Characterization and Technological Potential of Underutilized Ancestral Andean Crop Flours from Ecuador
Abstract
:1. Introduction
2. Materials and Methods
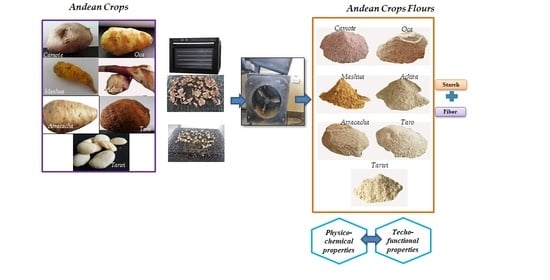
2.1. Andean Crop Flours
2.2. Proximate Composition of Flours and Energy Value
2.3. Total Starch and Amylose Content
2.4. Physico-Chemical Properties of Flours
2.4.1. pH and Titratable Acidity
2.4.2. Color
2.4.3. Granulometry
2.4.4. Bulk Density
2.4.5. Scanning Electron Microscopic (SEM)
2.4.6. Differential Scanning Calorimetry
2.4.7. Swelling Power and Solubility
2.4.8. Water Absorption Capacity (WAC)
2.5. Rheological Properties
2.6. Total Phenolic Content and Antioxidant Activity
2.6.1. Total Phenolic Content
2.6.2. Antioxidant Activity
2.7. Experimental Design
3. Results
3.1. Proximal Composition
3.2. pH and Acidity
3.3. Color and Visual Appearance
3.4. Granulometry
3.5. Bulk Density
3.6. Scanning Electron Microscopy (SEM)
3.7. Differential Scanning Calorimetry (DSC)
3.8. Swelling Power and Solubility
3.9. Water Absorption Capacity (WAC)
3.10. Rheological Characterization
3.11. Antioxidant and Total Phenol Content
3.12. Cluster Analysis and Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flores, H.E.; Walker, T.S.; Guimarães, R.L.; Bais, H.P.; Vivanco, J.M. Andean Root and Tuber Crops: Underground Rainbows. HortScience 2003, 38, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Málaga, J.; Avila-Santamaría, J.J.; Carpio, C.E. The Andean Region: An Important and Growing Us Agricultural Trade Partner. Choices 2019, 34, 1–9. [Google Scholar]
- Bravo, K.; Alzate, F.; Osorio, E. Fruits of selected wild and cultivated Andean plants as sources of potential compounds with antioxidant and anti-aging activity. Ind. Crop. Prod. 2016, 85, 341–352. [Google Scholar] [CrossRef]
- Rubio, A.L.; Rodríguez, J.; Fernández, C.; Crespo, J.F. Storage Proteins: Physiological and Antigenic Effects. Pub. Eur. Assoc. Anim. Prod. 2004, 110, 159–176. [Google Scholar]
- Silva, A.; Caro, J.C.; Magaña-Lemus, D. Household food security: Perceptions, behavior and nutritional quality of food purchases. J. Econ. Psychol. 2016, 55, 139–148. [Google Scholar] [CrossRef]
- Hermann, M. The impact of the European Novel Food Regulation on trade and food innovation based on traditional plant foods from developing countries. Food Policy 2009, 34, 499–507. [Google Scholar] [CrossRef]
- Andrade-Mahecha, M.M.; Tapia-Blácido, D.; Menegalli, F.C. Physical-chemical, thermal, and functional properties of achira (Canna indica L.) flour and starch from different geographical origin. Starch 2012, 64, 348–358. [Google Scholar] [CrossRef]
- Pinto, L.C.; de Oliveira, T.P.; de Souza, R.; Santos, N.B.F.; Santos, L.F.P.; de Assis Santos, A.; de Santos, T.X.; Santos, C.T.; Nunes, C.J.; Costa, I.B. Probiotic Kefir-Fermented Beverage-Based Colocasia Esculenta, L.: Development, Characterization, and Microbiological Stability During Chilled Storage. J. Food Process. Preserv. 2021, 45, e15113. [Google Scholar] [CrossRef]
- Wongsagonsup, R.; Nateelerdpaisan, T.; Gross, C.; Suphantharika, M.; Belur, P.D.; Agoo, E.M.G.; Janairo, J.I.B. Physicochemical Properties and in Vitro Digestibility of Flours and Starches from Taro Cultivated in Different Regions of Thailand. Int. J. Food Sci. Technol. 2021, 56, 2395–2406. [Google Scholar] [CrossRef]
- Cui, R.; Zhu, F. Physicochemical and functional properties of sweetpotato flour. J. Sci. Food Agric. 2019, 99, 4624–4634. [Google Scholar] [CrossRef]
- Manzelli, M.; Romagnoli, S.; Ghiselli, L.; Benedettelli, S.; Palchetti, E.; Andrenelli, L.; Vecchio, V. Typicity in Potato: Characterization of Geographic Origin. Ital. J. Agron. 2010, 5, 61–68. [Google Scholar] [CrossRef]
- Sulistiani, R.; Siregar, L.A.; Harahap, F. The Effects of Temperature and Potassium Fertilizer on the Growth, Yield, and Biochemical Parameters of Ipomoea Batatas Var. Antin-1. Acta Agrobot. 2020, 73, 7337. [Google Scholar] [CrossRef]
- Calle, J.; Benavent-Gil, Y.; Rosell, C.M. Use of Flour from Cormels of Xanthosoma sagittifolium (L.) Schott and Colocasia esculenta (L.) Schott to Develop Pastes Foods: Physico-Chemical, Functional and Nutritional Characterization. Food Chem. 2021, 344, 128666. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.G.; Viana, D.J.S.; Dos Santos, A.S.; Júnior, V.C.D.A.; Rosa, D.D.S. Structure and properties of starch and flour of four Brazilian sweet potatoes (Ipomoea batatas) cultivars. Matéria 2020, 25. [Google Scholar] [CrossRef]
- Sanchez, R.G.R.; Juan, G.A.C.S.; Lim, P.A.A.; Gabriel, A.A. Effect of flour processing on the proximate composition, polyphenolic content, and antioxidant activity of 19 Philippine sweet potato cultivars. J. Food Process. Preserv. 2020, 44. [Google Scholar] [CrossRef]
- Ali, M.I.; Mousa, E.A.; Hassan, N.A. Production of Healthy Chips Ready to Eat Using Potato, Green Pea and Lupine Flour for Malnourished Children. Age 2019, 1, 2. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC, Ed.; The Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- FAO. Food Energy—Methods of Analysis and Conversion Factors. In Report of a Technical Workshop, Rome, 3–6 December 2002; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003. [Google Scholar]
- Salazar, D.; Arancibia, M.; Raza, K.; López-Caballero, M.; Montero, M. Influence of Underutilized Unripe Banana (Cavendish) Flour in the Formulation of Healthier Chorizo. Foods 2021, 10, 1486. [Google Scholar] [CrossRef] [PubMed]
- Salazar, D.; Arancibia, M.; Lalaleo, D.; Rodríguez-Maecker, R.; López-Caballero, M.E.; Montero, M.P. Physico-Chemical Properties and Filmogenic Aptitude for Edible Packaging of Ecuadorian Discard Green Banana Flours (Musa acuminanta AAA) Food Hydrocolloids. Food Hydrocoll. 2021, 122, 107048. [Google Scholar] [CrossRef]
- Gujska, E.; Khan, K. Effect of Temperature on Properties of Extrudates from High Starch Fractions of Navy, Pinto and Garbanzo Beans. J. Food Sci. 1990, 55, 466–469. [Google Scholar] [CrossRef]
- JJinapong, N.; Suphantharika, M.; Jamnong, P. Production of Instant Soymilk Powders by Ultrafiltration, Spray Drying and Fluidized Bed Agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Henshaw, F.O.; McWatters, K.H.; Akingbala, J.O.; Chinnan, M.S. Thermal properties of cowpea flour: A study by differential scanning calorimetry. Food/Nahrung 2003, 47, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.T.; Moreno, F.J.; Moreno, R.; Villamiel, M.; Hernandez-Hernandez, O. Morphological, technological and nutritional properties of flours and starches from mashua (Tropaeolum tuberosum) and melloco (Ullucus tuberosus) cultivated in Ecuador. Food Chem. 2019, 301, 125268. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic—Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Villacrés, E.; Quelal, M.B.; Fernández, E.; Garcìa, G.; Cueva, G.; Rosell, C.M. Impact of debittering and fermentation processes on the antinutritional and antioxidant compounds in Lupinus mutabilis sweet. LWT 2020, 131, 109745. [Google Scholar] [CrossRef]
- Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality; Kluwer Academic/Plenum: New York, NY, USA, 2005. [Google Scholar]
- Bernabé, Y.; Fanny, C. Caracterización Fisicoquímica, Fitoquímica y Funcional de la Harina De Khaya y Oca (Oxalis tuberosa) Para Uso Industrial; Universidad Nacional del Centro del Peru: Huancayo, Peru, 2017. [Google Scholar]
- Villacrés, E.; Rubio, A.; Egas, L.; Segovia, G. Usos Alternativos Del Chocho: Chocho (Lupinus Mutabilis Sweet) Alimento Andino Redescubierto; INIAP, Estación Experimental Santa Catalina, Departamento de Nutrición y Calidad: Quito, Ecuador, 2006. Available online: http://repositorio.iniap.gob.ec/handle/41000/298 (accessed on 21 August 2021).
- Sulas, L.; Canu, S.; Ledda, L.; Carroni, A.M.; Salis, M. Yield and nitrogen fixation potential from white lupine grown in rainfed Mediterranean environments. Sci. Agric. 2016, 73, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Tiuquinga, J. Utilización de Diferentes Niveles de Harina de Canna Edulis (Achira) en la Alimentación de Cuyes en la Etapa de Gestación–Lactancia. Bachelor’s Thesis, Facultad De Ciencias Pecuarias—Escuela De Ingeniería Zootécnica, Riobamba, Ecuador, 2017. [Google Scholar]
- Ayusman, S.; Duraivadivel, P.; Gowtham, H.G.; Sharma, S.; Hariprasad, P. Bioactive Constituents, Vitamin Analysis, Antioxidant Capacity and A-Glucosidase Inhibition of Canna indica L. Rhizome Extracts. Food. Biosci. 2020, 35, 100544. [Google Scholar] [CrossRef]
- Yadav, A.R.; Guha, M.; Tharanathan, R.; Ramteke, R. Changes in characteristics of sweet potato flour prepared by different drying techniques. LWT 2006, 39, 20–26. [Google Scholar] [CrossRef]
- Valcárcel-Yamani, B.; Rondan-Sanabria, G.G.; Finardi-Filho, F. The physical, chemical and functional characterization of starches from Andean tubers: Oca (Oxalis tuberosa Molina), olluco (Ullucus tuberosus Caldas) and mashua (Tropaeolum tuberosum Ruiz & Pavón). Braz. J. Pharm. Sci. 2013, 49, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, S.M.L.; Rincón, N.; Contreras-Padilla, M.; Millan-Malo, B.M.; Rodriguez-Garcia, M.E. Morphological, structural, thermal, compositional, vibrational, and pasting characterization of white, yellow, and purple Arracacha Lego-like starches and flours (Arracacia xanthorrhiza). Int. J. Biol. Macromol. 2018, 113, 1188–1197. [Google Scholar] [CrossRef]
- Okonwu, K.; Ariaga, C.A. Nutritional Evaluation of Various Parts of Canna indica L. Annu. Res. Rev. Biol. 2016, 11, 1–5. [Google Scholar] [CrossRef]
- Choquechambi, L.A.; Callisaya, I.R.; Ramos, A.; Bosque, H.; Mújica, A.; Jacobsen, S.-E.; Sørensen, M.; Leidi, E.O. Assessing the Nutritional Value of Root and Tuber Crops from Bolivia and Peru. Foods 2019, 8, 526. [Google Scholar] [CrossRef] [Green Version]
- No, R. Regulation (Ec) No 1924/2006 of the European Parlament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods. Corrigendum to Regulation (Ec) No 1924/2006. OJEU 2007, L12, 3–17. [Google Scholar]
- Leidi, E.O.; Altamirano, A.M.; Mercado, G.; Rodriguez, J.P.; Ramos, A.; Alandia, G.; Sørensen, M.; Jacobsen, S.-E. Andean roots and tubers crops as sources of functional foods. J. Funct. Foods 2018, 51, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Matsuguma, L.S.; Lacerda, L.G.; Schnitzler, E.; Carvalho Filho, M.A.D.S.; Franco, C.M.L.; Demiate, I.M. Characterization of Native and Oxidized Starches of Two Varieties of Peruvian Carrot (Arracacia xanthorrhiza, B.) from Two Production Areas of Paraná State, Brazil. Braz. Arch. Biol. Technol. 2009, 52, 701–713. [Google Scholar] [CrossRef] [Green Version]
- Gross, R.; Von Baer, E.; Koch, F.; Marquard, R.; Trugo, L.; Wink, M. Chemical Composition of a New Variety of the Andean Lupin (Lupinus mutabilis Cv. Inti) with Low-Alkaloid Content. J. Food Compos. Anal. 1988, 1, 353–361. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Mujica, A. El Tarwi (Lupinus mutabilis Sweet.) Y Sus Parientes Silvestres. Bot. Econ. Andes. Cent. 2006, 28, 458–482. [Google Scholar]
- Moorthy, S.N.; Sajeev, M.S.; Anish, R.J. Chapter 11—Functionality of Tuber Starches. In Starch in Food, 2nd ed.; Malin, S., Lars, N., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 421–508. [Google Scholar]
- Azeem, M.; Mu, T.-H.; Zhang, M. Influence of Particle Size Distribution on Nutritional Composition, Microstructural and Antioxidant Properties of Orange and Purple-Fleshed Sweet Potato Flour. J. Food Process. Preserv. 2021, 45, e15283. [Google Scholar] [CrossRef]
- Escalante-Aburto, A.; Ponce-García, N.; Ramírez-Wong, B.; Santiago-Ramos, D.; Véles-Medina, J.J.; Cárdenas, J.D.D.F. Effect of extrusion factors and particle size on starch properties of nixtamalized whole blue corn snacks. Starch-Stärke 2016, 68, 1111–1120. [Google Scholar] [CrossRef]
- Aliaga, P.; Cecilia, N. Contenido De Azúcares En Raíces Reservantes De 106 Clones De Camote (Ipomoea batatas (L.) Lam.) De La Colección De Germoplasma. Anales Científicos 2009, 70, 1–10. [Google Scholar]
- Khajehei, F.; Merkt, N.; Claupein, W.; Graeff-Hoenninger, S. Yacon (Smallanthus sonchifolius Poepp. & Endl.) as a Novel Source of Health Promoting Compounds: Antioxidant Activity, Phytochemicals and Sugar Content in Flesh, Peel, and Whole Tubers of Seven Cultivars. Molecules 2018, 23, 278. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Villarreal, V.; Serna-Saldivar, S.O.; García-Lara, S. Effects of Parboiling and Other Hydrothermal Treatments on the Physical, Functional, and Nutritional Properties of Rice and Other Cereals. Cereal Chem. 2018, 95, 79–91. [Google Scholar] [CrossRef]
- Torres, A.; Durán, M.; Montero, P. Evaluación De Las Propiedades Funcionales Del Almidón Obtenido a Partir De Malanga (Colocasia esculenta). Cienc. Ing. Dia 2013, 8, 29–38. [Google Scholar]
- Aboubakar, N.Y.; Scher, J.; Mbofung, C. Physicochemical, thermal properties and microstructure of six varieties of taro (Colocasia esculenta L. Schott) flours and starches. J. Food Eng. 2008, 86, 294–305. [Google Scholar] [CrossRef]
- Moorthy, S. Tuber Crop Starches. Tech. Bull 2000, 18. [Google Scholar]
- Ledezma, C.C.Q. Los almidones resistentes y la salud. Investig. Desarro. 2008, 8, 130–141. [Google Scholar] [CrossRef] [Green Version]
- Ramón, F. Efecto del Estrés Abiótico Post-Cosecha en las Características Físico-Químicas y de Algunos Metabolitos Primarios de Mashua Morada (Tropaeolum tuberosum Ruíz & Pavón). Bachelor’s Thesis, Universidad Nacional Agraria La Molina, Lima, Peru, 2017. [Google Scholar]
- Lim, T.K. Oxalis Tuberosa. In Edible Medicinal and Non-Medicinal Plants; Springer: New York, NY, USA, 2016; pp. 139–146. [Google Scholar]
- FAO; WHO. “Codex Alimentarius”. In Codex Standard 151-1989 (Rev. 1-1995); Food and Agriculture Organization of the United Nations (FAO), World Health Organization (WHO), Eds.; FAO; WHO: Rome, Italy, 2007. [Google Scholar]
- Kraithong, S.; Lee, S.; Rawdkuen, S. Physicochemical and functional properties of Thai organic rice flour. J. Cereal Sci. 2018, 79, 259–266. [Google Scholar] [CrossRef]
- Alkarkhi, A.; bin Ramli, S.; Yong, Y.S.; Easa, A.M. Comparing physicochemical properties of banana pulp and peel flours prepared from green and ripe fruits. Food Chem. 2011, 129, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Flórez, S.; González-Gallego, J.; Culebras, J.M.; Tuñón, M. Los Flavonoides: Propiedades Y Acciones Antioxidantes. Nutr. Hosp. 2002, 17, 271–278. [Google Scholar] [PubMed]
- Villacrés, E.; Cuadrado, L.; Quelal, M.B. Actividad Antimicrobiana De Extractos De Granos Andinos El Chocho, La Quinua, Amaranto Y Sangorache, Fuente De MoleéCulas Bioactivas; Académica Española: Madrid, Spain, 2016. [Google Scholar]
- Velásquez-Barreto, F.F.; A Bello-Pérez, L.; Nuñez-Santiago, C.; Yee-Madeira, H.; Sánchez, C.E.V. Relationships among molecular, physicochemical and digestibility characteristics of Andean tuber starches. Int. J. Biol. Macromol. 2021, 182, 472–481. [Google Scholar] [CrossRef]
- Cooperstone, J.; Schwartz, S. Recent Insights into Health Benefits of Carotenoids Elvesier; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Young, A.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Campos, D.; Chirinos, R.; Ranilla, L.G.; Pedreschi, R. Chapter Eight—Bioactive Potential of Andean Fruits, Seeds, and Tubers. In Advances in Food and Nutrition Research; Fidel, T., Ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Savlak, N.; Türker, B.; Yeşilkanat, N. Effects of Particle Size Distribution on Some Physical, Chemical and Functional Properties of Unripe Banana Flour. Food Chem. 2016, 213, 180–186. [Google Scholar] [CrossRef]
- Martín, J.C.; Higuera, B.L. Glucosinolate composition of Colombian accessions of mashua (Tropaeolum tuberosum Ruíz & Pavón), structural elucidation of the predominant glucosinolate and assessment of its antifungal activity. J. Sci. Food Agric. 2016, 96, 4702–4712. [Google Scholar] [CrossRef]
- Kalyankar, S.D.; Deshmukh, M.A.; Chopde, S.S.; Khedkar, C.D.; Lule, V.K.; Deosarkar, S.S. Milk Powder. In Encyclopedia of Food and Health; Benjamin, C., Paul, M.F., Fidel, T., Eds.; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Subramanian, S.; Viswanathan, R. Bulk density and friction coefficients of selected minor millet grains and flours. J. Food Eng. 2007, 81, 118–126. [Google Scholar] [CrossRef]
- Rayo, L. Processo de Aglomeração de Farinha de Banana Verde Com Alto Conteúdo de Amido Resistente em Leito Fluidizado Pulsado. Master’s Thesis, Escola Politécnica Chemical Engineerin, Sao Paulo, Brasil, 2013. [Google Scholar]
- Abdullah, E.C.; Geldart, D. The use of bulk density measurements as flowability indicators. Powder Technol. 1999, 102, 151–165. [Google Scholar] [CrossRef]
- Hernández-Uribe, J.P.; Meza-Nieto, M.; Palma-Rodríguez, H.M.; Navarro-Cortez, R.O.; Guzmán-Ortiz, F.A.; Bello-Pérez, L.A.; Vargas-Torres, A. Physicochemical, Morphological, and Molecular Properties of Starch Isolated from Dioscorea and Oxalis Tubers from Hidalgo State, Mexico. Starch-Stärke 2020, 72. [Google Scholar] [CrossRef]
- Lima, D.C.; Villar, J.; Castanha, N.; Maniglia, B.C.; Junior, M.D.M.; Augusto, P.E.D. Ozone modification of arracacha starch: Effect on structure and functional properties. Food Hydrocoll. 2020, 108, 106066. [Google Scholar] [CrossRef]
- Pinzon, M.I.; Sanchez, L.T.; Villa, C.C. Chemical, structural, and thermal characterization of starches from four yellow Arracacha (Arracacia xanthorriza) roots produced in Colombia. Heliyon 2020, 6, e04763. [Google Scholar] [CrossRef]
- Pang, Y.; Ali, J.; Wang, X.; Franje, N.J.; Revilleza, J.E.; Xu, J.; Li, Z. Relationship of Rice Grain Amylose, Gelatinization Temperature and Pasting Properties for Breeding Better Eating and Cooking Quality of Rice Varieties. PLoS ONE 2016, 11, e0168483. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, M.T.; Hernández-Hernández, O.; Moreno, F.J.; Villamiel, M. Andean tubers grown in Ecuador: New sources of functional ingredients. Food Biosci. 2020, 35, 100601. [Google Scholar] [CrossRef]
- Zhang, Y.H.P. Starch: A High-Density Chemical Hydrogen Storage Compound for Pem Fuel Cells. In Portable Hydrogen Energy Systems; Paloma, F.-A., Antonio, M.C., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 161–173. [Google Scholar]
- Wang, S.; Copeland, L. Phase Transitions of Pea Starch over a Wide Range of Water Content. J. Agric. Food Chem. 2012, 60, 6439–6446. [Google Scholar] [CrossRef]
- Wright, D.J.; Boulter, D. Differential scanning calorimetric study of meals and constituents of some food grain Legumes. J. Sci. Food Agric. 1980, 31, 1231–1241. [Google Scholar] [CrossRef]
- Lii, C.; Shao, Y.; Tseng, K. Gelations Mechanism and Rheological of Rice Starch. Cereal Chem. 1995, 72, 393–400. [Google Scholar]
- Sasaki, T.; Matsuki, J. Effect of Wheat Starch Structure on Swelling Power. Cereal Chem. J. 1998, 75, 525–529. [Google Scholar] [CrossRef]
- Zhang, P.; Whistler, R.L.; BeMiller, J.N.; Hamaker, B.R. Banana Starch: Production, Physicochemical Properties, and Digestibility—A Review. Carbohydr. Polym. 2005, 59, 443–458. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Igwe, V.S.; Echeta, C.K. The Functional Properties of Foods and Flours. Int. J. Adv. Acad. 2019, 5, 139–160. [Google Scholar]
- Agama-Acevedo, E.; Islas-Hernandez, J.J.; Osorio-Díaz, P.; Rendón-Villalobos, R.; Utrilla-Coello, R.G.; Angulo, O.; Bello-Pérez, L.A. Pasta with Unripe Banana Flour: Physical, Texture, and Preference Study. J. Food Sci. 2009, 74, S263–S67. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lim, S.; Yoo, B. Dynamic rheology of corn starch–sugar composites. J. Food Eng. 2004, 64, 521–527. [Google Scholar] [CrossRef]
- Rosalina, I.; Bhattacharya, M. Dynamic rheological measurements and analysis of starch gels. Carbohydr. Polym. 2002, 48, 191–202. [Google Scholar] [CrossRef]
- Arıcı, M.; Özülkü, G.; Kahraman, B.; Yıldırım, R.M.; Toker, Ö.S. Taro Flour Usage in Wheat Flour Bread and Gluten-Free Bread: Evaluation of Rheological, Technological and Some Nutritional Properties. J. Food Process Eng. 2020, 43, e13454. [Google Scholar] [CrossRef]
- Zi, Y.; Shen, H.; Dai, S.; Ma, X.; Ju, W.; Wang, C.; Guo, J.; Liu, A.; Cheng, D.; Li, H.; et al. Comparison of starch physicochemical properties of wheat cultivars differing in bread- and noodle-making quality. Food Hydrocoll. 2019, 93, 78–86. [Google Scholar] [CrossRef]
- Khatkar, B.S.; Schofield, J.D. Dynamic Rheology of Wheat Flour Dough. I. Non-Linear Viscoelastic Behaviour. J. Sci. Food Agric. 2002, 82, 827–829. [Google Scholar] [CrossRef]
- Zhang, D.; Mu, T.; Sun, H. Comparative study of the effect of starches from five different sources on the rheological properties of gluten-free model doughs. Carbohydr. Polym. 2017, 176, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Cui, R. Comparison of Physicochemical Properties of Oca (Oxalis tuberosa), Potato, and Maize Starches. Int. J. Biol. Macromol. 2020, 148, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Albano, K.M.; Franco, C.M.; Telis, V.R. Rheological behavior of Peruvian carrot starch gels as affected by temperature and concentration. Food Hydrocoll. 2014, 40, 30–43. [Google Scholar] [CrossRef]
- Georgopoulos, T.; Larsson, H.; Eliasson, A.-C. Influence of native lipids on the rheological properties of wheat flour dough and gluten. J. Texture Stud. 2006, 37, 49–62. [Google Scholar] [CrossRef]
- Santacruz, S.; Ruales, J.; Eliasson, A.C. Three under-Utilised Sources of Starch from the Andean Region in Ecuador. Part Ii. Rheological Characterisation. Carbohydr. Polym. 2003, 51, 85–92. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, L. Morphological, thermal, rheological and retrogradation properties of potato starch fractions varying in granule size. J. Sci. Food Agric. 2004, 84, 1241–1252. [Google Scholar] [CrossRef]
- Singh, A.; Geveke, D.J.; Yadav, M.P. Improvement of rheological, thermal and functional properties of tapioca starch by using gum arabic. LWT 2017, 80, 155–162. [Google Scholar] [CrossRef]
- Xu, J.; Mohamed, A.A. Thermal and rheological properties of Lupinus albus flour. J. Am. Oil Chem. Soc. 2003, 80, 763–766. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Williams, P.A. Rheological Properties of Sago Starch. J. Agric. Food Chem. 1998, 46, 4060–4065. [Google Scholar] [CrossRef]
- Choi, H.-M.; Yoo, B. Rheology of Mixed Systems of Sweet Potato Starch and Galactomannans. Starch-Stärke 2008, 60, 263–269. [Google Scholar] [CrossRef]
- Chaisawang, M.; Suphantharika, M. Effects of guar gum and xanthan gum additions on physical and rheological properties of cationic tapioca starch. Carbohydr. Polym. 2005, 61, 288–295. [Google Scholar] [CrossRef]
- Liu, S.; Chen, D.; Xu, J. The Effect of Partially Substituted Lupin, Soybean, and Navy Bean Flours on Wheat Bread Quality. Food Nutr. Sci. 2018, 9, 840–854. [Google Scholar] [CrossRef] [Green Version]
- Boonpichai, A.; Sirivongpaisal, P. Rheological Properties of Purple Sweet Potato Flour and Its Application to Noodle Product. In Proceedings of the 16th ASEAN Food Conference, Bali, Indonesia, 15–18 October 2019; Science and Technology Publications: Setúbal, Portugal, 2020. [Google Scholar]
- Fonseca-Florido, H.A.; Gómez-Aldapa, C.A.; López-Echevarría, G.; Velazquez, G.; Morales-Sánchez, E.; Castro-Rosas, J.; Méndez-Montealvo, G. Effect of Granular Disorganization and the Water Content on the Rheological Properties of Amaranth and Achira Starch Blends. LWT 2018, 87, 280–286. [Google Scholar] [CrossRef]
- Sánchez, A.; del Pilar, B. Caracterización Fisicoquimica Y Reologica De Almidon De Arracacha (Arracacia xanthorrhiza) Variedad Amarilla Procedente De La Provincia San Ignacio-Departamento De Cajamarca. Bachelor’s Thesis, Universidad Cesar Vallejo, Trujillo, Peru, 2015. [Google Scholar]
- Graf, B.L.; Zhang, L.; Corradini, M.; Kuhn, P.; Newman, S.S.; Salbaum, J.M.; Raskin, I. Physicochemical differences between malanga (Xanthosoma sagittifolium) and potato (Solanum tuberosum) tubers are associated with differential effects on the gut microbiome. J. Funct. Foods 2018, 45, 268–276. [Google Scholar] [CrossRef]
- Saleh, M.; Al-Ismail, K.; Ajo, R. Pasta quality as impacted by the type of flour and starch and the level of egg addition. J. Texture Stud. 2016, 48, 370–381. [Google Scholar] [CrossRef]
- Catunta, D. Efecto De La Deshidratación Osmótica Sobre Los Compuestos Antioxidantes En Dos Accesiones De Mashua (Tropaeolum tuberosum R&P). Bachelor’s Thesis, Universidad Nacional del Altiplano, Puno, Peru, 2016. [Google Scholar]
- Doyle, R.; Rodriguez, L. Estudio Comparativo De La Composición Química Y Carácter Reductor De Dos Variedades De Tropaeolum Tuberosum (Ruíz Y Pavón, Kuntze) Mashua. Bachelor’s Thesis, Universidad de Guayaquil, Guayaquil, Ecuador, 2018. [Google Scholar]
- Aleman, A.; Blanco-Pascual, N.; Montero, M.; Gómez-Guillén, M. Simple and efficient hydrolysis procedure for full utilization of the seaweed Mastocarpus stellatus to produce antioxidant films. Food Hydrocoll. 2016, 56, 277–284. [Google Scholar] [CrossRef]
- Pérez-Balladares, D.; Castañeda-Terán, M.; Granda-Albuja, M.G.; Tejera, E.; Iturralde, G.; Granda-Albuja, S.; Jaramillo-Vivanco, S.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J. Chemical Composition and Antioxidant Activity of the Main Fruits, Tubers and Legumes Traditionally Consumed in the Andean Regions of Ecuador as a Source of Health-Promoting Compounds. Plant Foods Hum. Nutr. 2019, 74, 350–357. [Google Scholar] [CrossRef]
- Córdova-Ramos, J.S.; Glorio-Paulet, P.; Hidalgo, A.; Camarena, F. Efecto Del Proceso Tecnológico Sobre La Capacidad Antioxidante Y Compuestos Fenólicos Totales Del Lupino Andino (Lupinus mutabilis Sweet). Sci. Agropecu. 2020, 11, 157–165. [Google Scholar] [CrossRef]







| Parameters | Camote | Oca | Mashua | Achira | Arracacha | Taro | Tarwi |
|---|---|---|---|---|---|---|---|
| Moisture | 6.20 ± 0.27 d | 16.40 ± 0.18 b | 18.87 ± 0.06 a | 5.94 ± 0.12 d | 6.16 ± 0.19 d | 6.22 ± 0.26 d | 7.00 ± 0.03 c |
| Protein | 4.65 ± 0.24 d | 1.63 ± 0.07 e | 9.12 ± 0.13 b | 4.80 ± 0.15 d | 2.10 ± 0.18 e | 8.37 ± 0.14 c | 52.82 ± 0.02 a |
| Fat | 0.37 ± 0.06 b | 1.06 ± 0.06 b | 0.59 ± 0.07 b | 0.63 ± 0.29 b | 0.69 ± 0.08 b | 0.73 ± 0.08 b | 17.78 ± 0.64 a |
| Ash | 3.24 ± 0.49 d | 3.04 ± 0.24 d | 4.94 ± 0.05 bc | 8.04 ± 0.04 a | 4.49 ± 0.18 c | 5.55 ± 0.01 b | 2.95 ± 0.07 d |
| Fiber | 11.19 ± 0.11 d | 5.33 ± 0.05 g | 9.60 ± 0.05 e | 11.51 ± 0.05 c | 9.25 ± 0.02 f | 14.90 ± 0.01 a | 12.58 ± 0.01 b |
| Total CH | 74.35 ± 0.36 b | 72.54 ± 0.10 c | 56.89 ± 0.25 f | 69.09 ± 0.04 d | 77.31 ± 0.45 a | 64.23 ± 0.17 e | 6.86 ± 0.60 g |
| Starch | 40.05 ± 0.55 d | 28.12 ± 0.39 e | 22.21 ± 0.30 f | 52.18 ± 0.72 b | 42.82 ± 0.59 c | 59.98 ± 0.82 a | 5.90 ± 0.08 g |
| Amylose | 6.23 ± 0.13 d | 9.87 ± 0.36 b | 9.68 ± 0.13 b | 15.20 ± 0.22 a | 4.49 ± 0.07 e | 8.94 ± 0.08 c | 1.95 ± 0.01 f |
| Amylopectin | 33.82 ± 0.42 c | 18.25 ± 0.03 d | 12.53 ± 0.17 e | 36.99 ± 0.50 b | 38.33 ± 0.52 b | 51.04 ± 0.74 a | 3.95 ± 0.09 f |
| Calories | 341.73 ± 1.65 b | 316.88 ± 0.05 d | 288.48 ± 0.98 e | 324.18 ± 0.55 c | 343.66 ± 0.01 b | 326.77 ± 0.57 c | 423.92 ± 0.49 a |
| pH | 5.85 ± 0.01 e | 6.35 ± 0.01 ab | 5.88 ± 0.01 de | 6.05 ± 0.01 c | 6.29 ± 0.02 b | 5.97 ± 0.01 cd | 6.41 ± 0.09 a |
| Acidity | 0.25 ± 0.01 e | 0.24 ± 0.02 e | 1.03 ± 0.03 a | 0.50 ± 0.03 b | 0.31 ± 0.01 d | 0.43 ± 0.03 c | 0.12 ± 0.01 f |
| Sample | Camote | Oca | Mashua | Achira | Arracacha | Taro | Tarwi |
|---|---|---|---|---|---|---|---|
| L* | 64.18 ± 0.12 e | 69.00 ± 0.28 d | 59.20 ± 0.21 g | 61.78 ± 0.62 f | 72.08 ± 0.26 c | 78.66 ± 0.37 a | 77.37 ± 0.14 b |
| a* | 8.17 ± 0.20 b | 5.26 ± 0.14 c | 10.83 ± 0.12 a | 5.89 ± 0.35 c | 4.46 ± 0.11 d | 2.31 ± 0.52 e | 0.32 ± 0.13 f |
| b* | 7.89 ± 0.60 g | 22.71 ± 0.24 c | 39.34 ± 0.18 a | 21.40 ± 0.15 d | 18.98 ± 0.14 e | 12.62 ± 0.07 f | 25.06 ± 0.11 b |
| C* | 11.37 ± 0.30 g | 23.49 ± 0.16 c | 40.81 ± 0.18 a | 22.20 ± 0.15 d | 19.45 ± 0.12 e | 12.84 ± 0.11 f | 25.36 ± 0.14 b |
| °H | 44.40 ± 0.50 g | 83.95 ± 0.40 c | 77.30 ± 0.30 e | 68.82 ± 0.60 f | 78.94 ± 0.50 d | 86.80 ± 0.60 b | 92.59 ± 0.80 a |
| WI | 62.43 ± 0.35 c | 61.12 ± 0.47 d | 42.30 ± 0.23 f | 55.80 ± 0.30 e | 65.97 ± 0.43 b | 75.10 ± 0.40 a | 66.97 ± 0.35 b |
| Sample | Fineness Index | Uniformity Index (g/10 g Sample) | Bulk Density (g/cm3) | ||
|---|---|---|---|---|---|
| Thick | Medium | Fine | |||
| Camote | 3.29 ± 0.05 b | 6.05 ± 0.01 b | 1.39 ± 0.03 f | 2.55 ± 0.01 c | 0.62 ± 0.01 bc |
| Oca | 2.88 ± 0.01 d | 5.31 ± 0.05 e | 1.32 ± 0.04 f | 3.37 ± 0.04 b | 0.71 ± 0.01 a |
| Mashua | 3.26 ± 0.08 bc | 5.89 ± 0.06 c | 1.50 ± 0.05 e | 2.49 ± 0.11 c | 0.65 ± 0.01 bc |
| Achira | 2.87 ± 0.07 d | 3.22 ± 0.02 f | 1.75 ± 0.01 d | 5.03 ± 0.03 a | 0.63 ± 0.01 bc |
| Arracacha | 3.12 ± 0.02 c | 5.64 ± 0.06 d | 1.85 ± 0.01 c | 2.51 ± 0.05 c | 0.67 ± 0.04 ab |
| Taro | 3.33 ± 0.01 b | 6.01 ± 0.01 bc | 1.96 ± 0.01 b | 2.04 ± 0.01 d | 0.60 ± 0.01 c |
| Tarwi | 3.84 ± 0.08 a | 6.39 ± 0.03 a | 2.99 ± 0.03 a | 0.53 ± 0.07 e | 0.47 ± 0.01 d |
| Particle Size * (%) | Camote | Oca | Mashua | Achira | Arracacha | Taro | Tarwi |
|---|---|---|---|---|---|---|---|
| 0–10 µm | 43.3 ± 0.02 | - | 40 ± 0.05 | - | 21.7 ± 0.02 | 90.6 ± 0.02 | 50.0 ± 0.01 |
| 10–20 µm | 53.3 ± 0.01 | 25.0 ± 0.02 | 40 ± 0.04 | - | 60.9 ± 0.01 | 6.3 ± 0.01 | - |
| 20–30 µm | 3.3 ± 0.04 | 9.4 ± 0.06 | 3.2 ± 0.01 | 17.4 ± 0.03 | 3.1 ± 0.02 | - | |
| 30–40 µm | - | 34.4 ± 0.03 | 20 ± 0.02 | 38.7 ± 0.05 | - | - | 50.0 ± 0.01 |
| 40–50 µm | - | 18.8 ± 0.04 | 19.4 ± 0.03 | - | - | - | |
| 50–60 µm | - | 12.5 ± 0.02 | 38.7 ± 0.02 | - | - | - | |
| Average particle size (µm) | 12.14 | 34.54 | 14.8 | 44.89 | 8.97 | 5.62 | 20 |
| Piled-up Size * (%) | |||||||
| 0–30 µm | - | - | - | - | - | 100 | - |
| 30–60 µm | - | - | - | - | - | - | 100 |
| 60–90 µm | - | - | 100 | - | - | - | - |
| Average piled-up particles size | - | - | 80.36 | - | - | 19.51 | 42.22 |
| Samples | To | Tm | Tc | ΔH |
|---|---|---|---|---|
| (°C) | (°C) | (°C) | (mJ/mg) | |
| Camote | 75.76 ± 0.7 a | 81.48 ± 0.1 a | 87.21 ± 0.4 ab | 0.55 ± 0.1 c |
| Oca | 68.58 ± 0.6 bc | 72.89 ± 0.6 bc | 78.03 ± 0.6 b | 0.87 ± 0.1 ab |
| Mashua | 69.86 ± 0.1 b | 73.81 ± 0.7 b | 78.29 ± 0.9 b | 1.42 ± 0.5 a |
| Achira | 64.03 ± 0.1 cd | 68.74 ± 0.4 c | 73.02 ± 0.1 b | 0.54 ± 0.1 c |
| Arracacha | 62.26 ± 0.3 d | 68.46 ± 0.1 c | 74.26 ± 0.4 b | 0.76 ± 0.1 b |
| Taro | - | - | - | - |
| Tarwi | 64.54 ± 0.9 cd | 79.89 ± 0.6 ab | 97.22 ± 0.6 a | 1.41 ± 0.5 a |
| Rheological Parameters | Camote | Oca | Mashua | Achira | Arracacha | Taro | Tarwi | |
|---|---|---|---|---|---|---|---|---|
| n | 0.1513 ± 0.02 ab | 0.1651 ± 0.01 ab | 0.1558 ± 0.01 ab | 0.1394 ± 0.02 ab | 0.1813 ± 0.01 a | 0.1201 ± 0.02 b | 0.1201 ± 0.02 b | |
| ω (1 rad/s) | G′ | 3259.00 ± 0.49 cz | 347.02 ± 0.06 gz | 635.21 ± 0.10 fz | 3837.40 ± 0.53 az | 1130.00 ± 0.20 ez | 2259.30 ± 0.39 dz | 3528.00 ± 0.42 bz |
| G″ | 2465.90 ± 0.45 cz | 296.43 ± 0.06 gz | 578.45 ± 0.10 fz | 2347.00 ± 0.42 dz | 818.99 ± 0.17 ez | 2609.00 ± 0.42 bz | 2692.60 ± 0.41 az | |
| Tanδ | 0.76 ± 0.00 dz | 0.85 ± 0.00 cz | 0.91 ± 0.00 bz | 0.61 ± 0.00 fz | 0.72 ± 0.00 ey | 1.15 ± 0.00 aw | 0.76 ± 0.00 dz | |
| ω (30 rad/s) | G′ | 5452.21 ± 0.03 by | 608.45 ± 0.00 gy | 1.079.08 ± 0.01 fy | 6165.21 ± 0.03 ay | 2093.54 ± 0.01 ey | 4063.73 ± 0.02 dy | 5308.00 ± 0.02 cy |
| G″ | 4556.13 ± 0.03 ay | 602.83 ± 0.00 gy | 1.019.08 ± 0.01 fy | 4295.34 ± 0.03 dy | 1638.56 ± 0.01 ey | 4509.65 ± 0.02 by | 4496.99 ± 0.02 cy | |
| Tanδ | 0.84 ± 0.00 ey | 0.99 ± 0.00 by | 0.94 ± 0.00 cy | 0.70 ± 0.00 gy | 0.78 ± 0.00 fx | 1.11 ± 0.00 ax | 0.85 ± 0.00 dy | |
| ω (60 rad/s) | G′ | 6055.06 ± 0.02 bx | 682.22 ± 0.00 gx | 1.202.14 ± 0.00 fx | 6790.65 ± 0.02 ax | 2373.87 ± 0.01 ex | 4580.18 ± 0.01 dx | 5768.79 ± 0.01 cx |
| G″ | 5163.35 ± 0.02 ax | 696.66 ± 0.00 gx | 1.143.74 ± 0.00 fx | 4858.37 ± 0.01 dx | 1887.31 ± 0.01 ex | 5041.72 ± 0.01 bx | 4992.49 ± 0.01 cx | |
| Tanδ | 0.85 ± 0.00 ex | 1.02 ± 0.00 bx | 0.95 ± 0.00 cx | 0.72 ± 0.00 gx | 0.80 ± 0.00 fw | 1.10 ± 0.00 ay | 0.87 ± 0.00 dx | |
| ω (90 rad/s) | G′ | 6438.15 ± 0.01 bw | 729.46 ± 0.00 gw | 1.280.53 ± 0.00 fw | 7185.52 ± 0.01 aw | 2554.95 ± 0.01 ew | 4912.20 ± 0.01 dw | 6056.66 ± 0.01 cw |
| G″ | 5555.41 ± 0.01 aw | 758.18 ± 0.00 gw | 1.223.62 ± 0.00 fw | 5221.34 ± 0.01 dw | 2049.98 ± 0.00 ew | 5381.60 ± 0.01 bw | 5307.28 ± 0.01 cw | |
| Tanδ | 0.86 ± 0.00 ew | 1.04 ± 0.00 bw | 0.96 ± 0.00 cw | 0.73 ± 0.00 gw | 0.80 ± 0.00 fw | 1.10 ± 0.00 ay | 0.88 ± 0.00 dw | |
| Variables | CP 1 | CP 2 | CP 3 | CP 4 |
|---|---|---|---|---|
| Variance (%) | 43.4 | 24.3 | 13.9 | 9.2 |
| Cumulative (%) | 43.4 | 67.7 | 81.6 | 90.8 |
| Moisture | −0.84 | −0.41 | 0.16 | −0.05 |
| Protein | 0.59 | −0.8 | −0.06 | −0.08 |
| Fat | 0.57 | −0.8 | −0.16 | −0.05 |
| Ash | 0.07 | 0.48 | 0.69 | −0.39 |
| Fiber | 0.81 | 0.12 | 0.24 | −0.24 |
| Total CH | −0.5 | 0.85 | −0.03 | 0.14 |
| Starch | 0.1 | 0.96 | 0.12 | −0.24 |
| Amylose | −0.43 | −0.7 | 0.51 | −0.1 |
| Amylopectin | 0.43 | 0.7 | −0.51 | 0.1 |
| L* | 0.55 | −0.07 | −0.68 | −0.45 |
| a* | −0.8 | 0.14 | 0.36 | 0.44 |
| b* | −0.54 | −0.68 | 0.36 | −0.22 |
| C* | −0.57 | −0.67 | 0.39 | −0.13 |
| H | 0.12 | −0.53 | −0.13 | −0.79 |
| WI | 0.63 | 0.3 | −0.63 | −0.25 |
| Fineness Index | 0.75 | −0.59 | 0.09 | 0.01 |
| Bulk Density | −0.82 | 0.54 | −0.05 | 0.06 |
| Granular Size | −0.02 | −0.01 | 0.47 | −0.01 |
| Tm | −0.01 | −0.55 | 0.02 | 0.81 |
| FH | 0.06 | 0.53 | 0.68 | −0.44 |
| Solubility | −0.54 | 0.75 | 0.1 | 0.23 |
| Water Absorption Capacity | −0.49 | 0.58 | 0.02 | −0.48 |
| n | −0.81 | 0.17 | −0.32 | 0.31 |
| G′1 | 0.88 | 0.06 | 0.37 | 0.21 |
| G″1 | 0.95 | 0.11 | 0.24 | 0.04 |
| G′30 | 0.89 | 0.16 | 0.36 | 0.21 |
| G″30 | 0.94 | 0.17 | 0.24 | 0.08 |
| G′60 | 0.89 | 0.18 | 0.35 | 0.2 |
| G″60 | 0.94 | 0.19 | 0.25 | 0.09 |
| G′90 | 0.89 | 0.19 | 0.35 | 0.2 |
| G″90 | 0.94 | 0.2 | 0.25 | 0.1 |
| Phenols | −0.63 | −0.14 | 0.64 | 0.04 |
| ABTS | 0.42 | −0.24 | −0.47 | 0.35 |
| Cophenetic correlation = 0.988 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, D.; Arancibia, M.; Ocaña, I.; Rodríguez-Maecker, R.; Bedón, M.; López-Caballero, M.E.; Montero, M.P. Characterization and Technological Potential of Underutilized Ancestral Andean Crop Flours from Ecuador. Agronomy 2021, 11, 1693. https://doi.org/10.3390/agronomy11091693
Salazar D, Arancibia M, Ocaña I, Rodríguez-Maecker R, Bedón M, López-Caballero ME, Montero MP. Characterization and Technological Potential of Underutilized Ancestral Andean Crop Flours from Ecuador. Agronomy. 2021; 11(9):1693. https://doi.org/10.3390/agronomy11091693
Chicago/Turabian StyleSalazar, Diego, Mirari Arancibia, Iván Ocaña, Roman Rodríguez-Maecker, Mauricio Bedón, María Elvira López-Caballero, and María Pilar Montero. 2021. "Characterization and Technological Potential of Underutilized Ancestral Andean Crop Flours from Ecuador" Agronomy 11, no. 9: 1693. https://doi.org/10.3390/agronomy11091693
APA StyleSalazar, D., Arancibia, M., Ocaña, I., Rodríguez-Maecker, R., Bedón, M., López-Caballero, M. E., & Montero, M. P. (2021). Characterization and Technological Potential of Underutilized Ancestral Andean Crop Flours from Ecuador. Agronomy, 11(9), 1693. https://doi.org/10.3390/agronomy11091693