Inoculation of Klebsiella variicola Alleviated Salt Stress and Improved Growth and Nutrients in Wheat and Maize
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Samples, Plant Seeds, and Klebsiella variicola SURYA6
2.2. Analysis of Physicochemical Parameters of the Soil before and after Sowing
2.3. Evaluation of Salinity Tolerance in K. variicola SURYA6
2.4. Screening for Salinity Ameliorating Metabolites of K. variicola SURYA6
2.4.1. Production of ACCD
2.4.2. Screening and Production of Indole-3-Acetic Acid (IAA)
2.4.3. Screening and Production of Exopolysaccharide (EPS)
2.5. Confirmation of the Non-Pathogenic Nature of the Potent Isolate
2.5.1. Antibiotic Sensitivity and Blood Agar Test
2.5.2. Hemolysis Test
2.5.3. Catalase and Oxidase Tests
2.6. Plant Growth Promotion Studies
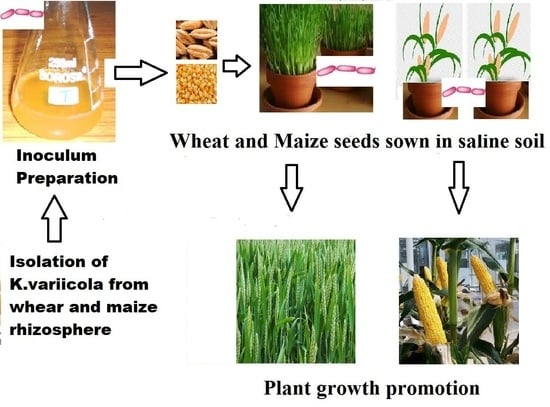
2.6.1. Experimental Design
2.6.2. Making of Saline Soil
2.6.3. Seed Bacterization and Application of K. variicola SURYA6 to the Soil
2.6.4. Measurement of Plant Growth Parameters
2.6.5. Estimation of Osmolyte, Sugar, Protein, and Amino Acid Contents in Plants
2.6.6. Analysis of Plant Nutrients and Mineral Content
2.7. Statistical Analyses
3. Results
3.1. Screening for Salinity Stress Tolerance
3.2. Screening and Production of Salinity Ameliorating Traits
3.3. Confirmation of Non-Pathogenicity of K. variicola SURYA6
3.4. Plant Growth Promotion Studies—Pot Assay
3.5. Analysis of Osmolytes and Biochemical Contents in Wheat and Maize Plants
3.6. Analysis of Mineral Content of Wheat and Maize Plants
3.7. Analysis of Soil Physical Parameters and Nutrients
4. Discussion
4.1. Screening for Salinity Stress Tolerance
4.2. Screening and Production of Salinity Ameliorating Traits
4.3. Confirmation of Non-Pathogenicity of K.variicola SURYA6
4.4. Plant Growth Promotion Studies—Pot Assay
4.5. Analysis of Osmolytes and Biochemical Contents in Wheat and Maize Plants
4.6. Analysis of Mineral Content of Wheat and Maize Plants
4.7. Analysis of Soil Physical Parameters and Nutrients
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield Trends Are Insufficient to Double Global Crop Production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, K.; Mubeenand, F.; Naqqash, T. Halotolerant PGPR: A hope for the cultivation of saline soils. J. King Saud Univ. Sci. 2019, 3, 1195–1204. [Google Scholar] [CrossRef]
- Sardouei-Nasab, S.; Mohammadi-Nejad, G.; Nakhoda, B. Yield stability in bread wheat germplasm across drought stress and non-stress conditions. Agronomy 2019, 111, 175–181. [Google Scholar] [CrossRef]
- Maize Production. Available online: https://farmer.gov.in/imagedefault/pestanddiseasescrops/normalmaizeproductiontechnologies.pdf (accessed on 8 March 2021).
- Farahat, M.G.; Mahmoud, M.K.; Youseif, S.H.; Saleh, A.S.; Kamel1, Z. Alleviation of salinity stress in wheat by ACC deaminase-producing Bacillus aryabhattai EWR29 with multifarious plant growth-promoting attributes. Plant Arch. 2020, 20, 417–429. [Google Scholar]
- Kumar Arora, N.; Fatima, T.; Mishra, J. Halo-tolerant plant growth-promoting rhizobacteria for improving productivity and remediation of saline soils. J. Adv. Res. 2020. [Google Scholar] [CrossRef]
- Al Omron, A.M.; El-Maghraby, S.E.; Nadeem, M.E.A.; El-Eter, A.M.; Al-Mohani, H. Long term effect of irrigation with the treated sewage effluent on some soil properties of Al-Hassa Governorate, Saudi Arabia. J. Saudi. Soc. Agric. Sci. 2012, 11, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Sagar, A.; Riyazuddin, R.; Shukla, P.K.; Ramteke, P.W.; Sayyed, R.Z. Heavy metal stress tolerance in Enterobacter sp. PR14 is mediated by plasmid. Indian J. Exp. Biol. 2020, 58, 115–121. Available online: http://nopr.niscair.res.in/handle/123456789/53518 (accessed on 26 February 2021).
- Yadav, A.N.; Kour, D.; Sharma, S.; Sachan, S.G.; Singh, B.; Chauhan, V.S.; Sayyed, R.Z.; Kaushik, R. Psychrotrophic Microbes: Biodiversity, Mechanisms of Adaptation and Biotechnological Implications in Alleviation of Cold Stress. In Plant, Plant Growth Promoting Rhizobacteria for Sustainable Stress Management Vol 1 Abiotic Stress Management; Sayyed, R.Z., Arora, N.K., Reddy, M.S., Eds.; Springer: Singapore, 2019; Volume 1, pp. 219–253. [Google Scholar]
- Nia, S.H.; Zarea, M.J.; Rejali, F.; Varma, A. Yield and yield components of wheat as affected by salinity and inoculation with Azospirillum strains from saline or non-saline soil. J. Saudi Soc. Agric. Sci. 2012, 11, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt tolerant plant growth-promoting rhizobacteria for enhancing crop productivity of saline soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [Green Version]
- Cantrell, I.C.; Linderman, R.G. Pre-inoculation of lettuce and onion with VA mycorrhizal fungi reduce deleterious effects of soil salinity. Plant Soil. 2001, 233, 269–281. [Google Scholar] [CrossRef]
- Wheat in the World. Available online: https://wheat.org/wheat-in-the-world/ (accessed on 8 March 2021).
- Kohler, J.; Hernandez, J.A.; Caravaca, F.; Roldana, A. Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuce to severe salt stress. Environ. Exp. Bot. 2009, 65, 245–252. [Google Scholar] [CrossRef]
- Sagar, A.; Sayyed, R.Z.; Ramteke, P.W.; Sharma, S.; Marraiki, N.; Elgorban, A.M.; Syed, A. ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. ameliorates salt stress and promotes the growth of rice and millets under salt stress. Physiol. Mol. Biol. Plants 2020, 26, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.; Shukla, P.K.; Ramteke, P.W.; Sayyed, R.Z. Stimulation of seed germination and growth parameters of rice var. Sahbhagi by Enterobacter cloacae (KP226569) in presence of ammonia sulfate as a substitute of 1-aminocyclopropane-1-carboxylate. In Plant Growth-Promoting Rhizobacteria (PGPR): Prospects for Sustainable Agriculture; Sayyed, R.Z., Reddy, M.S., Antonious, S., Eds.; Springer-Nature: Singapore, 2019; pp. 117–124. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Timmusk, S.; El-Daim, I.A.A.; Copolovici, L.; Tanilas, T.; Kännaste, A.; Behers, L. Drought-tolerance of wheat improved by rhizosphere bacteria from harsh environments: Enhanced biomass production and reduced emissions of stress volatiles. PLoS ONE 2014, 9, 96086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodd, I.C.; Pérez-Alfocea, F. Microbial amelioration of crop salinity stress. J. Exp. Bot. 2012, 63, 3415–3428. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, D.K.; Kasotia, A.; Jain, S.; Vaishnav, A.; Kumari, S.; Sharma, K.P.; Varma, A. Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J. Plant Growth Regul. 2015, 35, 276–300. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Barrios, C.H.; Aguilar, V.A.; Beltran, R.M.; Aguilar, V.E.; Duran, B.J.; Rodriguez, M.N.; Lozano, A.L.; Perez, O.M.; Rojas, J.; Garza, R.U. Molecular epidemiology of Klebsiella variicola obtained from different sources. Sci. Rep. 2019, 9, 10610. [Google Scholar] [CrossRef]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Holliday, V.T.; Gartner, W.G. Methods of soil P analysis in archaeology. J. Arch. Sci. 2007, 34, 301–333. [Google Scholar] [CrossRef]
- Baghel, S.S. Determination of Potassium in Soil and Plant. In CAFT on Advances in Agrotechnologies for Improving Soil, Plant, Atmosphere System; Jawahrlal Nehru Krishi Vishwa Vidyalaya: Jabalpur, India, 2012; pp. 23–25. [Google Scholar]
- Gupta, R.; Mohohaptra, H. Microbial biomass an economical alternative for the removal of heavy metals from wastewater. Ind. J. Exp. Biol. 2003, 41, 945–966. Available online: http://hdl.handle.net/123456789/17155 (accessed on 1 March 2021).
- Egamberdieva, D.; Berg, G.; Lindström, K.; Räsänen, L.A. Alleviation of salt stress of symbiotic Galega officinalis L. (goat’s rue) by co-inoculation of Rhizobium with root-colonizing. Pseudomonas. Plant Soil 2013, 369, 453–465. [Google Scholar] [CrossRef]
- Safronova, V.I.; Stepanok, V.V.; Engqvist, G.L.; Alekseyev, Y.V.; Belimov, A.A. Root-associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil. Biol. Fertil. Soils 2006, 42, 267–272. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Levels of 1-aminocyclopropane-1-carboxylic acid (ACC) in exudates and extracts of canola seeds treated with plant growth-promoting bacteria. Can. J. Microbiol. 2001, 47, 368–372. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951, 26, 192–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Dynamism of PGPR in bioremediation and plant growth promotion in heavy metal contaminated soil. Indian J. Exp. Biol. 2016, 54, 286–290. Available online: http://hdl.handle.net/123456789/34070 (accessed on 1 March 2021).
- Sayyed, R.Z.; Chincholkar, S.B. Production of Exo-polysaccharide (EPS): A biopolymer from A. faecalis. J. Food Sci. Technol. 2008, 45, 531–533. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Rebecca, B. Blood Agar Plates and Hemolysis Protocols; American Society for Microbiology: Washington, DC, USA, 2005; pp. 1–9. Available online: www.asmscience.org/content/education/protocol/protocol.3229 (accessed on 1 March 2021).
- Karen, R. Catalase Test Protocol; American Society of Microbiology: Washington, DC, USA, 2010; pp. 1–9. Available online: https://asm.org/getattachment/72a871fc-ba92-4128-a194-6f1bab5c3ab7/Catalase-Test-Protocol.pdf (accessed on 1 March 2021).
- Patricia, S.; Laura, C. Oxidae Test Protocol; American Society of Microbiology: Washington, DC, USA, 2010; pp. 1–9. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts: Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cockin, E.C.; Ricketts, R.E. The determination of amino acid with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Parker, R.E. Continuous distribution: Tests of significance. In Introductory Statistics for Biology, 2nd ed.; Arnold BS Ed: London, UK, 1979; pp. 18–42. [Google Scholar]
- Khan, A.; Sayyed, R.Z.; Seifi, S. Rhizobacteria: Legendary Soil Guards in Abiotic Stress Management. In Plant, Plant Growth Promoting Rhizobacteria for Sustainable Stress Management Vol 1 Abiotic Stress Management; Sayyed, R.Z., Arora, N.K., Reddy, M.S., Eds.; Springer: Singapore, 2019; Volume 1, pp. 327–342. [Google Scholar]
- Oren, A. Microbial life at high salt concentrations: Phylogenetic and metabolic diversity. Saline Syst. 2008, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Kulkarni, J.; Jha, B. Halotolerant Rhizobacteria Promote Growth and Enhance Salinity Tolerance in Peanut. Front. Microbiol. 2016, 7, 1600. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Jha, P.; Jha, P.N. The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J. Plant Physiol. 2015, 184, 57–67. [Google Scholar] [CrossRef]
- Shahid, S.; Kim, G.; Johnson, N. MicroRNAs from the parasitic plant Cuscutacampestris target host messenger RNAs. Nature 2018, 553, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation, and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef] [Green Version]
- Kruasuwan, W.; Thamchaipenet, A. 1-Aminocyclopropane-1-carboxylate (ACC) Deaminase-Producing Endophytic Diazotrophic Enterobacter sp. EN-21 Modulates Salt–Stress Response in Sugarcane. J. Plant Growth Regul. 2018, 37, 849–858. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Shahzad, R.; Kang, S.-M.; Seo, C.-W.; Park, Y.-G.; Park, H.-J.; Lee, I.-J. IAA-producing Klebsiella variicola AY13 reprograms soybean growth during flooding stress. J. Crop. Sci. Biotechnol. 2017, 20, 235–242. [Google Scholar] [CrossRef]
- Mandal, A.K.; Yadav, K.K.; Sen, I.K.; Kumar, A.; Chakraborti, S.; Islam, S.S.; Chakraborty, R. Partial characterization and flocculating behavior of an exopolysaccharide produced in nutrient-poor medium by a facultative oligotroph Klebsiella sp. PB12. J. Biosci. Bioeng. 2013, 115, 76–81. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Martínez, L.; Silva, J.; Martínez-Romero, E. Klebsiella variicola, A Novel Species with Clinical and Plant-Associated Isolates. Syst. Appl. Microbiol. 2004, 27, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Medina, N.; Barrios-Camacho, H.; Duran-Bedolla, J.; Garza-Ramos, U. Klebsiella variicola: An emerging pathogen in humans. Emerg. Microbes Infect. 2019, 8, 973–988. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.P.; Jha, P.; Jha, P.N. Bio-inoculation of plant growth-promoting rhizobacterium Enterobacter cloacae ZNP-3 increased resistance against salt and temperature stresses in wheat plant (Triticum aestivum L.). J. Plant Growth Regul. 2017, 36, 783–798. [Google Scholar] [CrossRef]
- Villamizar, G.A.C.; Nacke, H.; Boehning, M.; Herz, K.; Daniel, R. Functional Metagenomics Reveals an Overlooked Diversity and Novel Features of Soil-Derived Bacterial Phosphatases and Phytases. mBio 2019, 10, e01966-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinsinger, P.; Herrmann, L.; Lesueur, D.; Agnes, R.; Jean, T.; Kittima, W.; Claude, R. Impact of roots, microorganisms, and microfauna on the fate of soil phosphorus in the rhizosphere. Ann. Plant Rev. Online 2018, 377–407. [Google Scholar] [CrossRef]
- Kusale, S.P.; Attar, Y.C. Optimization and production of phytase by Bacillus subtilis and its agricultural application. Intl. J. Res. Appl. Sci. Eng. Technol. 2017, 5, 2321–9653. [Google Scholar]
- Kaur, G.; Reddy, M.S. Improvement of crop yield by phosphate solubilizing Aspergillus species in organic farming. Arch. Agron. Soil Sci. 2016, 63, 24–34. [Google Scholar] [CrossRef]
- Dardanelli, M.S.; de Córdoba, F.J.F.; Espuny, M.R.; Carvajal, M.A.R.; Díaz, M.E.S.; Gil Serrano, A.M.; Okon, Y.; Megías, M. Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol. Biochem. 2008, 40, 2713–2721. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved Salinity Tolerance of Arachis hypogaea (L.) by the Interaction of Halotolerant Plant-Growth-Promoting Rhizobacteria. J. Plant Growth Regul. 2012, 31, 195–206. [Google Scholar] [CrossRef]
- Vaishnav, A.; Kumari, S.; Jain, S.M.; Varma, A.; Choudhary, D.K. Putative bacterial volatile-mediated growth in soybean (Glycine max L. Merrill) and expression of induced proteins under salt stress. J. Appl. Microbiol. 2015, 119, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Berge, S.H.; Mahmood, O.T. Inoculating wheat seedling with exopolysaccharides-producing bacteria restrict sodium uptake and stimulates plant growth under salt stress. Biol. Fertil. Soils 2004, 40, 157–162. [Google Scholar] [CrossRef]
- Upadhyay, S.; Singh, J.; Singh, D. Exopolysaccharide-Producing Plant Growth-Promoting Rhizobacteria under Salinity Condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Kumari, S.; Vaishnav, A.; Jain, S.; Varma, A.; Choudhary, D.K. Bacterial-Mediated Induction of Systemic Tolerance to Salinity with Expression of Stress Alleviating Enzymes in Soybean (Glycine max L. Merrill). J. Plant Growth Regul. 2015, 34, 558–573. [Google Scholar] [CrossRef]
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in Phototrophic Biofilms and in Complex Microbial Mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Kim, M.-S.; Sun, Y.; Dowd, S.E.; Shi, H.; Paré, P.W. Soil Bacteria Confer Plant Salt Tolerance by Tissue-Specific Regulation of the Sodium Transporter HKT1. Mol. Plant-Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Kloepper, J.W.; Ryu, C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Balaban, N.P.; Suleimanova, A.D.; Valeeva, L.R.; Chastukhina, I.B.; Rudakova, N.L.; Sharipova, M.R.; Shakirov, E.V. Microbial Phytases and Phytate: Exploring Opportunities for Sustainable Phosphorus Management in Agriculture. Am. J. Mol. Biol. 2017, 7, 11–29. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth-promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]






| Antibiotics | Symbol | Diameter Zone (mm) | Sensitivity/Resistivity | Diameter Zone (mm) | Antibiotics | Symbol | Diameter Zone(mm) | Sensitivity/Resistivity | Diameter Zone(mm) |
|---|---|---|---|---|---|---|---|---|---|
| Amikacin | AK | 14–17 | S | 20 | Co-Trimoxazole | COT | 10–16 | S | 25 |
| Amoxyclav | AMC | 13–18 | INT | 18 | Doxycycline HCL | DO | 12–16 | INT | 16 |
| Ampicillin | A/S | 11–15 | S | 22 | Ertapenem | Etp | 18–22 | INT | 20 |
| Azithromycin | AZM | 13–18 | INT | 18 | Gentamicin | GEN | 12–15 | S | 21 |
| Carbenicillin | CB | 19–23 | INT | 23 | Imepenem | IPM | 13–16 | S | 24 |
| Cefaperazone | SCF | 14–20 | S | 32 | Levofloxacin | LE | 13–17 | INT | 14 |
| Cefazolin | CZ | 14–18 | S | 27 | Linezolid | LZ | 20–21 | INT | 21 |
| Cefepime | CPM | 14–18 | S | 25 | Meropenem | MEM | 13–16 | S | 24 |
| Cefpirome | CFP | 19–23 | S | 28 | Norfloxacin | NOR | 12–17 | S | 20 |
| Ceftazidime | CAZ | 14–18 | S | 28 | Novobiocin | NV | 17–12 | INT | 16 |
| Ceftazidime | CTZ | 14–18 | S | 28 | Ofloxacin | OFX | 12–16 | S | 22 |
| Ceftizoxime | CZX | 14–20 | S | 24 | Piperacillin/Tazo. | PIT | 17–21 | S | 28 |
| Ceftriaxone | CFS | 13–18 | S | 32 | Polymyxin B | PB | 8–12 | INT | 12 |
| Cefuroxime | CXM | 14–18 | R | 10 | Teicoplanin | TEI | 10–14 | R | 10 |
| Cephadroxil | CFR | 12–18 | R | 11 | Tetracycline | TE | 14–19 | S | 22 |
| Chloramphenicol | C | 13–17 | S | 19 | Ticarcillin | TI | 14–20 | R | 10 |
| Ciprofloxacin | CIP | 15–21 | S | 36 | Tigecycline | TGC | 7–13 | S | 22 |
| Clindamycin | CD | 14–21 | R | 14 | Tobramycin | TOB | 12–15 | INT | 15 |
| Colistin | CL | 10–11 | S | 16 | Ceftriaxone | CTR | 18–22 | S | 32 |
| Nalidixic Acid | NA | 14–18 | S | 26 | Cefixime | CFM | 16–18 | S | 32 |
| Nitrofurointoin | NIT | 12–14 | R | 11 | Ceftazidime/clavu | CAC | 18–20 | S | 24 |
| Mineral Content | Seedling | DAS | Before Sowing | After Sowing | |||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||||
| Total Nitrogen (%) | Wheat | 30 | 2.01 ± 0.02a | 1.35 ± 0.03d | 2.99 ± 0.06a | 2.31 ± 0.02a | 3.91 ± 0.08e |
| 45 | 3.03 ± 0.05b | 2.19 ± 0.0e | 1.67 ± 0.02c | 3.78 ± 0.05d | 4.99 ± 0.07b | ||
| Maize | 30 | 2.81 ± 0.03c | 1.82 ± 0.06b | 3.15 ± 0.07d | 3.91 ± 0.03c | 4.01 ± 0.09d | |
| 45 | 3.94 ± 0.04c | 2.67 ± 0.07d | 4.27 ± 0.02d | 4.87 ± 0.04c | 6.22 ± 0.04a | ||
| P (%) | Wheat | 30 | 1.9 ± 0.04b | 0.99 ± 0.05b | 2.01 ± 0.02d | 2.8 ± 0.04b | 2.61 ± 0.06a |
| 45 | 2.5 ± 0.03d | 1.52 ± 0.04d | 3.12 ± 0.07a | 3.4 ± 0.03d | 3.81 ± 0.03b | ||
| Maize | 30 | 1.87 ± 0.05d | 1.05 ± 0.03b | 2.91 ± 0.06c | 2.79 ± 0.05d | 3.41 ± 0.02d | |
| 45 | 2.78 ± 0.06d | 1.99 ± 0.08c | 3.66 ± 0.04d | 4.82 ± 0.06d | 4.89 ± 0.04a | ||
| Na (%) | Wheat | 30 | 1.12 ± 0.02d | 0.85 ± 0.06d | 1.99 ± 0.04c | 2.92 ± 0.02c | 2.85 ± 0.11d |
| 45 | 2.08 ± 0.03b | 1.85 ± 0.03d | 2.88 ± 0.02d | 4.48 ± 0.03b | 3.89 ± 0.13a | ||
| Maize | 30 | 2.01 ± 0.05c | 1.42 ± 0.02b | 2.99 ± 0.06d | 3.11 ± 0.05c | 3.32 ± 0.15c | |
| 45 | 2.31 ± 0.06c | 1.32 ± 0.05c | 3.01 ± 0.12a | 4.42 ± 0.06b | 3.12 ± 0.09a | ||
| K (%) | Wheat | 30 | 1.32 ± 0.04b | 0.99 ± 0.03c | 2.01 ± 0.07c | 2.41 ± 0.04b | 3.21 ± 0.07c |
| 45 | 2.27 ± 0.01b | 1.97 ± 0.02d | 3.32 ± 0.03b | 4.87 ± 0.01b | 4.31 ± 0.05a | ||
| Maize | 30 | 2.31 ± 0.06b | 1.42 ± 0.06b | 2.99 ± 0.08a | 3.42 ± 0.06b | 3.32 ± 0.04b | |
| 45 | 2.31 ± 0.05b | 1.42 ± 0.04b | 2.99 ± 0.02a | 4.54 ± 0.05b | 3.32 ± 0.08b | ||
| Mg (%) | Wheat | 30 | 0.51 ± 0.05a | 0.11 ± 0.02c | 1.01 ± 0.07b | 0.91 ± 0.05a | 1.51 ± 0.06e |
| 45 | 0.71 ± 0.08b | 0.11 ± 0.06c | 0.75 ± 0.04d | 1.02 ± 0.08b | 1.99 ± 0.02e | ||
| Maize | 30 | 0.97 ± 0.03d | 0.48 ± 0.03b | 1.75 ± 0.06a | 1.27 ± 0.03d | 1.99 ± 0.05e | |
| 45 | 1.75 ± 0.07d | 0.98 ± 0.05d | 2.61 ± 0.08c | 4.94 ± 0.07a | 2.87 ± 0.04a | ||
| Before Sowing | After Sowing | ||||
|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||
| Electrical conductivity (dS/m) | 4.0±0.10a | 3.9± 0.03e | 4.2 ± 0.09d | 4.7 ± 0.07c | 5.7 ± 0.11b |
| pH | 9.2± 0.11a | 7.7± 0.11g | 7.3 ± 0.09d | 6.9 ± 0.10f | 6.8 ± 0.12o |
| Organic carbon (%) | 0.93± 0.12c | 0.93 ± 0.12c | 1.51 ± 0.13b | 0.43 ± 0.11e | 2.91 ± 0.14c |
| Available nitrogen (Kg/ha) | 50.87 ± 0.13d | 50.87 ± 0.13d | 68.57 ± 0.12d | 25.87 ± 0.11b | 98.57 ± 0.10c |
| Available P (Kg/ha) | 26.16 ± 0.14d | 26.16 ± 0.14a | 27.21 ± 0.11c | 17.16 ± 0.12a | 47.21 ± 0.13b |
| Available K (Kg/ha) | 44.11 ± 0.13a | 44.11 ± 0.13c | 56.12 ± 0.10d | 34.22 ± 0.13c | 78.12 ± 0.12b |
| Available Ca++ (ppm) | 1650 ± 0.09d | 1650 ± 0.09c | 2640 ± 0.09d | 1230 ± 0.12a | 6400 ± 0.13c |
| Available Mg++ (ppm) | 400 ± 0.05d | 400 ± 0.05c | 900 ± 0.08d | 300 ± 0.10b | 1254 ± 0.05c |
| Available S++ (ppm) | 21.67 ± 0.04c | 21.67 ± 0.04d | 39.68 ± 0.07a | 15.67 ± 0.11d | 51.68 ± 0.10a |
| Available Fe++ (ppm) | 11.1 ± 0.02c | 11.1 ± 0.02c | 15.5 ± 0.06b | 8.1 ± 0.12 a | 17.5 ± 0.09c |
| Available Mn++ (ppm) | 19.7 ± 0.03a | 19.7 ± 0.03c | 12.5 ± 0.08c | 7.7 ± 0.13d | 25.5 ± 0.07b |
| Available Zn++ (ppm) | 6.81 ± 0.09a | 6.81 ± 0.09d | 8.32 ± 0.07c | 5.81 ± 0.10c | 8.32 ± 0.06d |
| Available Cu++ (ppm) | 4.45 ± 0.11b | 4.45 ± 0.11b | 6.85 ± 0.09c | 4.45 ± 0.13a | 7.22 ± 0.10d |
| Available B (ppm) | 0.05 ± 0.09a | 0.05 ± 0.09c | 0.28 ± 0.06c | 0.03 ± 0.11c | 1.35 ± 0.12b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusale, S.P.; Attar, Y.C.; Sayyed, R.Z.; El Enshasy, H.; Hanapi, S.Z.; Ilyas, N.; Elgorban, A.M.; Bahkali, A.H.; Marraiki, N. Inoculation of Klebsiella variicola Alleviated Salt Stress and Improved Growth and Nutrients in Wheat and Maize. Agronomy 2021, 11, 927. https://doi.org/10.3390/agronomy11050927
Kusale SP, Attar YC, Sayyed RZ, El Enshasy H, Hanapi SZ, Ilyas N, Elgorban AM, Bahkali AH, Marraiki N. Inoculation of Klebsiella variicola Alleviated Salt Stress and Improved Growth and Nutrients in Wheat and Maize. Agronomy. 2021; 11(5):927. https://doi.org/10.3390/agronomy11050927
Chicago/Turabian StyleKusale, Supriya P., Yasmin C. Attar, R. Z. Sayyed, Hesham El Enshasy, Siti Zulaiha Hanapi, Noshin Ilyas, Abdallah M. Elgorban, Ali H. Bahkali, and Najat Marraiki. 2021. "Inoculation of Klebsiella variicola Alleviated Salt Stress and Improved Growth and Nutrients in Wheat and Maize" Agronomy 11, no. 5: 927. https://doi.org/10.3390/agronomy11050927
APA StyleKusale, S. P., Attar, Y. C., Sayyed, R. Z., El Enshasy, H., Hanapi, S. Z., Ilyas, N., Elgorban, A. M., Bahkali, A. H., & Marraiki, N. (2021). Inoculation of Klebsiella variicola Alleviated Salt Stress and Improved Growth and Nutrients in Wheat and Maize. Agronomy, 11(5), 927. https://doi.org/10.3390/agronomy11050927