“Beyond the Source of Bioenergy”: Microalgae in Modern Agriculture as a Biostimulant, Biofertilizer, and Anti-Abiotic Stress
Abstract
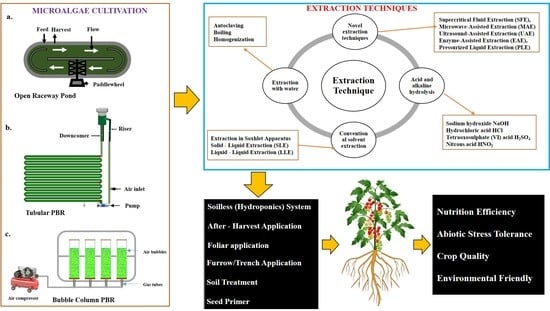
:1. Introduction
Biology of Microalgae
2. Growing Microalgae
2.1. Growth Parameters
2.2. The Biochemical Constituent of Microalgae
2.3. Production Schemes
2.3.1. Open Pond Systems
2.3.2. Racetrack System
2.3.3. Closed System (Photobioreactor)
3. Microalgae-Derived Extracts (Bioactive Compound and High-Value Product)
3.1. Extraction Methods of Microalgae Extract
3.1.1. Novel Techniques of Extraction
Supercritical Fluid Extraction (SFE)
Pressurized Liquid Extraction (PLE)
Microwave-Assisted Extraction (MAE)
Ultrasound-Assisted Extraction (UAE)
Enzyme-Assisted Extraction (EAE)
3.2. Chemical Constituents of Microalgae Extracts
3.3. Application Methods of Microalgae Extracts
4. Microalgae Extracts as Biostimulant and Biofertilizer
5. As Alleviator of Abiotic Stress
6. Future Direction
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelczar, M.J.; Chan, E.C.S.; Krieg, N.R. Microbiology: Concepts and Applications; McGraw-Hill: New York, NY, USA, 1993; Volume 182. [Google Scholar]
- Barsanti, L.; Gualtieri, P. Algae: Anatomy, Biochemistry, and Biotechnology; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Mutanda, T.; Ramesh, D.; Karthikeyan, S.; Kumari, S.; Anandraj, A.; Bux, F. Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour. Technol. 2011, 102, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, E.G.; Sigee, D.C. Freshwater Algae: Identification, Enumeration and Use as Bioindicators; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments Content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of Different Commercial Dried Algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Pereira, T.; Barroso, S.; Mendes, S.; Amaral, R.; Dias, J.R.; Baptista, T.; Saraiva, J.A.; Alves, N.; Gil, M.M. Optimization of phycobiliprotein pigments extraction from red algae Gracilaria gracilis for substitution of synthetic food colorants. Food Chem. 2020, 321, 126688. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravikumar, R.; Carvalho, I.S. Microalgae taxonomy and breeding. In Biofuel Crops: Production, Physiology and Genetics; CABI: Wallingford, UK, 2013; pp. 44–53. [Google Scholar] [CrossRef]
- Chapman, D. The Algae; Springer: Berlin/Heidelberg, Germany, 1973. [Google Scholar]
- Wehr, J.D.; Sheath, R.G.; Kociolek, J.P. Freshwater Algae of North America: Ecology and Classification; Academic Press: San Diego, CA, USA, 2015. [Google Scholar]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Candido, C.; Lombardi, A.T. Mixotrophy in green microalgae grown on an organic and nutrient rich waste. World J. Microbiol. Biotechnol. 2020, 36, 20. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.; Caetano, N. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Hultberg, M.; Carlsson, A.; Gustafsson, S. Treatment of drainage solution from hydroponic greenhouse production with microalgae. Bioresour. Technol. 2013, 136, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, M.; Jönsson, H.L.; Bergstrand, K.-J.; Carlsson, A. Impact of light quality on biomass production and fatty acid content in the microalga Chlorella vulgaris. Bioresour. Technol. 2014, 159, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Paliwal, C.; Ghosh, T.; Pancha, I.; Chokshi, K.; Mitra, M.; Ghosh, A.; Mishra, S. Applications of de-oiled microalgal biomass towards development of sustainable biorefinery. Bioresour. Technol. 2016, 214, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Correa, I.; Drews-Jr, P.; Botelho, S.; De Souza, M.S.; Tavano, V.M. Deep Learning for Microalgae Classification. In Proceedings of the 2017 16th IEEE International Conference on Machine Learning and Applications (ICMLA), Cancun, Mexico, 18–21 December 2017; pp. 20–25. [Google Scholar]
- Acién, F.G.; Gómez, C.; Morales-Amaral, M.M.; Fernandez, F.G.A.; Molina-Grima, E. Wastewater treatment using microalgae: How realistic a contribution might it be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 2016, 100, 9013–9022. [Google Scholar] [CrossRef]
- Hossain, N.; Mahlia, T.M.I. Progress in physicochemical parameters of microalgae cultivation for biofuel production. Crit. Rev. Biotechnol. 2019, 39, 835–859. [Google Scholar] [CrossRef]
- Lavens, P.; Sorgeloos, P. Manual on the Production and Use of Live Food for Aquaculture; FAO: Rome, Italy, 1993. [Google Scholar]
- Richmond, A.; Hu, Q. Handbook of microalgal culture: Applied Phycology and Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- FAO. Algal Production—Physical and Chemical Conditions (Culture Medium/Nutrients); FAO: Rome, Italy, 2013. [Google Scholar]
- Enzing, C.; Ploeg, M.; Barbosa, M.; Sijtsma, L. Microalgae-Based Products for the Food And Feed Sector: An Outlook for Europe; JRC Scientific and Policy Reports; European Comission: Brussels, Belgium, 2014; pp. 19–37. [Google Scholar]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge University Press: Cambridge, UK, 1994; Volume 10. [Google Scholar]
- Kaliaperumal, N.; Ramalingam, J.R.; Kalimuthu, S.; Ezhilvalavan, R. Seasonal changes in growth, biochemical constituents and phycocolloid of some marine algae of Mandapam coast. Seaweed Res. Util. 2002, 24, 73–77. [Google Scholar]
- Ha, G.-S.; El-Dalatony, M.M.; Kim, D.-H.; Salama, E.-S.; Kurade, M.B.; Roh, H.-S.; Abomohra, A.E.-F.; Jeon, B.-H. Biocomponent-based microalgal transformations into biofuels during the pretreatment and fermentation process. Bioresour. Technol. 2020, 302, 122809. [Google Scholar] [CrossRef]
- Machana, K.; Kanokrung, A.; Srichan, S.; Vongsak, B.; Kutako, M.; Siafha, E. Monitoring of Biochemical Compounds and Fatty Acid in Marine Microalgae from the East Coast of Thailand. Walailak J. Sci. Technol. 2018, 17, 334–347. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhang, Y.; Qi, X. Biogas from microalgae: Technologies, challenges and opportunities. Renew. Sustain. Energy Rev. 2020, 117, 109503. [Google Scholar] [CrossRef]
- Priyadarshani, I.; Rath, B. Commercial and industrial applications of micro algae—A review. J. Algal Biomass Util. 2012, 3, 89–100. [Google Scholar]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Show, P.L.; Tang, M.S.Y.; Nagarajan, D.; Ling, T.C.; Ooi, C.-W.; Chang, J.-S. A Holistic Approach to Managing Microalgae for Biofuel Applications. Int. J. Mol. Sci. 2017, 18, 215. [Google Scholar] [CrossRef] [Green Version]
- Petruk, G.; Gifuni, I.; Illiano, A.; Roxo, M.; Pinto, G.; Amoresano, A.; Marzocchella, A.; Piccoli, R.; Wink, M.; Olivieri, G.; et al. Simultaneous production of antioxidants and starch from the microalga Chlorella sorokiniana. Algal Res. 2018, 34, 164–174. [Google Scholar] [CrossRef]
- Dasan, Y.K.; Lam, M.K.; Yusup, S.; Lim, J.W.; Lee, K.T. Life cycle evaluation of microalgae biofuels production: Effect of cultivation system on energy, carbon emission and cost balance analysis. Sci. Total Environ. 2019, 688, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Yaakob, M.; Mohamed, R.; Al-Gheethi, A.; Ravishankar, G.; Ambati, R. Influence of Nitrogen and Phosphorus on Microalgal Growth, Biomass, Lipid, and Fatty Acid Production: An Overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Roy, S.S.; Pal, R. Microalgae in Aquaculture: A Review with Special References to Nutritional Value and Fish Dietetics. In Proceedings of the Zoological Society; Springer: Berlin/Heidelberg, Germany, 2015; Volume 68, pp. 1–8. [Google Scholar]
- Satyanarayana, K.G.; Mariano, A.B.; Vargas, J.V.C. A review on microalgae, a versatile source for sustainable energy and materials. Int. J. Energy Res. 2011, 35, 291–311. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.; Skill, S.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Ajeej, A.; Thanikal, J.V.; Narayanan, C.M.; Kumar, R.S. An overview of bio augmentation of methane by anaerobic co-digestion of municipal sludge along with microalgae and waste paper. Renew. Sustain. Energy Rev. 2015, 50, 270–276. [Google Scholar] [CrossRef]
- Demirbas, A.; Demirbas, M.F. Importance of algae oil as a source of biodiesel. Energy Convers. Manag. 2011, 52, 163–170. [Google Scholar] [CrossRef]
- Lee, Y.-K. Microalgal mass culture systems and methods: Their limitation and potential. J. Appl. Phycol. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- García, L.M.; Adjallé, K.; Barnabé, S.; Raghavan, G. Microalgae biomass production for a biorefinery system: Recent advances and the way towards sustainability. Renew. Sustain. Energy Rev. 2017, 76, 493–506. [Google Scholar] [CrossRef]
- Olaizola, M. Commercial development of microalgal biotechnology: From the test tube to the marketplace. Biomol. Eng. 2003, 20, 459–466. [Google Scholar] [CrossRef]
- Junying, Z.; Junfeng, R.; Baoning, Z. Factors in mass cultivation of microalgae for biodiesel. Chin. J. Catal. 2013, 34, 80–100. [Google Scholar]
- Carlsson, A.S.; Van Beilen, J.B.; Möller, R.; Clayton, D. Micro-and Macro-Algae: Utility for Industrial Applications; CPL Press: Berkshire, UK, 2007. [Google Scholar]
- Costa, J.A.V.; Freitas, B.C.B.; Santos, T.D.; Mitchell, B.G.; de Morais, M.G. Open pond systems for microalgal culture In. Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 199–223. [Google Scholar]
- Vonshak, A.; Richmond, A. Mass production of the blue-green alga Spirulina: An overview. Biomass 1988, 15, 233–247. [Google Scholar] [CrossRef]
- Razzak, S.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; de Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Qin, L.; Alam, A.; Wang, Z. Open Pond Culture Systems and Photobioreactors for Microalgal Biofuel Production. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2019; pp. 45–74. [Google Scholar]
- Benemann, J.R.; Oswald, W.J. Systems and Economic Analysis of Microalgae Ponds For Conversion of CO2 to Biomass; Nasa Sti/recon Technical Report N; US Department of Energy: Washington, DC, USA, 1994; p. 95.
- Singh, N.K.; Dhar, D.W. Microalgae as second generation biofuel. A review. Agron. Sustain. Dev. 2011, 31, 605–629. [Google Scholar] [CrossRef] [Green Version]
- Han, T.; Lu, H.F.; Ma, S.S.; Zhang, Y.H.; Liu, Z.D.; Duan, N. Progress in microalgae cultivation photobioreactors and applications in wastewater treatment: A review. Int. J. Agric. Biol. Eng. 2017, 10, 1–29. [Google Scholar]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.-S. Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: A review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Tredici, M.R. Mass production of microalgae: Photobioreactors. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley-Blackwell: Hoboken, NJ, USA, 2004; Volume 1, pp. 178–214. [Google Scholar]
- Pulz, O. Photobioreactors: Production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 2001, 57, 287–293. [Google Scholar] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Sharma, S. Development of suitable photobioreactor for algae production—A review. Renew. Sustain. Energy Rev. 2012, 16, 2347–2353. [Google Scholar] [CrossRef]
- Janssen, M.; Tramper, J.; Mur, L.R. Enclosed outdoor photobioreactors: Light regime, photosynthetic efficiency, scale-up, and future prospects. Biotechnol. Bioeng. 2002, 81, 193–210. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Vasumathi, K.; Premalatha, M.; Subramanian, P. Parameters influencing the design of photobioreactor for the growth of microalgae. Renew. Sustain. Energy Rev. 2012, 16, 5443–5450. [Google Scholar] [CrossRef]
- Wang, B.; Lan, C.Q.; Horsman, M. Closed photobioreactors for production of microalgal biomasses. Biotechnol. Adv. 2012, 30, 904–912. [Google Scholar] [CrossRef]
- Aishvarya, V.; Pradhan, N.; Nayak, R.R.; Sukla, L.B.; Mishra, B.K. Enhanced inorganic carbon uptake by Chlorella sp. IMMTCC-2 under autotrophic conditions for lipid production and CO2 sequestration. Environ. Biol. Fishes 2012, 24, 1455–1463. [Google Scholar] [CrossRef]
- Slegers, P.; Lösing, M.; Wijffels, R.; van Straten, G.; van Boxtel, A. Scenario evaluation of open pond microalgae production. Algal Res. 2013, 2, 358–368. [Google Scholar] [CrossRef]
- Harun, R.; Singh, M.; Forde, G.M.; Danquah, M.K. Bioprocess engineering of microalgae to produce a variety of consumer products. Renew. Sustain. Energy Rev. 2010, 14, 1037–1047. [Google Scholar] [CrossRef]
- Barbosa, M.J.; Albrecht, M. Hydrodynamic stress and lethal events in sparged microalgae cultures. Biotechnol. Bioeng. 2003, 83, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; Alvim-Ferraz, M.D.C.; Martins, F. Photobioreactor design for microalgae production through computational fluid dynamics: A review. Renew. Sustain. Energy Rev. 2017, 79, 248–254. [Google Scholar] [CrossRef]
- Eriksen, N.T. The technology of microalgal culturing. Biotechnol. Lett. 2008, 30, 1525–1536. [Google Scholar] [CrossRef]
- Das, P.; Aziz, S.S.; Obbard, J.P. Two phase microalgae growth in the open system for enhanced lipid productivity. Renew. Energy 2011, 36, 2524–2528. [Google Scholar] [CrossRef]
- Suali, E.; Sarbatly, R. Conversion of microalgae to biofuel. Renew. Sustain. Energy Rev. 2012, 16, 4316–4342. [Google Scholar] [CrossRef]
- Norsker, N.-H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Acién, F.; Molina, E.; Reis, A.; Torzillo, G.; Zittelli, G.; Sepúlveda, C.; Masojídek, J. Photobioreactors for the production of microalgae. In Microalgae-Based Biofuels and Bioproducts; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 1–44. [Google Scholar]
- Suparmaniam, U.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T.; Shuit, S.H. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 2019, 115, 109361. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Dmytryk, A.; Wilk, R.; Gramza, M.; Rój, E. Evaluation of Supercritical Extracts of Algae as Biostimulants of Plant Growth in Field Trials. Front. Plant Sci. 2016, 7, 1591. [Google Scholar] [CrossRef] [Green Version]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Abu-Ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Lafarga, T. Cultured Microalgae and Compounds Derived Thereof for Food Applications: Strain Selection and Cultivation, Drying, and Processing Strategies. Food Rev. Int. 2020, 36, 559–583. [Google Scholar] [CrossRef]
- Subashini, G.; Bhuvaneswari, S. Novel Products from Microalgae. In Bioactive Natural Products in Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 451–465. [Google Scholar]
- Samarasinghe, N.; Fernando, S.; Faulkner, W.B. Effect of high pressure homogenization on aqueous phase solvent extraction of lipids from Nannochloris oculata microalgae. J. Energy Nat. Resour. 2012, 1, 1–7. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.; Rouphael, Y. Renewable Sources of Plant Biostimulation: Microalgae as a Sustainable Means to Improve Crop Performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [Green Version]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Ramluckan, K.; Moodley, K.G.; Bux, F. An evaluation of the efficacy of using selected solvents for the extraction of lipids from algal biomass by the soxhlet extraction method. Fuel 2014, 116, 103–108. [Google Scholar] [CrossRef]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and Characterization of Bioactive Compounds with Health Benefits from Marine Resources: Macro and Micro Algae, Cyanobacteria, and Invertebrates. In Marine Bioactive Compounds; Hayes, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 55–98. [Google Scholar]
- Kadam, S.; Tiwari, B.K.; O’Donnell, C. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Sovova, H. Steps of supercritical fluid extraction of natural products and their characteristic times. J. Supercrit. Fluids 2012, 66, 73–79. [Google Scholar] [CrossRef]
- Herrero, M.; Sánchez-Camargo, A.D.P.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Zinnai, A.; Sanmartin, C.; Taglieri, I.; Andrich, G.; Venturi, F. Supercritical fluid extraction from microalgae with high content of LC-PUFAs. A case of study: Sc-CO2 oil extraction from Schizochytrium sp. J. Supercrit. Fluids 2016, 116, 126–131. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Mendiola, J.A.; Santoyo, S.; Cifuentes, A.; Reglero, G.; Ibanez, E.; Señoráns, F.J. Antimicrobial Activity of Sub- and Supercritical CO2 Extracts of the Green Alga Dunaliella salina. J. Food Prot. 2008, 71, 2138–2143. [Google Scholar] [CrossRef]
- Steytler, D. Supercritical fluid extraction and its application in the food industry. In Separation Processes in the Food and Biotechnology Industries: Principles and Applications; Woodhead Publishing: Sawston, UK, 1996; p. 17. [Google Scholar]
- Schütz, E. Supercritical fluids and applications—A patent review. In Chemical Engineering & Technology: Industrial Chemistry-Plant Equipment-Process Engineering-Biotechnology; Wiley-VCH GmbH: Weinheim, Germany, 2007; Volume 6, pp. 685–688. [Google Scholar]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibanez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, B.; Jones, B.A.; Ezzell, A.J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Nieto, A.; Borrull, F.; Pocurull, E.; Marcé-Recasens, R.M. Pressurized liquid extraction: A useful technique to extract pharmaceuticals and personal-care products from sewage sludge. TrAC Trends Anal. Chem. 2010, 29, 752–764. [Google Scholar] [CrossRef]
- Shang, Y.F.; Kim, S.M.; Lee, W.J.; Um, B.-H. Pressurized liquid method for fucoxanthin extraction from Eisenia bicyclis (Kjellman) Setchell. J. Biosci. Bioeng. 2011, 111, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Cha, K.H.; Song, D.-G.; Chung, D.; Pan, C.-H. Optimization of pressurized liquid extraction of zeaxanthin from Chlorella ellipsoidea. Environ. Biol. Fishes 2011, 24, 725–730. [Google Scholar] [CrossRef]
- Ganzler, K.; Salgó, A.; Valkó, K. Microwave extraction: A novel sample preparation method for chromatography. J. Chromatogr. A 1986, 371, 299–306. [Google Scholar] [CrossRef]
- Ohring, M.; Kasprzak, L. Reliability and Failure of Electronic Materials and Devices; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Ying, Z.; Han, X.; Li, J. Ultrasound-assisted extraction of polysaccharides from mulberry leaves. Food Chem. 2011, 127, 1273–1279. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Microwave-Assisted Extraction of Flavonoids: A Review. Food Bioprocess Technol. 2011, 5, 409–424. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef]
- Pasquet, V.; Chérouvrier, J.-R.; Farhat, F.; Thiéry, V.; Piot, J.M.; Bérard, J.-B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.-P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process. Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Jasso, R.M.; Mussatto, S.I.; Pastrana, L.; Aguilar, C.N.; Teixeira, J.A. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed. Carbohydrate. Polymers 2011, 86, 1137–1144. [Google Scholar]
- Wang, B.; Tong, G.Z.; Le Qu, Y.; Li, L. Microwave-Assisted Extraction and In Vitro Antioxidant Evaluation of Polysaccharides from Enteromorpha prolifera. Appl. Mech. Mater. 2011, 79, 204–209. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Zhang, Q.; Qu, Y.; Xu, H.; Li, G. Preparation and antioxidant property of extract and semipurified fractions of Caulerpa racemosa. Environ. Biol. Fishes 2012, 24, 1527–1536. [Google Scholar] [CrossRef]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-J.; Wijesinghe, W.A.J.P. Enzyme-Assisted Extraction and Recovery of Bioactive Components from Seaweeds; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 221–228. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, Q.; Cong, W. Enzyme-Assisted Aqueous Extraction of Lipid from Microalgae. J. Agric. Food Chem. 2012, 60, 11771–11776. [Google Scholar] [CrossRef]
- Puglisi, I.; Barone, V.; Sidella, S.; Coppa, M.; Broccanello, C.; Gennari, M.; Baglieri, A. Biostimulant activity of humic-like substances from agro-industrial waste on Chlorella vulgaris and Scenedesmus quadricauda. Eur. J. Phycol. 2018, 53, 433–442. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Kaleem, I.; Chun, L.; Tong, J. Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy 2010, 35, 5406–5411. [Google Scholar] [CrossRef]
- Maddi, B.; Viamajala, S.; Varanasi, S. Comparative study of pyrolysis of algal biomass from natural lake blooms with lignocellulosic biomass. Bioresour. Technol. 2011, 102, 11018–11026. [Google Scholar] [CrossRef]
- Anand, V.; Gautam, R.; Vinu, R. Non-catalytic and catalytic fast pyrolysis of Schizochytrium limacinum microalga. Fuel 2017, 205, 1–10. [Google Scholar] [CrossRef]
- Li, B.; Yang, L.; Wang, C.-Q.; Zhang, Q.-P.; Liu, Q.-C.; Li, Y.-D.; Xiao, R. Adsorption of Cd(II) from aqueous solutions by rape straw biochar derived from different modification processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.; Batista, F.; Lira, T.; Barrozo, M.; Vieira, L. Characterization and product formation during the catalytic and non-catalytic pyrolysis of the green microalgae Chlamydomonas reinhardtii. Renew. Energy 2017, 119, 731–740. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of microalgae: A critical review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Becker, E. Microalgae for Human and Animal Nutrition. In Handbook of Microalgae Culture: Applied Phycology and Biotechnology, 2nd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2013; pp. 461–503. [Google Scholar]
- Colla, G.; Svecova, E.B.; Cardarelli, M.; Rouphael, Y.; Reynaud, H.; Canaguier, R.; Planques, B. Effectiveness of a plant-derived protein hydrolysate to improve crop performances under different growing conditions. Acta Hortic. 2013, 175–179. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.B.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colla, G.; Rouphael, Y.; Lucini, L.; Canaguier, R.; Stefanoni, W.; Fiorillo, A.; Cardarelli, M. Protein hydrolysate-based biostimulants: Origin, biological activity and application methods. Acta Hortic. 2016, 1148, 27–34. [Google Scholar] [CrossRef]
- Bhalamurugan, G.L.; Valerie, O.; Mark, L. Valuable bioproducts obtained from microalgal biomass and their commercial applications: A review. Environ. Eng. Res. 2018, 23, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80. [Google Scholar] [CrossRef] [Green Version]
- Oancea, F.; Velea, S.; Fãtu, V.; Mincea, C.; Ilie, L. Microalgae-based plant biostimulant and its effect on water stressed tomato plants. Rom. J. Plant Prot. 2013, 6, 104–117. [Google Scholar]
- Dias, G.A.; Rocha, R.H.C.; Araújo, J.L.; Lima, J.F.; Guedes, W.A. Growth, yield, and postharvest quality in eggplant produced under different foliar fertilizer (Spirulina platensis) treatments. Semina 2016, 37, 3893. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gonzalez, J.; Sommerfeld, M.R. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. Environ. Biol. Fishes 2015, 28, 1051–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza, B.M.; Gómez-Serrano, C.; Fernandez, F.G.A.; Jimenez-Becker, S. Effect of microalgae hydrolysate foliar application (Arthrospira platensis and Scenedesmus sp.) on Petunia × hybrida growth. Environ. Biol. Fishes 2018, 30, 2359–2365. [Google Scholar] [CrossRef]
- Berry, Z.C.; Emery, N.C.; Gotsch, S.G.; Goldsmith, G.R. Foliar water uptake: Processes, pathways, and integration into plant water budgets. Plant. Cell Environ. 2018, 42, 410–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppens, J.; Grunert, O.; Hende, S.V.D.; Vanhoutte, I.; Boon, N.; Haesaert, G.; De Gelder, L. The use of microalgae as a high-value organic slow-release fertilizer results in tomatoes with increased carotenoid and sugar levels. Environ. Biol. Fishes 2015, 28, 2367–2377. [Google Scholar] [CrossRef]
- Renuka, N.; Guldhe, A.; Prasanna, R.; Singh, P.; Bux, F. Microalgae as multi-functional options in modern agriculture: Current trends, prospects and challenges. Biotechnol. Adv. 2018, 36, 1255–1273. [Google Scholar] [CrossRef] [PubMed]
- De Morais, M.G.; Vaz, B.D.S.; de Morais, E.G.; Costa, J.A.V. Biologically active metabolites synthesized by microalgae. BioMed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowitzka, M. Chemically-Mediated Interactions in Microalgae; Springer: Berlin/Heidelberg, Germany, 2016; pp. 321–357. [Google Scholar] [CrossRef]
- Paudel, Y.; Pradhan, S.; Pant, B.; Prasad, B.N. Role of blue green algae in rice productivity. Agric. Biol. J. N. Am. 2012, 3, 332–335. [Google Scholar] [CrossRef]
- Shalaby, T.A.; El-Ramady, H. Effect of foliar application of bio-stimulants on growth, yield, components, and storability of garlic (‘Allium sativum’ L.). Aust. J. Crop. Sci. 2014, 8, 271. [Google Scholar]
- Tarraf, S.A.; Talaat, I.M.; El-Sayed, A.E.-K.B.; Balbaa, L.K. Influence of foliar application of algae extract and amino acids mixture on fenugreek plants in sandy and clay soils. Nusant. Biosci. 1970, 7, 33–37. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.-H.; Mahlia, T.M.I. State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Zhou, Q. Co-cultivation of Chlorella spp and tomato in a hydroponic system. Biomass Bioenergy 2017, 97, 132–138. [Google Scholar] [CrossRef]
- EL Arroussi, H.; Benhima, R.; Elbaouchi, A.; Sijilmassi, B.; EL Mernissi, N.; Aafsar, A.; Meftah-Kadmiri, I.; Bendaou, N.; Smouni, A. Dunaliella salina exopolysaccharides: A promising biostimulant for salt stress tolerance in tomato (Solanum lycopersicum). Environ. Biol. Fishes 2018, 30, 2929–2941. [Google Scholar] [CrossRef]
- Faheed, F.A.; Fattah, Z.A. Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J. Agric. Soc. Sci. 2008, 4, 165–169. [Google Scholar]
- Barone, V.; Baglieri, A.; Stevanato, P.; Broccanello, C.; Bertoldo, G.; Bertaggia, M.; Cagnin, M.; Pizzeghello, D.; Moliterni, V.M.C.; Mandolino, G.; et al. Root morphological and molecular responses induced by microalgae extracts in sugar beet (Beta vulgaris L.). Environ. Biol. Fishes 2017, 30, 1061–1071. [Google Scholar] [CrossRef]
- Shariatmadari, Z.; Riahi, H.; Hashtroudi, M.S.; Ghassempour, A.; Aghashariatmadary, Z. Plant growth promoting cyanobacteria and their distribution in terrestrial habitats of Iran. Soil Sci. Plant Nutr. 2013, 59, 535–547. [Google Scholar] [CrossRef]
- Dmytryk, A.; Roj, E.; Wilk, R.; Chojnacka, K. Innovative bioformulations for seed treatment. Preliminary assessment of functional properties in the initial plant growth phase. Przem. Chem. 2014, 93, 959–963. [Google Scholar]
- Al-Saman, M.A.; Farfour, S.A.; Hamouda, R.A. Effects of some red Algae on antioxidant and phytochemical contents of Maize (Zea mays L.) plants. Int. J. Agric. 2015, 5, 393–398. [Google Scholar]
- El-Eslamboly, A.A.S.A.; El-Wanis, M.M.A.; Amin, A.W. Algal application as a biological control method of root-knot nematode Meloidogyne incognita on cucumber under protected culture conditions and its impact on yield and fruit quality. Egypt. J. Biol. Pest Control 2019, 29, 18. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Sornchai, P.; Saithong, N.; Srichompoo, Y.; Unartngam, A.; Iamtham, S. Effect of Spirulina maxima aqueous extract on seed germination and seedling growth of mung bean, Vigna radiata and rice, Oryza sativa var. Japonica. J. ISSAAS 2014, 20, 77–84. [Google Scholar]
- Chanda, M.-J.; Redouane, B.; Najib, E.; Yassine, K.; Lyamlouli, K.; Laila, S.; Zeroual, Y. Screening of microalgae liquid extracts for their bio stimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Sci. Rep. 2020, 10, 2820. [Google Scholar]
- Enan, S.A.A.M.; El-Saady, A.M.; El-Sayed, A.B. Impact of foliar feeding with alga extract and boron on yield and quality of sugar beet grown in sandy soil. Egypt J. Agronematol. 2016, 38, 319–336. [Google Scholar]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Suzuki, N. Hormone signaling pathways under stress combinations. Plant Signal. Behav. 2016, 11, e1247139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.-J. Synergistic association of endophytic fungi enhances Glycine max L. resilience to combined abiotic stresses: Heavy metals, high temperature and drought stress. Ind. Crop. Prod. 2020, 143, 111931. [Google Scholar] [CrossRef]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and Drought Stresses in Crops and Approaches for Their Mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Cammalleri, C.; Naumann, G.; Mentaschi, L.; Formetta, G.; Forzieri, G.; Gosling, S.; Bisselink, B.; De Roo, A.; Feyen, L. Global Warming and Drought Impacts in the EU; European Comission: Brussels, Belgium, 2020. [Google Scholar]
- FAO. Damage and Losses from Climate-Related Disasters in Agricultural Sectors; FAO: Rome, Italy, 2016; Available online: http://www.fao.org/3/i6486e/i6486e.pdf (accessed on 6 July 2021).
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal Biostimulants and Biofertilisers in Crop Productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Vernieri, P.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. Application of biostimulants in floating system for improving rocket quality. J. Food Agric. Environ. 2005, 3, 86. [Google Scholar]
- Abd El-Baky, H.H.; El-Baz, F.K.; El Baroty, G.S. Enhancing antioxidant availability in wheat grains from plants grown under seawater stress in response to microalgae extract treatments. J. Sci. Food Agric. 2010, 90, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y. Microalgae: New Source of Plant Biostimulants. Agronomy 2020, 10, 1240. [Google Scholar] [CrossRef]





| ALGAE | Chlorophyll | Other Pigments | Organelle Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taxonomic Class | Size | a | b | c | Bili Protein | Carotenoids | Eukaryote | Mesokaryote | Prokaryote | Storage Product (s) |
| Bacillariophyceae | 5–2000 μm | ✓ | - | ✓ | Nil | β-Carotene, Fucoxanthin | ✓ | ✓ | ✓ | Lipids and Chrysolaminarin |
| Charophyceae | May exceed 30 cm in length | ✓ | ✓ | - | Nil | β-Carotene, Zeaxanthin, Lutein, Violaxanthin, Neoxanthin | ✓ | ✓ | ✓ | Starch |
| Chlorophyceae | 10–80 μm | ✓ | ✓ | - | Phytochrome | Zeaxanthin, Lutein, Violaxanthin, Neoxanthin, Loroxanthin. | ✓ | ✓ | ✓ | Lipids and Starch |
| Chrysophyceae | ca. 2 μm–ca. 2 mm | ✓ | - | ✓ | Nil | β-Carotene, fucoxanthin | ✓ | ✓ | ✓ | Lipids and Chrysolaminarin |
| Cryptophyceae | 10–50 μm | ✓ | - | ✓ | Phycoerythrin | α-Carotene, Alloxanthin, Crocoxanthin | ✓ | ✓ | ✓ | Starch |
| Cyanophyceae | Vary considerably in size | ✓ | - | ✓ | Allophycocyanin, c-Phycocyanin | Flavacene β-Carotene, | - | - | ✓ | Polyphosphate Phycobilins |
| Dinophyceae | 50–59 µm | ✓ | - | ✓ | Nil | β-Carotene, Diatoxanthin, Monadoxanthin, Dinoxanthin | - | ✓ | - | Starch (amylose or amylopectin) |
| Euglenophyceae | ~35 μm | ✓ | ✓ | - | Nil | β-Carotene, Diatoxanthin, Diadinoxanthin, Monadoxanthin | - | ✓ | - | Paramylon, β-1,3 polymer of glucose |
| Haptophyceae | 3–7.5 μm | ✓ | - | ✓ | Nil | β-Carotene, Diatoxanthin, Diadinoxanthin, Fucoxanthin | ✓ | ✓ | ✓ | Water-soluble 1–3 glucan chrysolaminarin |
| Phaeophyceae | Range of morphologies and sizes | ✓ | - | ✓ | Nil | β-Carotene, Fucoxanthin, Violaxanthin | ✓ | ✓ | ✓ | Luminaria, Lipids |
| Prasinophyceae | ca. 0.95 μm | ✓ | ✓ | - | Nil | β-Carotene, Micronone, Neoxanthin, Zeaxanthin, Lutein, Violaxanthin | ✓ | - | - | Starch |
| Rhodophyceae | maximum is ~50 cm | ✓ | - | - | Allophycocyanin, c-Phycocyanin, Phytochrome | β-Carotene, Diatoxanthin, Monadoxanthin, Dinoxanthin | ✓ | ✓ | ✓ | Floridean starch |
| Xanthophyceae | ca. 2 μm–ca. 2 mm | ✓ | - | ✓ | Nil | β-Carotene, Diatoxanthin, Diadinoxanthin, Heteroxanthin | ✓ | ✓ | ✓ | Lipids, chrysolaminarin. |
| Parameters | Temp °C | Salinity (g L−1) | Light Intensity (mmol m−2 s−1) | Photoperiod (Light: Dark, h) | pH |
|---|---|---|---|---|---|
| Range | 16–27 | 12.0–40 | 15–135 (depends on volume and density) | NR | 7.0–9.0 |
| Optimum | 18–24 | 20–24 | 40–70 | 16:8 (minimum) 24:0 (maximum) | 8.2–8.7 |
| Algae | Carbohydrate (%) | Lipid (%) | Protein (%) | References |
|---|---|---|---|---|
| Arthrospira platensis | 8–20 | 4–9 | 49–65 | [27,34] |
| Chlorella species | 12–30 | 10 | 30–35 | [34,35,36,37] |
| Scenedesmus species | 13–16 | 12–14 | 60–71 | [34,37,38] |
| Dunaliella species | 3–17 | 14–21 | 48–57 | [27,34] |
| Synechococcus species | 9–17 | 14–55 | 10–63 | [34,38] |
| Euglena species | 14–18 | 14–20 | 39–61 | [34] |
| Prymnesium species | 14–18 | 14–20 | 39–61 | [27,38] |
| Anabaena species | 25–30 | 9–14 | 24–29 | [27,35,38] |
| Chlamydomonas species | 2–17 | 9–21 | 28–56 | [27,34] |
| Porphyridium species | 40–57 | 9–14 | 28–45 | [27,38] |
| Arthrospira maxima | 13–13 | 6–7 | 60–71 | [27,34] |
| Spirogyra porticalis | 33–64 | 11–21 | 6–20 | [38] |
| Tetraselmis maculata | 15 | 3 | 52 | [27,38] |
| Pavlovaceae | 6–9 | 9–14 | 24–29 | [34] |
| Characteristics | Open System (Raceway) | Closed System (Photobioreactor) | References | ||
|---|---|---|---|---|---|
| Paddlewheel | Stirred Tank Reactor | Tubular Reactor | Column Reactor | ||
| Light use efficiency | Good | Good | Best | Good | [55,61] |
| Transfer of gas | Normal | Lower–higher | Lower–higher | Higher | [12,62] |
| Mixing potential | Partial uniformity | Nearly uniformity | Perfect/absolute mixing | Partial mixing | [54,63] |
| Control of species | Nil | Best | Good | Good | [55,64] |
| Loss through evaporation | High | Moderate | Nil | Nil | [61,65] |
| Quality of biomass | Variable | Reproducible | Reproducible | Reproducible | [54,57] |
| Energy demand for mixing | Low | High | High | High | [64,66,67] |
| Maintenance | Easy | Difficult | Difficult | Difficult | [62,64,67] |
| Required space | Large area | Moderate | Moderate | Moderate | [54,57,63] |
| Type of operation | Batch | Batch | Batch | Batch | [54,65,67] |
| Setup capital | Low | High | High | High | [64,67] |
| Limitations | Requires a huge area of land | Requires large setup capital | Possible formation of fouling/scale along the bend regions | High maintenance cost | [64,66,67] |
| Production Technology (USD) | Capital Costs kg−1 (USD) | Labour kg−1 (USD) | Other Variable Costs (Utilities, Fertilizer) kg−1 (USD) | Total Costs/kg for a Large (100 ha–200 ha) Plant (USD) | Optimal Theoretical Total Costs kg−1 Dry Weight (USD) | References |
|---|---|---|---|---|---|---|
| Open ponds | 3.58 | 0.18 | 1.86 | 25 (2004) 7.07 | 0.81 | [43,68,69] |
| 5.87 | 0.25 | |||||
| 8–11 | ||||||
| Horizontal tubular PBR | 3.25 | 1.04 | 1.09 | 4.92 | (NA) | [68,69] |
| 11.63 | 0.43 | 1.96 | 14.95 | |||
| Flat panel PBR | 12.38 | 0.42 | 1.20 | 7.07 | 2.14 | [68,69,70] |
| Crop | Greenhouse | M/Species | Extraction/Process Method | Conc. Of AE | Parameters | Reference |
|---|---|---|---|---|---|---|
| Lettuce | Soil | Chlorella vulgaris | Fresh and dried algal were applied in the field to vegetables | Biofertilizer—1/2, 1, 2, and 3 g of fresh algal and dry algal cells/1 kg soil Biomass | Chlorophyll a, b, and carotenoids. Plant growth (root dry wt. and length) | [138] |
| Tomato | Petri plates | Acutodesmus dimorphus | 1 kg of biomass freeze dried submerged in distilled water, DW (Conc. 150 g L−1) = the suspension + micro fluidizer (M-110EH-30) = intracellular extract. Intracellular extract + centrifugation (8989× g/10 min/22 °C). The collected supernatant in a flask covered with foil paper to reduce potential degradation was stored at 4 °C | Seed primers—different concentrations (0, 1, 5, 10, 25, 50, 75, and 100%) of aqueous cell extracts from DW OR 10 mL, 0.1/9.9 mL, 0.5/9.5 mL, 1/9 mL, 2.5/7.5 mL, 5/7.5 mL, 7.5/2.5 mL, 10 mL | Seed germination, germination energy, lateral root development, flower development | [125] |
| 3 types of vegetable—Chinese Cabbage, Chinese broccoli, and Protea White Crown. | Tissue towel | Arthrospira platensis | A desirable quantity of microalgae suspension (50 mL) was removed from growing flasks and then allowed to pass through centrifugation for a maximum of 10 min. The collected supernatants were examined to determine the level of ammonia, nitrate, and nitrite | Biofertilizer—seed germination study—Arthrospira biomass. T1 to T5, T0 (tap water only). (2, 4, 6, 8, and 10 g L−1, respectively) biomass | Rate of germination, root and shoot length, vigor index as well as dry weight of 100 seedlings | [144] |
| Arugula, Bayam Red, and Pak Choy plants | Potted plants experiment | Arthrospira platensis | A desirable quantity of microalgae suspension (50 mL) was removed from growing flasks. Then, it was allowed to pass through centrifugation for a maximum of 10 min. The collected supernatants were examined to determine the level of ammonia, nitrate, and nitrite | Biofertilizer—potted plants and control—Arthrospira platensis (5 g/500 g soil), inorganic fertilizer—Triple Pro 15/15/15 (3 × 10−1 g/500 g soil/week). Additionally, Arthrospira platensis + inorganic fertilizer (3 × 10−1 g/pot/week) biomass | Weekly measurement of plant growth (plant height and number of leaves per plant). After the completion of the experiment, parameters such as the number of leaves, the height of the plant, chlorophyll content, length of root, fresh, as well dry weights were determined. | [144] |
| Tomato | Potted plants experiment | Anabaena vaginicola ISC90 and Nostoc calcicola ISC89 | Harvested biomass—DW was used to wash the cells. The cell extraction was carried out by grinding algae with a pestle and blender in DW. The final extract made up of 5.0 g fresh algae as the raw material submerged in 500 mL of DW is assumed to be a 1% extract | The final extract application was conducted by spraying the potted treated soil while the control was irrigated with water every 7 days. The arrangement of pots was a complete randomized design in a fully controlled experimental greenhouse. 1% extract/spray | The morphological parameters measured after 40 days of the experiment include plant height, root length, dry and fresh weight of plant, as well as the number of leaves | [140] |
| Radish | Petri plates | BGA—Arthrospira platensis extract | Commercial dried biomass of SP used. Homogenate + centrifugation = supernatant considered to be 100% algal filtrate (1:10) | Foliar spray (5%, 7%, 10%, 15%, 20%, and 25%, v/v). Seed soaking—dose of 100, 300, 500, 700 μL per 1.5 g of seed | The longest and heaviest plant was observed at a dose of 300 μL/1.5 g seeds and 15% of filtrate as a foliar application. The chlorophyll content was higher at 100 μL/1.5 g seeds as well as 5% of filtrate as a foliar application. | [122] |
| Rice | Potted plants experiment | BGA—Arthrospira maxima extract | Extracts obtained from three types of solvent viz. DW, methanol, and hexane at 0, 2.5, 3.5, 4.5, and 5 g L−1 of biomass/solvent | The potted plants were treated with extracts at three different stages of seed development, the dry stage, the radicle emergence stage, and the vegetative growth stage | DW, methanol, and extracts affect the germination of seed while hexane reveals no impact on seed germination. | [145] |
| Wheat seeds | Petri plates | BGA—Arthrospira platensis extract | The seeds treated with extract were sown in a cotton base for the next 11 days, with nine replicates of each sample. | The coated seeds in three different doses (8, 14, and 20 μL g−1 of seeds) of formulation were used. Seed coated with 8 μL gave the best result | Seeds coated with the extract resulted in the increase in biomass yield by approx. 13% | [141] |
| Tomato | Soil | 18 Microalgae and Cyanobacteria species from the AlgoBioTech collection | Screening of microalgae liquid extracts | Application doses of 0.1, 0.5, and 1 g L−1 were tested | The effects on plant growth, chlorophyll content, and nutrient uptake were significant | [146] |
| Sugar beet | Hydroponic Hoagland solution | 1. Chlorella vulgaris 2. Scenedesmus quadricauda | Biomass of each species + was harvested by centrifugation + freeze-drying. The biomass + washed (distilled water)—final pellets + methanol (to lyse the cell wall) = intracellular extracts. Intracellular extracts + centrifugation + evaporation (organic solvent), the extract was collected with distilled water. | Growth promoter—2 mL L−1, 4 mL L−1 Extract/Hoagland | Root morphological analysis (total root length, root surface area, and the total number of root tips). Molecular analysis of root tissues | [139] |
| Wheat | Soil Field trial | Arthrospira plantensis biomass and extract | As described in the work of Chojnacka et al. (2014) | Application doses of 1.0, 1.5, and 1.8 L ha−1 were tested | Quantity of grains per ear, the quantity of grain, and shank length | [72] |
| Maize | Soil Field trial | Laurencia obtuse, Corallina elongate powder (biomass) | After collection, microalgae were washed, dried in shadow in the open air, and the drying process was completed in the oven at 60 °C for 5 h. The dried biomass was mechanically ground to the powdery form. | 3 g of powdered biomass of microalgae per kg soil. | Root improvement, Polyphenolic, and antioxidant contents | [142] |
| Cucumber | Soil | Arthrospira platensis, Amphora cofeaeformis | Microalgae extracts were prepared as previously reported by Enan et al. (2016) [147] | Soil application—5 g m−2 Foliar application—2 g L−1 | Vegetative growth, yield, fruit quality, and nematode control | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello, A.S.; Saadaoui, I.; Ben-Hamadou, R. “Beyond the Source of Bioenergy”: Microalgae in Modern Agriculture as a Biostimulant, Biofertilizer, and Anti-Abiotic Stress. Agronomy 2021, 11, 1610. https://doi.org/10.3390/agronomy11081610
Bello AS, Saadaoui I, Ben-Hamadou R. “Beyond the Source of Bioenergy”: Microalgae in Modern Agriculture as a Biostimulant, Biofertilizer, and Anti-Abiotic Stress. Agronomy. 2021; 11(8):1610. https://doi.org/10.3390/agronomy11081610
Chicago/Turabian StyleBello, Adewale Suraj, Imen Saadaoui, and Radhouane Ben-Hamadou. 2021. "“Beyond the Source of Bioenergy”: Microalgae in Modern Agriculture as a Biostimulant, Biofertilizer, and Anti-Abiotic Stress" Agronomy 11, no. 8: 1610. https://doi.org/10.3390/agronomy11081610
APA StyleBello, A. S., Saadaoui, I., & Ben-Hamadou, R. (2021). “Beyond the Source of Bioenergy”: Microalgae in Modern Agriculture as a Biostimulant, Biofertilizer, and Anti-Abiotic Stress. Agronomy, 11(8), 1610. https://doi.org/10.3390/agronomy11081610