Pilot-Scale H2S and Swine Odor Removal System Using Commercially Available Biochar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar and Its Physico-Chemical Characteristics
2.2. Bench-Scale Biochar Column
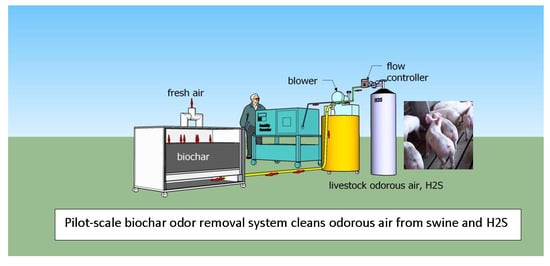
2.3. Pilot-Scale Odor Removal System
2.4. Analyses of Odorous VOCs, H2S, and SCOAVs
2.5. Statistical Methods
3. Results and Discussion
3.1. Biochar Characteristics
3.2. Pressure Drop
3.3. H2S Breakthrough Adsorption Capacity of CBC Using Bench-Scale Biochar Column
3.4. Performance of the Pilot-Scale Biochar Odor Removal System (PSBORS)
3.4.1. Removal of Odorous VOCs
3.4.2. Removal of H2S
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arogo, J.; Zhang, R.H.; Riskowski, G.L.; Day, D.L. Hydrogen Sulfide Production from Stored Liquid Swine Manure. Trans. ASABE 2000, 43, 1241–1245. [Google Scholar] [CrossRef]
- Elenbaas-Thomas, A.M.; Zhao, Y.; Hyun, Y.; Wang, X.; Anderson, B.; Riskowski, G.L.; Ellis, M.; Heber, A.J. Effects of Room Ozonation on Air Quality and Pig Performance. Trans. ASABE 2005, 48, 1167–1173. [Google Scholar] [CrossRef]
- Gay, S.W.; Schmid, D.R.; Clanton, C.J.; Janni, K.A.; Jacobson, L.D.; Weisberg, G.L. Odor, Total Reduced Sulfure, and Ammonia Emissions from Animal Housing Facilities and Manure Storage Units in Minnesota. Appl. Eng. Agric. 2003, 19, 347–360. [Google Scholar]
- NRC. Air Emissions from Animal Feeding Operations: Current Knowledge, Future Needs; The National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Koelsch, R.K.; Woodbury, B.L.; Stenberg, D.E.; Miller, D.N.; Schulte, D.D. Total Reduced Sulfur Concentrations in the Vicinity of Beef Cattle Feedlots. Appl. Eng. Agric. 2004, 20, 77–85. [Google Scholar] [CrossRef]
- Donham, K.J.; Wing, S.; Osterberg, D.; Flora, J.L.; Hodne, C.; Thu, K.M.; Thorne, P.S. Community Health and Socioeconomic Issues Surrounding Concentrated Feeding Operations. Environ. Health Perspect. 2007, 115, 317–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wing, S.; Horton, R.A.; Marshall, S.W.; Thu, K.M.; Taiik, M.; Schinasi, L.; Schiffman, S.S. Air Pollution and Odor in Communities near Industrial Swine Operations. Environ. Health Perspect. 2008, 116, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Blanes-Vidal, V.; Hansen, M.N.; Adamsen, A.P.S.; Feilberg, A.; Petersen, S.O.; Jensen, B.B. Characterization of Odor Released During Handling of Swine Slurry: Part I. Relationship between Odorants and Perceived Odor Concentrations. Atmos. Environ. 2009, 43, 2997–3005. [Google Scholar] [CrossRef]
- Blanes-Vidal, V.; Hansen, M.N.; Adamsen, A.P.S.; Feilberg, A.; Petersen, S.O.; Jensen, B.B. Characterization of Odor Released during Handling of Swine Slurry: Part II. Effect of Production Type, Storage and Physicochemical Characteristics of the Slurry. Atmos. Environ. 2009, 43, 3006–3014. [Google Scholar] [CrossRef]
- Jo, S.-H.; Kim, K.-H.; Jeon, B.-H.; Lee, M.-H.; Kim, Y.-H.; Kim, B.-W.; Cho, S.-B.; Hwang, O.-W.; Bhattacharay, S.S. Odor Characterization from Barns and Slurry Treatment Facilities at a Commercial Swine Facility in South Korea. Atmos. Environ. 2015, 119, 339–347. [Google Scholar] [CrossRef]
- Lo, Y.-C.M.; Koziel, J.A.; Cai, L.; Hoff, S.J.; Jenks, W.S.; Xin, H. Simultaneous Chemical Sensory Characterization of Volatile Organic Compounds and Semi-Volatile Organic Compounds Emitted from Swine Manure using Solid Phase Microextraction and Multidimensional Gas Chromatography-Mass Spectrometry-Olfactometry. J. Environ. Qual. 2008, 37, 521–534. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Bennett, J.L.; Raymer, J.H. Quantification of Odors and Odorants from Swine Operations in North Carolina. Agric. For. Meteorol. 2001, 108, 213–240. [Google Scholar] [CrossRef]
- Parker, D.B.; Gilley, J.; Woodbury, B.L.; Kim, K.-H.; Galvin, G.; Bartelt-Hunt, S.L.; Li, X.; Snow, D.D. Odorous VOC Emission Following Land Application of Swine Manure Slurry. Atmos. Environ. 2013, 66, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Maurer, D.L.; Koziel, J.A.; Harmon, J.D.; Hoff, S.J.; Rieck-Hinz, A.M.; Anderson, D.S. Summary of Performance Data for Technologies to Control Gaseous, Odor, and Particulate Emission from Livestock Operations: Air Management Practices Assessment tool (AMPAT). Data Brief 2016, 7, 1413–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalus, K.; Koziel, J.A.; Opalinski, S. A Review of Biochar Properties and their Utiliaiton in Crop Agriculture and Livestock Production. Appl. Sci. 2019, 9, 3494. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.-P.; Hagemann, N.; Draper, K.; Kammann, C. The Use of Biochar in Animal Feeding. Peer J. 2019, 7, e7373. [Google Scholar] [CrossRef] [Green Version]
- Novak, J.M.; Spokas, K.A.; Cantrell, K.; Ro, K.S.; Watts, D.W.; Glaz, B.; Busscher, W.J.; Hunt, P.G. Effects of Biochars and Hydrochars Produced from Lignocellulosic and Animal Manure on Fertility of a Mollisol and Entisol. Soil Use Manag. 2014, 30, 175–181. [Google Scholar] [CrossRef]
- Hwang, O.; Lee, S.-R.; Cho, S.; Ro, K.S.; Spiechs, M.; Woodbury, B.L.; Silva, P.J.; Han, D.-W.; Choi, H.; Kim, K.-Y.; et al. Efficacy of Different Biochars in Removing Odorous Volatile Organic Compounds (VOCs) Emitted from Swine Manure. ACS Sustain. Chem. Eng. 2018, 6, 14239–14247. [Google Scholar] [CrossRef]
- Ro, K.S.; Lima, I.M.; Reddy, G.B.; Jackson, M.A.; Gao, B. Removing Gaseous NH3 using Biochar as an Adsorbent. Agriculture 2015, 5, 991–1002. [Google Scholar] [CrossRef] [Green Version]
- Sikora, F.J.; Moore, K.P. (Eds.) Soil Test Methods from the Southeastern United States; Southern Cooperative Series Bulletin No. 419; University of Kentuky: Lexington, KY, USA, 2014; ISBN 1-58161-419-5. [Google Scholar]
- Kalus, K.; Konkol, D.; Korczynski, M.; Koziel, J.A.; Opalinski, S. Laying Hens Biochar Diet Supplementation–Effect on Performance, Excreta N Content, NH3, and VOCs Emissions, Egg Traits and Egg Consumers Acceptance. Agriculture 2020, 10, 237. [Google Scholar] [CrossRef]
- Kalus, K.; Konkol, D.; Korczynski, M.; Koziel, J.A.; Opalinski, S. Effect of Biochar Diet Supplementation on Chicken Broilers Performance, NH3 and Odor Emissions and Meat Consumer Acceptance. Animals 2020, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- BiocharNow. Available online: https://biocharnow.com/ (accessed on 8 March 2021).
- Pordesimo, L.O.; Igathinathane, C.; Bevans, B.D.; Holzgrafe, D.P. Potential of Dimensional Measurenments of Individual Pellets as Another Measure for Evaluating Pellet Quality. In American Society of Agricultural and Biological Engineers; ASABE, Ed.; ASABE: Pittsburg, PA, USA, 2010. [Google Scholar]
- Walker, J.; Knight, L.; Stein, L. A Plain English Guide to the EPA Part 503 Bioslids Rule. In U.S. Environmental Protection Agency; Report No.=EPA/832/R-93/003; U.S. Environmental Protection Agency: Washington, DC, USA, 1994. [Google Scholar]
- ALEC. Available online: https://www.alec.arizona.edu/ (accessed on 11 August 2021).
- Parker, D.B.; Malone, G.W.; Walter, W.D. Vegetative Environmental Buffers and Exhaust Fan Deflectors for Reducing Downwind Odor and VOCs from Tunnel-Ventilated Swine Barns. Trans. ASABE 2012, 55, 227–240. [Google Scholar] [CrossRef]
- Zahn, J.A.; Hatfield, J.L.; Do, Y.S.; DiSpirito, A.A.; Laird, D.A.; Pfeiffer, R.L. Characterization of Volatile Organic Emissions and Wastes from a Swine Production Facility. J. Environ. Qual. 1997, 26, 1687–1696. [Google Scholar] [CrossRef]
- Trabue, S.L.; Anhalt, J.C.; Zahn, J.A. Bias of Tedlar Bags in the Measurement of Agricultural Odorants. J. Environ. Qual. 2006, 35, 1668–1677. [Google Scholar] [CrossRef] [PubMed]
- Zahn, J.A.; DiSpirito, A.A.; Do, Y.S.; Brooks, B.E.; Cooper, E.E.; Hatfield, J.L. Correlation of Human Olfactory Responses to Airborne Concentrations of Malodorous Volatile Organic Compounds Emitted from Swine Effluent. J. Environ. Qual. 2001, 30, 624–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodbury, B.L.; Gilley, J.; Parker, D.B.; Marx, D.B.; Miller, D.N.; Eigenberg, R.A. Emission of Volatile Organic Compounds after Land Application of Cattle Manure. J. Environ. Qual. 2014, 43, 1207–1218. [Google Scholar] [CrossRef] [Green Version]
- Spokas, K.A.; Novak, J.M.; Stewart, C.E.; Cantrell, K.; Uchimiya, M.; DiSaire, M.G.; Ro, K.S. Qualitative Analysis of Volatile Organic Compounds on Biochar. Chemosphere 2011, 85, 869–882. [Google Scholar] [CrossRef]
- Ro, K.S. Kinetics and Energetics of Producing Animal Manure-based Biochar. Bioenergy Res. 2016, 9, 447–453. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Liu, R. Effects of Pyrolysis Temperature and Heating Time on Biochar Obtained from the Pyrolysis of Straw and Lignosulfonate. Bioresour. Technol. 2015, 176, 288–291. [Google Scholar] [CrossRef]
- Novak, J.M.; Watts, D.W.; Sigua, G.C.; Ducey, T.F. Corn Grain and Stover Nutrient Uptake Responses from Sandy Soil Treated with Designer Biochars and Compost. Agronomy 2021, 11, 942. [Google Scholar] [CrossRef]
- Ro, K.S.; McConnell, L.L.; Johnson, M.H.; Hunt, P.G.; Parker, D. Livestock Air Treatment using PVA-coated Powdered Activated Carbon Biofilter. Appl. Eng. Agric. 2008, 24, 791–798. [Google Scholar]
- Ergun, S. Fluid Flow through Packed Columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Classen, J.J.; Young, J.S.; Bottcher, R.W.; Westerman, P.W. Design and Analysis of a Pilot Scale Biofiltration System for Odorous Air. Trans. ASABE 2000, 43, 111–118. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Bagreev, A.; Adib, F.; Turk, A. Unmodified Versus Caustics-Impregnated Carbons for Control of Hydrogen Sulfide Emissions from Sewage Treatment Plants. Environ. Sci. Technol. 2000, 34, 1069–1074. [Google Scholar] [CrossRef]
- Shang, G.; Li, Q.; Liu, L.; Chen, P.; Huang, X. Adsorption of Hydorgen Sulfide by Biochars Derived from Pyrolysis of Different Agricultural/Forestry Wastes. J. Air Wastes Manag. Assoc. 2016, 66, 8–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandosz, T.J. On the Adsorption/Oxidation of Hydrogen Sulfide on Activated Carbons at Ambient Temperatures. J. Colloid Interface Sci. 2002, 246, 1–20. [Google Scholar] [CrossRef]
- Feilberg, A.; Nyord, T.; Hansen, M.N.; Lindholst, S. Chemical Evaluation of Odor Reduction by Soil Injection of Animal Manure. J. Environ. Qual. 2011, 40, 1674–1682. [Google Scholar] [CrossRef] [PubMed]





| Property | Commercial Pine Biochar † |
|---|---|
| pH | 6.7 ± 0.5 |
| Moisture content (%) | 2.8 ± 0.03 |
| BET surface area (m2/g) | 102 ± 22 |
| Sauter mean diameter (cm) | 1.90 ± 0.04 |
| Sphericity | 0.92 ± 0.01 |
| Proximate properties | |
| Volatile matter (%db ‡) | 14.0 ± 0.14 |
| Fixed carbon (%db ‡) | 81.7 ± 0.08 |
| Ash (%db ‡) | 4.3 ± 0.14 |
| Ultimate properties | |
| C (%db ‡) | 94.3 ± 0.06 |
| H (%db) | 2.3 ± 0.02 |
| N (%db) | 0.37 ± 0.02 |
| O (%db) | < 0.01 |
| Part 503 Elements | |
| As, μg/g (75) § | 0.30 ± 0.04 |
| Cr, μg/g (3000) § | 8.59 ± 1.18 |
| Cu, μg/g (4300) § | 3.91 ± 0.26 |
| Pb, μg/g (840) § | 0.88 ± 0.02 |
| Mo, μg/g (75) § | 0.21 ± 0.03 |
| Ni, μg/g (420) § | 1.26 ± 0.21 |
| Se, μg/g (100) § | 0.26 ± 0.05 |
| Zn, μg/g (7500) § | 25.76 ± 0.76 |
| Dry | Humid | |
|---|---|---|
| Number of samples | 3 | 3 |
| Relative humidity, RH (%) | 23.9 ± 1.7 | 64.0 ± 1.5 |
| Temperature, T (°C) | 18.6 ± 1.8 | 19.3 ± 1.0 |
| Superficial velocity, U (m/s) | 0.055 | 0.055 |
| Influent concentration (ppm) | 74.5 ± 0.8 | 72.0 ± 2.5 |
| Breakthrough capacity (mg/g) | 0.34 ± 0.13 | 2.51 ± 0.32 |
| Compounds | SCOT (ng/L) | DL (ng/L) | Influent Concentration (ng/L) | Influent SCOAV | Effluent Concentration (ng/L) | Effluent SCOAV |
|---|---|---|---|---|---|---|
| DMDS | 12 | 0.3 | 4.1 ± 2.4 | 0.34 | BDL † | 0.03 ‡ |
| DMTS | 2 | 0.0 | 0.5 ± 0.4 | 0.23 | BDL | 0 ‡ |
| Acetic acid | 578 | 98 | 347 ± 259 | 0.60 | 125 ± 83 | 0.22 |
| Propionic acid | 106 | 18 | 47 ± 15 | 0.44 | BDL | 0.17 ‡ |
| i-Butyric acid | 38 | 0.4 | 8.0 ± 4.3 | 0.21 | BDL | 0.01 ‡ |
| Butyric acid | 6.9 | 1.1 | 9.9 ± 9.6 | 1.43 | BDL | 0.16 ‡ |
| i-Valeric acid | 2.3 | 9.8 | BDL | - | BDL | - |
| Valeric acid | 8.8 | 2.0 | BDL | - | BDL | - |
| Hexanoic acid | 69 | 1.2 | BDL | - | BDL | - |
| Heptanoic acid | 60 | 4.0 | BDL | - | BDL | - |
| Phenol | 206 | 86 | BDL | - | BDL | - |
| 4-methyl phenol | 1.3 | 2.2 | 30 ± 28 | 13.65 | BDL | 1.69 ‡ |
| 4-ethyl phenol | 6.3 | 2.7 | BDL | - | BDL | - |
| Indole | 2.1 | 0.1 | 0.2 ± 0.4 | 0.10 | BDL | 0.05 ‡ |
| Skatole | 0.48 | 0.1 | 0.1 ± 0.2 | 0.25 | BDL | 0.21 ‡ |
| Average daily sum of influent SCOAVs | 26.5 ± 7.1 | Average daily sum of effluent SCOAVs | 2.5 ± 0.0 |
| Duration (h) | Flow Rate (LPM) | Biochar (kg) | SCDOT † (ppm) | DL (ppm) | RH (%) | Influent Concentration (ppm) | Effluent Concentration (ppm) |
|---|---|---|---|---|---|---|---|
| 39.3 | 20 | 27.4 | 0.002 | 0.1 | 61.6 ± 3.2 | 71.9 ± 5.2 | BDL |
| 9.2 | 25.5 | 10.2 | 0.002 | 0.1 | 64.2 ± 0.6 | 308.5 ± 33.0 | BDL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ro, K.S.; Woodbury, B.; Spiehs, M.; Szogi, A.A.; Silva, P.J.; Hwang, O.; Cho, S. Pilot-Scale H2S and Swine Odor Removal System Using Commercially Available Biochar. Agronomy 2021, 11, 1611. https://doi.org/10.3390/agronomy11081611
Ro KS, Woodbury B, Spiehs M, Szogi AA, Silva PJ, Hwang O, Cho S. Pilot-Scale H2S and Swine Odor Removal System Using Commercially Available Biochar. Agronomy. 2021; 11(8):1611. https://doi.org/10.3390/agronomy11081611
Chicago/Turabian StyleRo, Kyoung S., Bryan Woodbury, Mindy Spiehs, Ariel A. Szogi, Philip J. Silva, Okhwa Hwang, and Sungback Cho. 2021. "Pilot-Scale H2S and Swine Odor Removal System Using Commercially Available Biochar" Agronomy 11, no. 8: 1611. https://doi.org/10.3390/agronomy11081611
APA StyleRo, K. S., Woodbury, B., Spiehs, M., Szogi, A. A., Silva, P. J., Hwang, O., & Cho, S. (2021). Pilot-Scale H2S and Swine Odor Removal System Using Commercially Available Biochar. Agronomy, 11(8), 1611. https://doi.org/10.3390/agronomy11081611