. Publ. 2016, 13. [Google Scholar] [CrossRef]
- Mukhtar Ahmed, K.B.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production—A review. Carbohydr. Polym. 2020, 227. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, N.; Stojanovic, G.; Palic, R. Composition and antimicrobial activity of Equisetum arvense L. essential oil. Phytother. Res. 2006, 20, 85–88. [Google Scholar] [CrossRef]
- Čanadanović-Brunet, J.M.; Ćetković, G.S.; Djilas, S.M.; Tumbas, V.T.; Savatović, S.S.; Mandić, A.I.; Markov, S.L.; Cvetković, D.D. Radical scavenging and antimicrobial activity of horsetail (Equisetum arvense L.) extracts. Int. J. Food Sci. Technol. 2009, 44, 269–278. [Google Scholar] [CrossRef]
- Nagai, T.; Myoda, T.; Nagashima, T. Antioxidative activities of water extract and ethanol extract from field horsetail (tsukushi) Equisetum arvense L. Food Chem. 2005, 91, 389–394. [Google Scholar] [CrossRef]
- Radojevic, I.D.; Stankovic, M.S.; Stefanovic, O.D.; Topuzovic, M.D.; Comic, L.R.; Ostojic, A.M. Great horsetail (Equisetum telmateia Ehrh.): Active substances content and biological effects. Excli J. 2012, 11, 59–67. [Google Scholar]
- Rogozhin, E.A.; Tepkeeva, I.I.; Zaitsev, D.V.; Demushkin, V.P.; Smirnov, A.N. Biological activity of peptide extracts of medicinal plants against phytopathogenic fungi and oomycetes. Russ. Agric. Sci. 2011, 37, 314–317. [Google Scholar] [CrossRef]
- Sehari, M.; Kouadria, M.; Amirat, M.; Sehari, N.; Hassani, A. Phytochemistry and antifungal activity of plant extracts from Nettle (Urtica dioica L.). Ukr. J. Ecol. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Torun, B.; Biyik, H.H.; Ercin, Z.; Coban, E.P. Antifungal activities of Urtica dioica L., Sinapis arvensis L. and Apium graveolens Mill. leaves on Botrytis cinerea Pers. Ann. Phytomed.-Int. J. 2018, 7, 94–97. [Google Scholar] [CrossRef]
- Romanazzi, G.; Feliziani, E.; Santini, M.; Landi, L. Effectiveness of postharvest treatment with chitosan and other resistance inducers in the control of storage decay of strawberry. Postharvest Biol. Technol. 2013, 75, 24–27. [Google Scholar] [CrossRef]
- Hadizadeh, I.; Peivastegan, B.; Kolahi, M. Antifungal activity of nettle (Urtica dioica L.), colocynth (Citrullus colocynthis L. Schrad), oleander (Nerium oleander L.) and konar (Ziziphus spina-christi L.) extracts on plants pathogenic fungi. Pak. J. Biol. Sci. Pjbs 2009, 12, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Yalcin, M. Activity of commercial still waters from volatile oils production against wood decay fungi. Maderas-Cienc. Y Tecnol. 2010, 12, 127–133. [Google Scholar] [CrossRef]
- Cobos, R.; Mateos, R.M.; Alvarez-Perez, J.M.; Olego, M.A.; Sevillano, S.; Gonzalez-Garcia, S.; Garzon-Jimeno, E.; Coque, J.J. Effectiveness of natural antifungal compounds in controlling infection by grapevine trunk disease pathogens through pruning wounds. Appl. Environ. Microbiol 2015, 81, 6474–6483. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, T.; Rego, C.; Oliveira, H. Potential use of chitosan in the control of grapevine trunk diseases. Phytopathol. Mediterr. 2007, 46, 218–224. [Google Scholar]
- Matei, P.M.; Martín-Ramos, P.; Sánchez-Báscones, M.; Hernández-Navarro, S.; Correa-Guimaraes, A.; Navas-Gracia, L.M.; Rufino, C.A.; Ramos-Sánchez, M.C.; Martín-Gil, J. Synthesis of chitosan oligomers/propolis/silver nanoparticles composite systems and study of their activity against Diplodia seriata. Int. J. Polym. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- La Torre, A.; Righi, L.; Iovino, V.; Battaglia, V. Evaluation of copper alternative products to control grape downy mildew in organic farming. J. Plant. Pathol. 2019, 101, 1005–1012. [Google Scholar] [CrossRef]
- Garcia-Cela, E.; Ramos, A.J.; Sanchis, V.; Marin, S. Ochratoxigenic moulds and effectiveness of grape field antifungals in a climatic change scenario. J. Sci. Food Agric. 2012, 92, 1455–1461. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Pérez-Lebeña, E.; Martín-Ramos, P. On the applicability of chitosan oligomers-amino acid conjugate complexes as eco-friendly fungicides against grapevine trunk pathogens. Agronomy 2021, 11, 324. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Martín-Gil, J.; Pérez-Lebeña, E.; Ruano-Rosa, D.; Revuelta, J.L.; Casanova-Gascón, J.; Ramos-Sánchez, M.C.; Martín-Ramos, P. Antifungal agents based on chitosan oligomers, ε-polylysine and Streptomyces spp. secondary metabolites against three Botryosphaeriaceae species. Antibiotics 2019, 8, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzón-Durán, L.; Martín-Gil, J.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Pérez-Lebeña, E.; Martín-Ramos, P. Antifungal Activity of Chitosan Oligomers–Amino Acid Conjugate Complexes against Fusarium culmorum in Spelt (Triticum spelta L.). Agronomy 2020, 10, 1427. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clement, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing Grapevine Trunk Diseases With Respect to Etiology and Epidemiology: Current Strategies and Future Prospects. Plant. Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-Costet, M.-F. Grapevine trunk diseases. A review; OIV publications:: Paris, France, 2016; p. 25. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Luque, J.; Elena, G.; Garcia-Figueres, F.; Reyes, J.; Barrios, G.; Legorburu, F.J. Natural infections of pruning wounds by fungal trunk pathogens in mature grapevines in Catalonia (Northeast Spain). Aust. J. Grape Wine Res. 2014, 20, 134–143. [Google Scholar] [CrossRef]
- Aroca, Á.; Gramaje, D.; Armengol, J.; García-Jiménez, J.; Raposo, R. Evaluation of the grapevine nursery propagation process as a source of Phaeoacremonium spp. and Phaeomoniella chlamydospora and occurrence of trunk disease pathogens in rootstock mother vines in Spain. Eur. J. Plant. Pathol. 2010, 126, 165–174. [Google Scholar] [CrossRef]
- Garcia, D.; Garcia-Cela, E.; Ramos, A.J.; Sanchis, V.; Marin, S. Mould growth and mycotoxin production as affected by Equisetum arvense and Stevia rebaudiana extracts. Food Control. 2011, 22, 1378–1384. [Google Scholar] [CrossRef]
- Garcia, D.; Ramos, A.J.; Sanchis, V.; Marin, S. Effect of Equisetum arvense and Stevia rebaudiana extracts on growth and mycotoxin production by Aspergillus flavus and Fusarium verticillioides in maize seeds as affected by water activity. Int. J. Food Microbiol. 2012, 153, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rusin, C.; Cavalcanti, F.R.; Lima, P.C.G.d.; Faria, C.M.D.R.; Almança, M.A.K.; Botelho, R.V. Control of the fungi Lasiodiplodia theobromae, the causal agent of dieback, in cv. syrah grapevines. Acta Sci. Agron. 2020, 43. [Google Scholar] [CrossRef]
- Amponsah, N.T.; Jones, E.; Ridgway, H.J.; Jaspers, M.V. Evaluation of fungicides for the management of Botryosphaeria dieback diseases of grapevines. Pest. Manag. Sci. 2012, 68, 676–683. [Google Scholar] [CrossRef]
- Kotze, C.; Van Niekerk, J.; Halleen, F.; Mostert, L.; Fourie, P. Evaluation of biocontrol agents for grapevine pruning wound protection against trunk pathogen infection. Phytopathol. Mediterr. 2011, 50, 247–263. [Google Scholar] [CrossRef]
- Díaz, G.A.; Latorre, B.A. Efficacy of paste and liquid fungicide formulations to protect pruning wounds against pathogens associated with grapevine trunk diseases in Chile. Crop Prot. 2013, 46, 106–112. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological Control of Phytopathogenic Fungi by Fatty Acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Clitherow, K.H.; Binaljadm, T.M.; Hansen, J.; Spain, S.G.; Hatton, P.V.; Murdoch, C. Medium-Chain Fatty Acids Released from Polymeric Electrospun Patches Inhibit Candida albicans Growth and Reduce the Biofilm Viability. Acs Biomater. Sci. Eng. 2020, 6, 4087–4095. [Google Scholar] [CrossRef]
- Pohl, C.H.; Kock, J.L.; Thibane, V.S. Antifungal free fatty acids: A review. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 3, 61–71. [Google Scholar]
- Altieri, C.; Cardillo, D.; Bevilacqua, A.; Sinigaglia, M. Inhibition of Aspergillus spp. and Penicillium spp. by Fatty Acids and Their Monoglycerides. J. Food Prot. 2007, 70, 1206–1212. [Google Scholar] [CrossRef]
- Stopiglia, C.D.O.; Vieira, F.J.; Mondadori, A.G.; Oppe, T.P.; Scroferneker, M.L. In Vitro Antifungal Activity of Dihydroxyacetone Against Causative Agents of Dermatomycosis. Mycopathologia 2010, 171, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Shobana, S.; Vidhya, V.; Ramya, M. Antibacterial activity of garlic varieties (ophioscordon and sativum) on enteric pathogens. Curr. Res. J. Biol. Sci. 2009, 1, 123–126. [Google Scholar]
- Honda, T. Investigation of Innovative Synthesis of Biologically Active Compounds on the Basis of Newly Developed Reactions. Chem. Pharm. Bull. 2012, 60, 687–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komai, S.-i.; Hosoe, T.; Nozawa, K.; Okada, K.; de Campos Takaki, G.M.; Fukushima, K.; Miyaji, M.; Horie, Y.; Kawai, K.-i. Antifungal activity of pyranone and furanone derivatives, isolated from Aspergillus sp. IFM51759, against Aspergillus fumigatus. Mycotoxins 2003, 53, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Madison, V.; Chau, A.S.; Loebenberg, D.; Palermo, R.E.; McNicholas, P.M. Three-Dimensional Models of Wild-Type and Mutated Forms of Cytochrome P450 14α-Sterol Demethylases from Aspergillus fumigatus and Candida albicans Provide Insights into Posaconazole Binding. Antimicrob. Agents Chemother. 2004, 48, 568–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrios, G.N. Control of Plant Diseases. Plant Pathol. 2005, 293–353. [Google Scholar] [CrossRef]
- Xing, K.; Shen, X.; Zhu, X.; Ju, X.; Miao, X.; Tian, J.; Feng, Z.; Peng, X.; Jiang, J.; Qin, S. Synthesis and in vitro antifungal efficacy of oleoyl-chitosan nanoparticles against plant pathogenic fungi. Int. J. Biol. Macromol. 2016, 82, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Georgopapadakou, N.H.; Dix, B.A.; Smith, S.A.; Freudenberger, J.; Funke, P.T. Effect of antifungal agents on lipid biosynthesis and membrane integrity in Candida albicans. Antimicrob. Agents Chemother. 1987, 31, 46–51. [Google Scholar] [CrossRef] [Green Version]
- Lo, W.-H.; Deng, F.-S.; Chang, C.-J.; Lin, C.-H. Synergistic Antifungal Activity of Chitosan with Fluconazole against Candida albicans, Candida tropicalis, and Fluconazole-Resistant Strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef] [PubMed]
- Kolesnyk, I.; Konovalova, V.; Kharchenko, K.; Burban, A.; Kujawa, J.; Kujawski, W. Enhanced transport and antifouling properties of polyethersulfone membranes modified with α-amylase incorporated in chitosan-based polymeric micelles. J. Membr. Sci. 2020, 595. [Google Scholar] [CrossRef]
- Larignon, P.; Dubos, B. Le Black Dead Arm: Maladie nouvelle à ne pas confondre avec l′esca. Phytoma-La Défense Des Végétaux 2001, 538, 26–29. [Google Scholar]
- Urbez-Torres, J.R. The status of Botryosphaeriaceae species infecting grapevines. In Phytopathologia Mediterranea; University of Florence: Florence, Italy, 2011; Volume 50, pp. 5–45. [Google Scholar]
- Gramaje, D.; Armengol, J. Fungal Trunk Pathogens in the Grapevine Propagation Process: Potential Inoculum Sources, Detection, Identification, and Management Strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Marsberg, A.; Kemler, M.; Jami, F.; Nagel, J.H.; Postma-Smidt, A.; Naidoo, S.; Wingfield, M.J.; Crous, P.W.; Spatafora, J.W.; Hesse, C.N.; et al. Botryosphaeria dothidea: A latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 2017, 18, 477–488. [Google Scholar] [CrossRef]
- Moral, J.; Agustí-Brisach, C.; Pérez-Rodríguez, M.; Xaviér, C.; Raya, M.C.; Rhouma, A.; Trapero, A. Identification of fungal species associated with branch dieback of olive and resistance of table cultivars to Neofusicoccum mediterraneum and Botryosphaeria dothidea. Plant Dis. 2017, 101, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Olmo, D.; Gramaje, D.; Armengol, J. Evaluation of fungicides to protect pruning wounds from Botryosphaeriaceae species infections on almond trees. Phytopathol. Mediterr. 2017, 56, 77–86. [Google Scholar]
- Fan, K.; Wang, J.; Fu, L.; Li, X.; Zhang, Y.; Zhang, X.; Zhai, H.; Qu, J. Sensitivity of Botryosphaeria dothidea from apple to tebuconazole in China. Crop Prot. 2016, 87, 1–5. [Google Scholar] [CrossRef]



| Code | Isolate | Binomial Nomenclature | Geographical Origin | Host/Date |
|---|---|---|---|---|
| ITACYL_F098 | Y-084-01-01a | Diplodia seriata De Not. | Spain (DO Toro) | Grapevine (Tempranillo) 2004 |
| ITACYL_F111 | Y-091-03-01c | Neofusicoccum parvum (Pennycook & Samuels) Crous, Slippers & A.J.L.Phillips | Spain (Navarra, nursery) | Grapevine (Verdejo) 2006 |
| ITACYL_F141 | Y-127-02-01 | Botryosphaeria dothidea (Moug.) Ces. & De Not. | Spain (Galicia) | Grapevine 2005 |
| ITACYL_F066 | T-046-05-3B | Dothiorella iberica A.J.L.Phillips, J.Luque & A.Alves | Spain | Grapevine (Tempranillo) 2009 |
| ITACYL_F187 | Y-291-24-01 | Diplodia coryli Fuckel | Spain (Gordoncillo, León) | Grapevine (Prieto Picudo) 2010 |
| ITACYL_F081 | Y-051-04-03a | Dothiorella sarmentorum (Fr.) A.J.L.Phillips, A.Alves & J.Luque | Spain (DO Tierra de León) | Grapevine (Prieto Picudo) 2004 |
| ITACYL_F118 | Y-103-08-01 | Dothiorella viticola A.J.L.Phillips & J.Luque | Spain (Extremadura) | Grapevine 2004 |
| ITACYL_F080 | Y-050-05-01c | Diplodia mutila (Fr.) Mont. | Spain (DO Ribera de Duero) | Grapevine 2004 |
| Treatment | D. seriata | N. parvum | B. dothidea | D. iberica | D. coryli | D. sarmentorum | D. viticola | D. mutila | |
|---|---|---|---|---|---|---|---|---|---|
| COS | EC50 | 744.4 | 680.2 | 362.8 | 706.6 | 472.2 | 398.7 | 554.3 | 343.7 |
| EC90 | 1179.9 | 1326.6 | 1191.6 | 1196.4 | 972.4 | 1075.9 | 1138.7 | 1196.8 | |
| COS-E. arvense | EC50 | 173.9 | 214.1 | 109.4 | 304.1 | 155.3 | 198.2 | 148.2 | 118.6 |
| EC90 | 429.0 | 637.1 | 267.1 | 817.3 | 999.0 | 669.0 | 351.1 | 208.3 | |
| COS-U. dioica | EC50 | 211.5 | 215.2 | 72.6 | 253.0 | 162.9 | 203.0 | 175.3 | 100.3 |
| EC90 | 483.5 | 650.2 | 334.4 | 625.8 | 411.6 | 533.0 | 379.7 | 227.1 |
| Sample | Frequency | Sum of Ranks | Mean of Ranks | Groups | ||
|---|---|---|---|---|---|---|
| COS negative control | 48 | 3366.000 | 70.125 | A | ||
| COS-U. dioica negative control | 48 | 3458.500 | 72.052 | A | ||
| COS-E. arvense negative control | 40 | 3444.500 | 86.113 | A | ||
| COS-E. arvense | 64 | 15017.000 | 234.641 | B | ||
| COS-U. dioica | 72 | 17119.500 | 237.771 | B | ||
| COS | 64 | 16600.000 | 259.375 | B | ||
| Positive control | 64 | 21194.500 | 331.164 | C | ||
| Sample | Frequency | Sum of Ranks | Mean of Ranks | Groups | ||
|---|---|---|---|---|---|---|
| COS-U. dioica negative control | 48 | 4216.500 | 87.844 | A | ||
| COS negative control | 48 | 4255.000 | 88.646 | A | ||
| COS-E. arvense negative control | 40 | 4504.500 | 112.613 | A | ||
| COS-E. arvense | 80 | 16097.000 | 201.213 | B | ||
| COS-U. dioica | 80 | 18098.500 | 226.231 | B | ||
| COS | 64 | 20311.000 | 317.359 | C | ||
| Positive control | 56 | 19253.500 | 343.813 | C | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langa-Lomba, N.; Buzón-Durán, L.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; Sánchez-Hernández, E.; González-García, V. Assessment of Conjugate Complexes of Chitosan and Urtica dioica or Equisetum arvense Extracts for the Control of Grapevine Trunk Pathogens. Agronomy 2021, 11, 976. https://doi.org/10.3390/agronomy11050976
Langa-Lomba N, Buzón-Durán L, Martín-Ramos P, Casanova-Gascón J, Martín-Gil J, Sánchez-Hernández E, González-García V. Assessment of Conjugate Complexes of Chitosan and Urtica dioica or Equisetum arvense Extracts for the Control of Grapevine Trunk Pathogens. Agronomy. 2021; 11(5):976. https://doi.org/10.3390/agronomy11050976
Chicago/Turabian StyleLanga-Lomba, Natalia, Laura Buzón-Durán, Pablo Martín-Ramos, José Casanova-Gascón, Jesús Martín-Gil, Eva Sánchez-Hernández, and Vicente González-García. 2021. "Assessment of Conjugate Complexes of Chitosan and Urtica dioica or Equisetum arvense Extracts for the Control of Grapevine Trunk Pathogens" Agronomy 11, no. 5: 976. https://doi.org/10.3390/agronomy11050976
APA StyleLanga-Lomba, N., Buzón-Durán, L., Martín-Ramos, P., Casanova-Gascón, J., Martín-Gil, J., Sánchez-Hernández, E., & González-García, V. (2021). Assessment of Conjugate Complexes of Chitosan and Urtica dioica or Equisetum arvense Extracts for the Control of Grapevine Trunk Pathogens. Agronomy, 11(5), 976. https://doi.org/10.3390/agronomy11050976