Seed Coating with Biowaste Materials and Biocides—Environment-Friendly Biostimulation or Threat?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Coating Materials
2.2.1. Waste Bovine (BC) and Fish (FC) Collagen
2.2.2. Poly(hexamethylenebiguanide) Hydrochloride (PHMB) Biocide
2.2.3. Dolomite (D)
2.3. Experimental Setup and Seed Coating Method
2.4. Growth Conditions
2.5. Experiment 1: Effects of Seed Coating on Growth and Nutrition
2.5.1. Chlorophyll Content Determination
2.5.2. Fluorescence of Chlorophyll a Determination
2.5.3. Growth Measurements
2.5.4. Metal Content Determination
2.6. Experiment 2: Effects of Seed Coating on Emergence of Seedlings
2.7. Statistical Analysis
3. Results
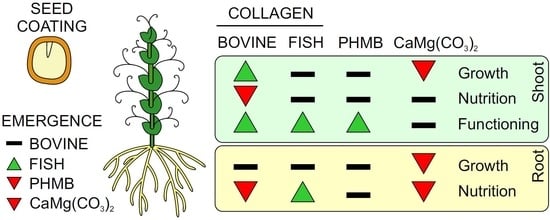
3.1. Effects of Seed Coating on Growth and Nutrition
3.2. Effect of Seed Coating on Emergence of Seedlings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buljan, J.; Reich, G.; Ludvik, J. Mass Balance. In Leather Processing, Regional Program for Pollution Control in the Tanning Industry in South-East Asia; US/RAS/92/120; United Nations Industrial Development Organization: Vienna, Austria, 2000. [Google Scholar]
- Sivaprakash, K.; Maharaja, P.; Pavithra, S.; Boopathy, R.; Sekaran, G. Preparation of light weight constructional materials from chrome containing buffing dust solid waste generated in leather industry. J. Mater. Cycles Waste Manag. 2017, 19, 928–938. [Google Scholar] [CrossRef]
- Konikkara, N.; Punithavelan, N.; Kennedy, J.; Vijaya, J.J. A new approach to solid waste management: Fabrication of super capacitor electrodes from solid leather wastes using aqueous KOH electrolyte. Clean Technol. Environ. 2017, 19, 1087–1098. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.V.; Lay, M.C.; Swan, J. Collagen extraction from various waste bovine hide sources. Waste Biomass Valori. 2019. [Google Scholar] [CrossRef]
- Silva, A.V.S.; Torquato, L.D.M.; Cruz, G. Potential application of fish scales as feedstock in thermochemical processes for the clean energy generation. Waste Manag. 2019, 100, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, P.K.; Dandge, P.B. Isolation, characterization and valorizable applications of fish scale collagen in food and agriculture industries. Biocat. Agric. Biotechnol. 2016, 7, 234–240. [Google Scholar] [CrossRef]
- Veses, A.; Sanahuja-Parejo, O.; Callén, M.S.; Murillo, R.; García, T. A combined two-stage process of pyrolysis and catalytic cracking of municipal solid waste for the production of syngas and solid refuse derived fuels. Waste Manag. 2020, 101, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.; Moreno, R.; Correia, J.; Ferro, V. A method for the rapid evaluation of leather biodegradability during the production phase. Waste Manag. 2019, 87, 661–671. [Google Scholar] [CrossRef]
- Khatoon, M.; Kashif, S.; Saad, S.; Umer, Z.; Rasheed, A. Extraction of Amino Acids and Proteins from Chrome Leather Waste. J. Waste Recycl. 2017, 2, 6. [Google Scholar]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, G.; Pallozzi, E.; Conversa, G.; Tufarelli, V.; De Lucia, B. Effects of an animal-derived biostimulant on the growth and physiological parameters of potted snapdragon (Antirrhinum majus L.). Front. Plant Sci. 2018, 9, 861. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Vidal, A.R.; Duarte, L.P.; Schmidt, M.M.; Cansian, R.L.; Fernandes, I.A.; Demiate, I.M.; Dornelles, R.C.P. Extraction and characterization of collagen from sheep slaughter by-products. Waste Manag. 2019, 102, 838–846. [Google Scholar] [CrossRef]
- Załuszniewska, A.; Nogalska, A. The Effect of Meat and Bone Meal (MBM) on the Seed Yield and Quality of Winter Oilseed Rape. Agronomy 2020, 10, 1952. [Google Scholar] [CrossRef]
- Corte, L.; Dell’Abate, M.T.; Magini, A.; Migliore, M.; Felici, B.; Roscini, L.; Sardella, R.; Tancini, B.; Emiliani, C.; Cardinali, G.; et al. Assessment of safety and efficiency of nitrogen organic fertilizers from animal-based protein hydrolysates—a laboratory multidisciplinary approach. J. Sci. Food Agric. 2014, 94, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oates, J.A.H. Lime and Limestone: Chemistry and Technology, Production and Uses; Wiley-VCH: Weinheim, Germany; New York, NY, USA, 1988. [Google Scholar] [CrossRef]
- Pesonen, J.; Myllymäki, P.; Tuomikoski, S.; Vervecken, G.; Hu, T.; Prokkola, H.; Tynjälä, P.; Lassi, U. Use of calcined dolomite as chemical precipitant in the simultaneous removal of ammonium and phosphate from synthetic wastewater and from agricultural sludge. Chem Eng. 2019, 3, 40. [Google Scholar] [CrossRef] [Green Version]
- Suntoro, S.; Widijanto, H.; Suryono Syamsiyah, J.; Afinda, D.W.; Dimasyuri, N.R.; Triyas, V. Effect of cow manure and dolomite on nutrient uptake and growth of corn (Zea mays L.). Bulg. J. Agric Sci. 2018, 24, 1020–1026. [Google Scholar]
- Cernay, C.; Pelzer, E.; Makowski, D. A global experimental dataset for assessing grain legume production. Sci. Data 2016, 3, 160084. [Google Scholar] [CrossRef] [Green Version]
- Kichigina, N.E.; Puhalsky, J.V.; Shaposhnikov, A.I.; Azarova, T.S.; Makarova, N.M.; Loskutov, S.I.; Safronova, V.I.; Tikhonovich, I.A.; Vishnyakova, M.A.; Semenova, E.V.; et al. Aluminum exclusion from root zone and maintenance of nutrient uptake are principal mechanisms of Al tolerance in Pisum sativum L. Physiol. Mol. Biol. Plants 2017, 23, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Mabhaudhi, T.; Chimonyo, V.G.P.; Hlahla, S.; Massawe, F.; Mayes, S.; Nhamo, L.; Modi, A.T. Prospects of orphan crops in climate change. Planta 2019, 250, 695–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okçu, G.; Kaya, M.D.; Atak, M. Effects of salt and drought stresses on germination and seedling growth of pea (Pisum sativum L.). Turk. J. Agric. For. 2005, 29, 237–242. [Google Scholar]
- Kangsopa, J.; Hynes, R.K.; Siri, B. Lettuce seeds pelleting: A new bilayer matrix for lettuce (Lactuca sativa) seeds. Seed Sci. Technol. 2018, 46, 521–531. [Google Scholar] [CrossRef]
- Amirkhani, M.; Netravali, A.N.; Huang, W.; Taylor, A.G. Investigation of soy protein–based biostimulant seed coating for broccoli seedling and plant growth enhancement. Hortscience 2016, 51, 1121–1126. [Google Scholar] [CrossRef] [Green Version]
- Pedrini, S.; Merritt, D.J.; Stevens, J.; Dixon, K. Seed Coating: Science or Marketing Spin? Trends Plant Sci. 2017, 22, 106–116. [Google Scholar] [CrossRef] [Green Version]
- Zeng, D.; Shi, Y. Preparation and application of a novel environmentally friendly organic seed coating for rice. J. Sci. Food Agric. 2009, 89, 2181–2185. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hort. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Waśkiewicz, A.; Stepień, Ł.; Wilman, K.; Kachlicki, P. Diversity of pea-associated F. proliferatum and F. verticillioides populations revealed by FUM1 sequence analysis and fumonisin biosynthesis. Toxins 2013, 5, 488–503. [Google Scholar] [CrossRef] [Green Version]
- De Paula, G.F.; Netto, G.I.; Mattoso, L.H.C. Physical and chemical characterization of poly(hexamethylenebiguanide) hydrochloride. Polymers 2011, 3, 928–941. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. The chlorophyll fluorescence ratio F735/F700 as an accurate measure of chlorophyll content in plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A.; Showater, A.M. Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte, Atriplex griffithii var. stocksii. Ann. Bot. 2000, 85, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejek, J.; Patykowski, J.; Wala, M. An experimental comparison of germination ecology and its implication for conservation of selected rare and endangered species of Dianthus (Caryophyllaceae). Botany 2018, 96, 319–328. [Google Scholar] [CrossRef]
- Khan, M.A.; Ungar, I.A. Effects of thermoperiod on recovery of seed germination 532 of halophytes from saline conditions. Am. J. Bot. 1997, 84, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Ranal, M.A.; Santana, D.G.D.E. How and why to measure the germination process? Revista Brasil Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Aravind, J. Germinationmetrics: Seed Germination Indices and Curve Fitting. R 538 Package. Version 0.1.3, 2019. Available online: https://github.com/aravind%20j/germinationmetricshttps://cran.r-539%20project.org/package=germinationmetrics (accessed on 9 March 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation 541 for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 11 March 2019).
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Sotelo, C.G.; Comesaña, B.M.; Ariza, R.P.; Pérez-Martín, R.I. Characterization of collagen from different discarded fish species of the west coast of the Iberian Peninsula. J. Aquat. Food Prod. Tech. 2016, 25, 388–399. [Google Scholar] [CrossRef] [Green Version]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Latesniloticus). Food Chem. 2004, 85, 81–89. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Dinesh Kumar, B.; Hemalatha, R.; Jyothirmayi, T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012, 135, 3020–3038. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil. 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Pajan, J.P.; Stall, W.M. Papaya (Carica papaya) response to foliar treatments with organic complexes of peptides and amino acids. Proc. FL. State Hort. Soc. 2003, 116, 30–32. [Google Scholar]
- Wilson, H.T.; Amirkhani, M.; Taylor, A.G. Evaluation of gelatin as a biostimulant seed treatment to improve plant performance. Front Plant Sci. 2018, 9, 1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the yield of spinach (Spinacia oleracea L.). Folia Hortic. 2010, 22, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Gajc-Wolska, J.; Kowalczyk, K.; Nowecka, M.; Mazur, K.; Metera, A. Effect of organic-mineral fertilizers on the yield and quality of Endive (Cichorium endivia L.). Acta Sci. Pol. 2012, 11, 189–200. [Google Scholar]
- Grabowska, A.; Kunicki, E.; Sekara, A.; Kalisz, A.; Wojciechowska, R. The effect of cultivar and biostimulant treatment on the carrot yield and its quality. Veg. Crops Res. Bull. 2012, 77, 37–48. [Google Scholar] [CrossRef]
- Wala, M.; Kołodziejek, J.; Mazur, J.; Patykowski, J. Differences in iron acquisition strategies of calcicole plant species from xerothermic grasslands. Geoderma 2020, 377, 114572. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Mou, B. Drench Application of Fish-derived Protein Hydrolysates Affects Lettuce Growth, Chlorophyll Content, and Gas Exchange. HortTechnology 2017, 27, 539–543. [Google Scholar] [CrossRef]
- López-Climent, M.F.; Arbona, V.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Relationship between salt tolerance and photosynthetic machinery performance in citrus. Environ. Exp. Bot. 2008, 62, 176–184. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Radziemska, M. Study of applying naturally occurring mineral sorbents of Poland (dolomite halloysite, chalcedonite) for aided phytostabilization of soil polluted with heavy metals. Catena 2018, 163, 123–129. [Google Scholar] [CrossRef]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant-soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Tomza-Marciniak, A.; Pilarczyk, B.; Marciniak, A.; Pilarczyk, R.; Bąkowska, M. Tin, Sn. In Mammals and Birds as Bioindicators of Trace Element Contaminations in Terrestrial Environments; Kalisińska, E., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Miedico, O.; Lammarino, M.; Paglia, G.; Tarallo, M.; Mangiacotti, M.; Chiaravalle, A.E. Environmental monitoring of the area surrounding oil wells in Val d’Agri (Italy): Element accumulation in bovine and ovine organs. Environ. Monit. Assess. 2016, 188, 338. [Google Scholar] [CrossRef]
- Vajpayee, P.; Tripathi, R.D.; Rai, U.N.; Ali, M.B.; Singh, S.N. Chromium (VI) accumulation reduces chlorophyll biosynthesis, nitrate reductase activity and protein content in Nymphaea alba L. Chemosphere 2000, 41, 1075–1082. [Google Scholar] [CrossRef]
- Nogueira, F.G.E.; Castro, I.A.; Bastos, A.R.R.; Souza, G.A.; de Carvalho, J.G.; Oliveira, L.C.A. Recycling of solid waste rich in organic nitrogen from leather industry: Mineral nutrition of rice plants. J. Hazard. Mater. 2011, 186, 1064–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, X.; Yang, M.; Zhang, B.; Chen, H.; Wang, Y.J. Recovery and utilization of collagen protein powder extracted from chromium leather scrap waste. Environ. Sci. Pollut. Res. 2019, 26, 7277–7283. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Drought and salinity: A comparison of their effects on mineral nutrition of plants. J. Plant Nutr. Soil Sci. 2005, 168, 541–549. [Google Scholar] [CrossRef]
- Coskan, A.; Dogan, K.; Demirbas, A.; Erdal, I.; Horzun, I.; Ok, E.C. Improvement of Ca and Mg uptake by application of dolomite and dolomite + leonardite. Sci. Papp. Ser. A Agron. 2017, 60, 67–72. [Google Scholar]
- Sulandjari Sakya, A.T.; Syamsiyah, J.; Panji, D. The application of amendments to increase nutrients absorption of Petiveria aleaceae L. (Singawalang) in peat soils. IOP Conf. Ser. Earth Environ. Sci. 2019, 250, 012017. [Google Scholar] [CrossRef]
- Loide, V.; Kõlli, R.; Reintam, E. Application of dolostone powder as industrial residual on magnesium-deficient arable soils in Estonia. Commun. Soil Sci. Plant Anal. 2010, 41, 207–218. [Google Scholar] [CrossRef]
- Mayland, H.F.; Wilkinson, S.R. Soil factors affecting magnesium availability in plant-animal systems: A review. J. Anim. Sci. 1989, 67, 3437–3444. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Wu, F.; Li, J.; Jiang, Y.; Duan, X. Antifungal activities of polyhexamethylene- biguanide and polyhexamethyleneguanide against the citrus sour rot pathogen Geotrichumcitri aurantii in vitro and in vivo. Postharvest Biol. Technol. 2011, 61, 160–164. [Google Scholar] [CrossRef]
- Mennan, H.; Ngouajio, M. Seasonal cycles in germination and seedling emergence of summer and winter populations of catch weed bedstraw (Galium aparine) and wild mustard (Brassica kaber). Weed Sci. 2006, 54, 114–120. [Google Scholar] [CrossRef]
- Kluyver, T.A.; Charles, M.; Jones, G.; Rees, M.; Osborne, C.P. Did greater burial depth increase the seed size of domesticated legumes? J. Exp. Bot. 2013, 64, 4101–4108. [Google Scholar] [CrossRef] [Green Version]
- Kołodziejek, J.; Patykowski, J. The effect of temperature, light and calcium carbonate on seed germination and radicle growth of the polycarpic perennial Galium cracoviense (Rubiaceae), a narrow endemic species from southern Poland. Acta Biol. Cracov Ser. Bot. 2015, 57, 70–81. [Google Scholar] [CrossRef] [Green Version]









| Parameter | Bovine Collagen (BC) | Fish Collagen (FC) |
|---|---|---|
| Acidity of 10% solution (pH) | 7.27 | 3.46 |
| Protein content (% DW) | 77.42 | 89.90 |
| Total ash content (% DW) | 5.36 | 1.81 |
| Total nitrogen content (% DW) | 13.78 | 14.30 |
| Heavy metal content (mg kg−1 DW) | ||
| Cr (III) | <0.1 | <0.1 |
| Zn | <0.05 | <0.05 |
| As | <0.005 | <0.05 |
| Pb | <0.10 | <0.10 |
| Hg | <0.10 | <0.10 |
| Cd | <0.10 | <0.10 |
| Cu | <0.02 | <0.02 |
| Sn | 6.60 ± 0.06 | - |
| Amino acid content (mg kg−1 DW) | ||
| Ala | 820.00 ± 70.00 | 931.33 ± 19.50 |
| Gly | 1961.33 ± 151.28 | 2768.33 ± 422.89 |
| Val | 105.00 ± 13.45 | 148.33 ± 6.66 |
| Leu | 222.67 ± 39.00 | 165.67 ± 14.98 |
| Ile | 52.67 ± 6.81 | 74.00 ± 4.00 |
| Thr | 151.00 ± 9.64 | 228.33 ± 10.41 |
| Ser | 137.00 ± 19.29 | 275.67 ± 5.13 |
| Pro | 2248.33 ± 480.63 | 3606.00 ± 129.00 |
| Asp | 447.00 ± 3.46 | 336.67 ± 10.41 |
| Met | 50.67 ± 5.13 | 82.67 ± 1.53 |
| Hyp | 1166.67 ± 251.66 | 1773.33 ± 30.55 |
| Glu | 554.33 ± 4.04 | 642.00 ± 12.12 |
| Phe | 83.33 ± 3.79 | 140.33 ± 7.51 |
| Lys | 92.00 ± 5.29 | 196.33 ± 1.53 |
| Element | Content (mg kg−1 DW) |
|---|---|
| Al | 469.51 ± 64.73 |
| B | 16.15 ± 11.41 |
| Ba | 2.53 ± 0.24 |
| Ca | 238,280.43 ± 7383.11 |
| Cr | 2.29 ± 0.24 |
| Cu | 1.08 ± 0.07 |
| Fe | 1242.19 ± 24.96 |
| K | 465.07 ± 60.31 |
| Mg | 137,983.20 ± 4673.20 |
| Mn | 279.07 ± 8.75 |
| Na | 25.25 ± 9.77 |
| Ni | 3.28 ± 0.42 |
| Sr | 29.66 ± 0.22 |
| Ti | 10.92 ± 1.81 |
| Zn | 18.38 ± 0.53 |
| Abbreviation | Binding Agent | Dolomite Addition a | Amount of Binding Agent or Binding Agent/Dolomite Mixture Applied to 500 g Seeds (g) | Seed Weight (mg) b |
|---|---|---|---|---|
| 0 | - | - | - | 237.92 ± 15.10 |
| 0 + D | - c | + | 16.08 | 240.12 ± 12.19 |
| BC | BC | - | 15.29 | 241.73 ± 19.60 |
| BC + D | BC | + | 19.10 | 249.88 ± 13.57 |
| FC | FC | - | 26.94 | 251.44 ± 11.63 |
| FC + D | FC | + | 33.67 | 288.86 ± 20.66 |
| PHMB | PHMB | - | 27.09 | 244.80 ± 14.10 |
| PHMB + D | PHMB | + | 33.87 | 248.00 ± 13.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skwarek, M.; Wala, M.; Kołodziejek, J.; Sieczyńska, K.; Lasoń-Rydel, M.; Ławińska, K.; Obraniak, A. Seed Coating with Biowaste Materials and Biocides—Environment-Friendly Biostimulation or Threat? Agronomy 2021, 11, 1034. https://doi.org/10.3390/agronomy11061034
Skwarek M, Wala M, Kołodziejek J, Sieczyńska K, Lasoń-Rydel M, Ławińska K, Obraniak A. Seed Coating with Biowaste Materials and Biocides—Environment-Friendly Biostimulation or Threat? Agronomy. 2021; 11(6):1034. https://doi.org/10.3390/agronomy11061034
Chicago/Turabian StyleSkwarek, Monika, Mateusz Wala, Jeremi Kołodziejek, Katarzyna Sieczyńska, Magdalena Lasoń-Rydel, Katarzyna Ławińska, and Andrzej Obraniak. 2021. "Seed Coating with Biowaste Materials and Biocides—Environment-Friendly Biostimulation or Threat?" Agronomy 11, no. 6: 1034. https://doi.org/10.3390/agronomy11061034
APA StyleSkwarek, M., Wala, M., Kołodziejek, J., Sieczyńska, K., Lasoń-Rydel, M., Ławińska, K., & Obraniak, A. (2021). Seed Coating with Biowaste Materials and Biocides—Environment-Friendly Biostimulation or Threat? Agronomy, 11(6), 1034. https://doi.org/10.3390/agronomy11061034