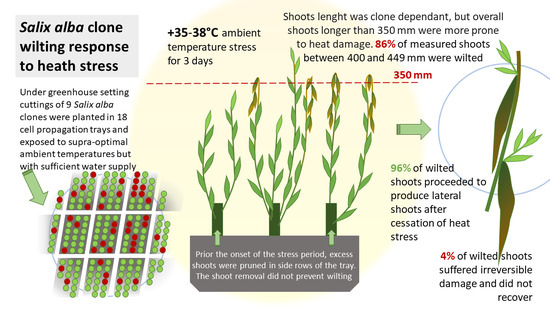
Salix alba Clone Wilting Response to Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Planting Material
2.2. Study Setting and Measurements
2.3. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. Societal Transformation for Peace in El Salvador; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Grossnickle, S.C.; Kiiskila, S.B.; Haase, D.L. Seedling ecophysiology: Five questions to explore in the nursery for optimizing subsequent field success. Tree Plant. Notes 2020, 63, 112–127. [Google Scholar]
- Lamaoui, M.; Jemo, M.; Datla, R.; Bekkaoui, F. Heat and drought stresses in crops and approaches for their mitigation. Front. Chem. 2018, 6, 26. [Google Scholar] [CrossRef]
- Punia, S.M.S.; Yadan, R. Evaluation of mungbean (Vigna radiata L. Wilczek) genotypes for high temperature and drought tolerance. In The Ecoscan, Proceedings of the National Conference on Harmony with Nature in Context of Resource Conservation and Climate Change, Hazaribag, India, 22–24 October 2016; ResearchGate: Berlin, Germany, 2016. [Google Scholar]
- Quintero-Calderón, E.H.; Sánchez-Reinoso, A.D.; Chávez-Arias, C.C.; Garces-Varon, G.; Restrepo-Díaz, H. Rice seedlings showed a higher heat tolerance through the foliar application of biostimulants. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 49, 12120. [Google Scholar] [CrossRef]
- Mancuso, S. Heat tolerance in olive. Adv. Hortic. Sci. 2002, 16, 125–130. [Google Scholar]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Yu, X.; Ottosen, C.-O.; Rosenqvist, E.; Zhao, L.; Wang, Y.; Yu, W.; Zhao, T.; Wu, Z. Drought stress had a predominant effect over heat stress on three tomato cultivars subjected to combined stress. BMC Plant Biol. 2017, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwivedi, S.; Basu, S.; Kumar, S.; Kumar, G.; Prakash, V.; Kumar, S.; Mishra, J.; Bhatt, B.; Malviya, N.; Singh, G.; et al. Heat stress induced impairment of starch mobilisation regulates pollen viability and grain yield in wheat: Study in Eastern Indo-Gangetic Plains. Field Crop. Res. 2017, 206, 106–114. [Google Scholar] [CrossRef]
- Chavan, S.G.; Duursma, R.A.; Tausz, M.; Ghannoum, O. Elevated CO2 alleviates the negative impact of heat stress on wheat physiology but not on grain yield. J. Exp. Bot. 2019, 70, 6447–6459. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, A.; Sita, K.; Siddique, K.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; Hanumantha Rao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and Heat-Stress Effects on Seed Filling in Food Crops: Impacts on Functional Biochemistry, Seed Yields, and Nutritional Quality. Front. Plant Sci. 2018, 871, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Helgerson, O.T. Heat damage in tree seedlings and its prevention. New For. 1989, 3, 333–358. [Google Scholar] [CrossRef]
- Li, M.; Jannasch, A.H.; Jiang, Y. Growth and hormone alterations in response to heat stress in perennial ryegrass accessions differing in heat tolerance. J. Plant Growth Regul. 2019, 39, 1022–1029. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Bhandari, K.; Kumar, S.; Kumar, J.; Prasad, P.V.; Siddique, K.; Nayyar, H. Influence of drought and heat stress, applied independently or in combination during seed development, on qualitative and quantitative aspects of seeds of lentil (Lens culinaris Medikus) genotypes, differing in drought sensitivity. Plant Cell Environ. 2019, 42, 198–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Song, H.; Tang, D.; Zhang, S. Sexually differential tolerance to water deficiency of Salix paraplesia—A female-biased alpine willow. Ecol. Evol. 2019, 9, 8450–8464. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Dong, N.; Maire, V.; Prentice, I.C.; Westoby, M.; Díaz, S.; Gallagher, R.V.; Jacobs, B.F.; Kooyman, R.; Law, E.A.; et al. Global climatic drivers of leaf size. Science 2017, 357, 917–921. [Google Scholar] [CrossRef] [Green Version]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total. Environ. 2021, 779, 146466. [Google Scholar] [CrossRef] [PubMed]
- R.C. Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 28 June 2021).
- Sreeman, S.M.; Vijayaraghavareddy, P.; Sreevathsa, R.; Rajendrareddy, S.; Arakesh, S.; Bharti, P.; Dharmappa, P.; Soolanayakanahally, R. Introgression of physiological traits for a comprehensive improvement of drought adaptation in crop plants. Front. Chem. 2018, 6, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olyslaegers, G.; Nijs, I.; Roebben, J.; Kockelbergh, F.; Vanassche, F.; Lakers, M.; Verbelen, J.-P.; Samson, R.; LeMeur, R.; Impens, I. Morphological and physiological indicators of tolerance to atmospheric stress in two sensitive and two tolerant tea clones in South Africa. Exp. Agric. 2002, 38, 397–410. [Google Scholar] [CrossRef]
- Andivia, E.; Villar-Salvador, P.; Oliet, J.A.; Puértolas, J.; Dumroese, R.K.; Ivetić, V.; Molina-Venegas, R.; Arellano, E.C.; Li, G.; Ovalle, J.F. Climate and species stress resistance modulate the higher survival of large seedlings in forest restorations worldwide. Ecol. Appl. 2021, 31, e2394. [Google Scholar] [CrossRef]
- Zotz, G.; Hietz, P.; Schmidt, G. Small plants, large plants: The importance of plant size for the physiological ecology of vascular epiphytes. J. Exp. Bot. 2001, 52, 2051–2056. [Google Scholar] [CrossRef]
- Wisniewski, M.; Sauter, J.; Fuchigami, L.; Stepien, V. Effects of near-lethal heat stress on bud break, heat-shock proteins and ubiquitin in dormant poplar (Populus nigra Charkowiensis x P. nigra incrassata). Tree Physiol. 1997, 17, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.; Shahwar, D. Role of heat shock proteins (HSPs) and heat stress tolerance in crop plants. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 211–234. [Google Scholar]
- Wang, G.; Zhou, Q.; He, M.; Zhong, X.; Tang, G. Wilting index and root morphological characteristics used as drought-tolerance variety selection at the seedling stage in soybean (Glycine max L.). Plant Growth Regul. 2020, 92, 29–42. [Google Scholar] [CrossRef]
- McGregor, I.R.; Helcoski, R.; Kunert, N.; Tepley, A.J.; Gonzalez-Akre, E.B.; Herrmann, V.; Zailaa, J.; Stovall, A.E.L.; Bourg, N.A.; McShea, W.J.; et al. Tree height and leaf drought tolerance traits shape growth responses across droughts in a temperate broadleaf forest. New Phytol. 2021, 231, 601–616. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Zhou, J.; Zhou, Y.-H.; Yu, J.-Q. Role of hormones in plant adaptation to heat stress. In Plant Hormones under Challenging Environmental Factors; Ahammed, G.J., Yu, J.-Q., Eds.; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive response of a native Mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Sarwar, M.; Saleem, M.F.; Ullah, N.; Ali, S.; Rizwan, M.; Shahid, M.R.; Alyemeni, M.N.; Alamri, S.; Ahmad, P. Role of mineral nutrition in alleviation of heat stress in cotton plants grown in glasshouse and field conditions. Sci. Rep. 2019, 9, 13022. [Google Scholar] [CrossRef] [Green Version]
- Del Castello, F.; Nejamkin, A.; Cassia, R.; Correa-Aragunde, N.; Fernández, B.; Foresi, N.; Lombardo, C.; Ramirez, L.; Lamattina, L. The era of nitric oxide in plant biology: Twenty years tying up loose ends. Nitric Oxide 2019, 85, 17–27. [Google Scholar] [CrossRef]
- Waraich, E.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef] [Green Version]
- Saha, G.; Mostofa, M.G.; Rahman, M.; Tran, L.-S.P. Silicon-mediated heat tolerance in higher plants: A mechanistic outlook. Plant Physiol. Biochem. 2021, 166, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Mathers, H.M. Summary of temperature stress issues in nursery containers and current methods of protection. HortTechnology 2003, 13, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.N.; Zhang, L.; Yang, L.; Islam, R.; Liu, Y.; Luo, H.; Yang, P.; Wang, Q.; Chan, Z. Transcriptomic profiling of tall fescue in response to heat stress and improved thermotolerance by melatonin and 24-epibrassinolide. BMC Genom. 2018, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Hussain, M.; Ejaz, M.; Yasmeen, F. Effect of silver nanoparticles on growth of wheat under heat stress. Iran J. Sci. Technol. Trans. A Sci. 2019, 43, 387–395. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Zhang, X.; Du, H.; Xu, B.; Huang, B. Melatonin suppression of heat-induced leaf senescence involves changes in abscisic acid and cytokinin biosynthesis and signaling pathways in perennial ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- DesRochers, A.; Tremblay, F. The effect of root and shoot pruning on early growth of hybrid poplars. For. Ecol. Manag. 2009, 258, 2062–2067. [Google Scholar] [CrossRef]
- Mexal, J.G.; Landis, T.D. Target seedling concepts: Height and diameter. In General Technical Report RM-200, Proceedings of the Target Seedling Symposium: Combined Meeting of the Western Forest Nursery Associations, Roseburg, OR, USA, 13–17 August 1990; Rose, R., Campbell, S.J., Landis, T.D., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Roseburg, OR, USA, 1990; pp. 17–35. [Google Scholar] [CrossRef] [Green Version]
- South, D.B. Effects of top-pruning on survival of southern pines and hardwoods. In General Technical Report SRS-20, Proceedings of the Ninth Biennial Southern Silvicultural Research Conference, Clemson, SC, USA, 25–27 February 1997; Waldrop, T.A., Ed.; USDA Forest Service Southern Research Station: Ashville, NC, USA, 1998. [Google Scholar]

| Clone | Number of Cuttings | Country of Origin | Name/Place of Origin |
|---|---|---|---|
| 0205K | 33 | Germany | Pyramidalis |
| 0206L | 72 | Germany | Steinach XI |
| 0207M | 80 | Germany | Rockanje |
| 0208N | 72 | Germany | Botanischer Garten München |
| 0211S | 63 | Germany | Isar IX |
| 0214W | 72 | Germany | Weide Godesberg |
| 0218B | 72 | Germany | Eckartsau |
| LVX1 | 72 | Latvia | Kalsnava |
| LVX2 | 54 | Latvia | Kalsnava |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celma, S.; Vendina, V.; Lazdina, D. Salix alba Clone Wilting Response to Heat Stress. Agronomy 2021, 11, 1821. https://doi.org/10.3390/agronomy11091821
Celma S, Vendina V, Lazdina D. Salix alba Clone Wilting Response to Heat Stress. Agronomy. 2021; 11(9):1821. https://doi.org/10.3390/agronomy11091821
Chicago/Turabian StyleCelma, Santa, Viktorija Vendina, and Dagnija Lazdina. 2021. "Salix alba Clone Wilting Response to Heat Stress" Agronomy 11, no. 9: 1821. https://doi.org/10.3390/agronomy11091821
APA StyleCelma, S., Vendina, V., & Lazdina, D. (2021). Salix alba Clone Wilting Response to Heat Stress. Agronomy, 11(9), 1821. https://doi.org/10.3390/agronomy11091821