A Review of the Biology, Ecology, and Management of the South American Locust, Schistocerca cancellata (Serville, 1838), and Future Prospects
Abstract
:1. Introduction
2. Biology and Ecology of S. cancellata
2.1. Phenotypic Plasticity Traits in S. cancellata
2.1.1. Nymphs
2.1.2. Adults
2.2. Habitats and Feeding Preferences
2.3. Population Dynamics
2.3.1. Life Cycle Parameters
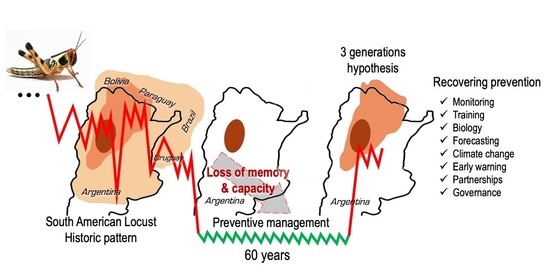
2.3.2. The Third Generation Hypothesis
2.4. Natural Enemies and Biological Control
3. Management of SAL: An Historical Perspective
3.1. Early Management Efforts against SAL Plagues
3.2. The Offensive Management Period
3.3. Advances That Formed the Basis for Preventive Management
3.4. The Preventive Management Program
4. Management under A New Plague Period
4.1. The Resurgence of SAL
4.2. Re-Establishing a Sustained SAL Preventive Management Program
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gastón, J. Síntesis histórica de las invasiones de langosta en la Argentina. Sec. Agric. Gan. Misc. 1969, 434, 1–32. [Google Scholar]
- Hunter, D.; Cosenzo, E. The origin of plagues and recent outbreaks of the South American locust, Schistocerca cancellata (Orthoptera: Acrididae) in Argentina. Bull. Èntomol. Res. 1990, 80, 295–300. [Google Scholar] [CrossRef]
- Medina, H.E.; Cease, A.J.; Trumper, E. The resurgence of the South American locust (Schistocerca cancellata). Metaleptea 2017, 37, 17–21. [Google Scholar]
- Zhang, L.; Lecoq, A.; Latchininsky, A.V.; Hunter, D.M. Locust and Grasshopper Management. Annual Rev. Entomol. 2019, 64, 15–34. [Google Scholar] [CrossRef]
- Cullen, D.A.; Cease, A.J.; Latchininsky, A.V.; Ayali, A.; Berry, K.; Buhl, J.; Rogers, S.M. From molecules to manangement: Mechanisms and consequences of locust phase polyphenism. Adv. Insect Physiol. 2017, 53, 167–285. [Google Scholar]
- Song, H.; Foquet, B.; Mariño-Pérez, R.; Woller, D.A. Phylogeny of locusts and grasshoppers reveals complex evolution of den-sity-dependent phenotypic plasticity. Sci. Rep. 2017, 7, 6606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, A.W. A reclassification of the Schistocerca americana complex (Orthoptera: Acrididae). Acrida 1981, 10, 61–77. [Google Scholar]
- Song, H.; Cigliano, M.M.; Lange, C.E. South American Locust. Schistocerca cancellata (Serville, 1838) (Acrididae). In Encyclopedia of Pest Orthoptera of the World; Lecoq, M., Zhang, L., Eds.; China Agricultural University Press: Beijing, China, 2019; pp. 198–203. [Google Scholar]
- Pocco, M.E.; Cigliano, M.M.; Foquet, B.; Lange, C.E.; Nieves, E.L.; Song, H. Density-Dependent Phenotypic Plasticity in the South American Locust, Schistocerca cancellata (Orthoptera: Acrididae). Ann. Èntomol. Soc. Am. 2019, 112, 458–472. [Google Scholar] [CrossRef]
- Simpson, S.J.; Despland, E.; Hägele, B.F.; Dodgson, T. Gregarious behavior in desert locusts is evoked by touching their back legs. Proc. Natl. Acad. Sci. USA 2001, 98, 3895–3897. [Google Scholar] [CrossRef] [Green Version]
- Köhler, P. Relación entre biosfera y ecosistemas de la langosta migratoria. Fund. Inst. Miguel Lillo Miscelánea 1979, 66, 37. [Google Scholar]
- Piou, C.; Zagaglia, G.; Medina, H.E.; Trumper, E.; Brizuela, X.R.; Maeno, K.O. Band movement and thermoregulation in Schistocerca cancellata. J. Insect Physiol. 2021, 104328. [Google Scholar] [CrossRef] [PubMed]
- Cigliano, M.M.; Pocco, M.; Lange, C.E. Acridoideos (Orthoptera) de Importancia Agroeconómica en la República Argentina. In Biodiversidad de Artrópodos Argentinos; Juñent, R., Ed.; Editorial INSUE—UNT: San Miguel de Tucumán, Argentina, 2014; Volume 3, pp. 11–36. [Google Scholar]
- Dirsh, V.M. Morphometrical Studies on Phases of the Desert Locust (Schistocerca gregaria, Forskal). Anti-Locust Bull. 1953, 16, 1–34. [Google Scholar]
- Uvarov, B.P. Grasshoppers and locusts: A handbook of general acridology. In Anatomy, Psysiology, Development Phase Polymorphism Introduction to Taxonomy; Cambridge University Press: Cambridge, UK, 1966; Volume 1. [Google Scholar]
- Le Gall, M.; Overson, R.; Cease, A. A Global Review on Locusts (Orthoptera: Acrididae) and Their Interactions with Livestock Grazing Practices. Front. Ecol. Evol. 2019, 7, 263. [Google Scholar] [CrossRef] [Green Version]
- COPR (Centre for Overseas Pest Research). The Locust and Grasshopper Agricultural Manual; COPR: London, UK, 1982. [Google Scholar]
- Köhler, P. Ecología de la zona central y gregarización de la langosta en la República Argentina. IDIA Supl. 1962, 7, 7–108. [Google Scholar]
- Waloff, Z.; Pedgley, D.E. Comparative biogeography and biology of the South American locust, Schistocerca cancellata (Serville), and the South African desert locust, S. gregaria flaviventris (Burmeister) (Orthoptera: Acrididae): A review. Bull. Entomol. Res. 1986, 76, 1–20. [Google Scholar]
- López, R.M.; Copa Bazán, A.F. Prospección y Ciclo Biológico de la Langosta Voladora Schistocerca cancellata (Orthoptera: Acridi-dae) en el Departamento de Santa Cruz, Bolivia, 2017. Tesis de Grado, UAGRM, Presente en la Biblioteca Rafael Peña de la Facultad de Ciencias Agrícolas con N° de registro T-2563, 2018; p. 73.
- Unterladstaetter, R. Malezas del Oriente Boliviano; CIAT: Santa Cruz de la Sierra, Bolivia, 2008; p. 542. [Google Scholar]
- Talal, S.; Cease, A.; Farington, R.; Medina, H.E.; Rojas, J.; Harrison, J. High carbohydrate diet ingestion increases post-meal lipid synthesis and drives respiratory exchange ratios above 1. J. Exp. Biol. 2021, 224, jeb240010. [Google Scholar] [CrossRef]
- White, T.C.R. The Inadequate Environment: Nitrogen and the Abundance of Animals; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Cease, A.J.; Elser, J.J.; Ford, C.F.; Hao, S.; Kang, L.; Harrison, J.F. Heavy Livestock Grazing Promotes Locust Outbreaks by Lowering Plant Nitrogen Content. Science 2012, 335, 467–469. [Google Scholar] [CrossRef]
- Word, M.L.; Hall, S.; Robinson, B.E.; Manneh, B.; Beye, A.; Cease, A.J. Soil-targeted interventions could alleviate locust and grasshopper pest pressure in West Africa. Sci. Total. Environ. 2019, 663, 632–643. [Google Scholar] [CrossRef]
- Le Gall, M.; Word, M.L.; Thompson, N.; Manneh, B.; Beye, A.; Cease, A.J. Linking land use and the nutritional ecology of herbivores: A case study with the Senegalese locust. Funct. Ecol. 2019, 34, 167–181. [Google Scholar] [CrossRef]
- Hunter, D.M.; McCulloch, L.; Wright, D.E. Lipid accumulation and migratory flight in the Australian plague locust, Chortoicetes terminifera (Walker) (Orthoptera: Acrididae). Bull. Èntomol. Res. 1981, 71, 543–546. [Google Scholar] [CrossRef]
- Herreid, C.F.; Full, R.J. Cockroaches on a treadmill: Aerobic running. J. Insect Physiol. 1984, 30, 395–403. [Google Scholar] [CrossRef]
- Rogowitz, G.; Chappell, M. Energy metabolism of eucalyptus-boring beetles at rest and during locomotion: Gender makes a difference. J. Exp. Biol. 2000, 203, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Raubenheimer, D. The Geometric Analysis of Nutrient-Allelochemical Interactions: A Case Study Using Locusts. Ecology 2001, 82, 422. [Google Scholar] [CrossRef]
- Zanotto, F.P.; Simpson, S.J.; Raubenheimer, D. The regulation of growth by locusts through post-ingestive compensation for variation in the levels of dietary protein and carbohydrate. Physiol. Èntomol. 1993, 18, 425–434. [Google Scholar] [CrossRef]
- Cease, A.J.; Harrison, J.F.; Hao, S.; Niren, D.C.; Zhang, G.; Kang, L.; Elser, J.J. Nutritional imbalance suppresses migratory phenotypes of the Mongolian locust (Oedaleus asiaticus). R. Soc. Open Sci. 2017, 4, 161039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Gall, M.; Word, M.L.; Thompson, N.; Beye, A.; Cease, A.J. Nitrogen Fertilizer Decreases Survival and Reproduction of Fe-male Locusts by Increasing Plant Protein to Carbohydrate Ratio. J. Animal Ecol. 2020, 89, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, N.E.; Wittenstein, E.; De Wysiecki, M.L.; Lange, C.E. Life History Parameters of the Gregarious Phase of the South American locust, Schistocerca cancellata (Serville) (Orthoptera: Acrididae), under Laboratory Conditions. J. Orthoptera Res. 1997, 121. [Google Scholar] [CrossRef]
- Uvarov, B.P. Biological and ecological basis of locust phases and their practical application. In Proceedings of the Fourth Inter-national Locust Conference, Cairo, Egypt, 22 April 1936; Government Press: Cairo, Egypt, 1936; Volume 7, p. 16. [Google Scholar]
- Berryman, A.A. The theory and classification of outbreaks. In Insect Outbreaks; Barbosa, P., Schultz, J.C., Eds.; Academic Press: New York, NY, USA, 1987; pp. 1–30. [Google Scholar]
- Akimenko, V.V.; Piou, C. Two-compartment age-structured model of solitarious and gregarious locust population dynamics. Math. Methods Appl. Sci. 2018, 41, 8636–8672. [Google Scholar] [CrossRef]
- Magor, J.; Lecoq, M.; Hunter, D. Preventive control and Desert Locust plagues. Crop. Prot. 2008, 27, 1527–1533. [Google Scholar] [CrossRef]
- Behmer, S.T.; Joern, A. Insect Herbivore Outbreaks Viewed through a Physiological Framework: Insights from Orthoptera. In Insect Outbreaks Reviited; Barbosa, F., Letourneau, D., Agrawaal, A., Eds.; Academic Press: Cambridge, MA, USA, 2012; pp. 3–29. [Google Scholar] [CrossRef]
- Dwyer, G.; Dushoff, J.; Yee, S.H. The combined effects of pathogens and predators on insect outbreaks. Nature 2004, 430, 341–345. [Google Scholar] [CrossRef]
- Hunter, D.M.; Elder, R.J. Rainfall sequences leading to population increases of Austracris guttulosa (Walker) (Orthoptera: Acrididae) in arid north-eastern Australia. Aust. J. Èntomol. 1999, 38, 204–218. [Google Scholar] [CrossRef]
- Barrera, M.; Turk, S. Estado actual de la langosta Schistocera cancellata paranensis (Burm.) en la Republica Argentina: Nuevos aportes a su bioecología. Acta Zool. Lilloana 1983, 27, 15–29. [Google Scholar]
- De Wysiecki, M.L.; Lange, C.E. La langosta Schistocerca cancellata Serville (Orthoptera: Acrididae: Cyrtacanthacridinae) en Argentina: Biologia, ecologia, historia y control. En: Barrientos Lozano, L. y P. In Manejo Integrado de la Langosta Centroamericana (Schistocerca piceifrons piceifrons, Walker) y Acridoideos Plaga en América Latina; Sierra, A., Ed.; Instituto Tecnológico de Ciudad Victoria: Tamaulipas, México, 2005; pp. 151–156. ISBN 970-18-7628-8. [Google Scholar]
- Daguerre, J.B. Nuestros actuales conocimientos sobre la langosta. III. Equilibrio biológico. Rev. Soc. Entomol. Argent. 1938, 10, 65–69. [Google Scholar]
- Daguerre, J.B. Informe de la Comisión Investigadora del Este. In Memoria de la Comisión Central de Investigaciones sobre la Langosta correspondiente al año; Ministerio de Agricultura de la Nación: Buenos Aires, Argentina, 1939; pp. 107–142. [Google Scholar]
- Fernández, M.L.; Copa Bazán, A.F. Evaluación de Hongos Entomopatógenos Nativos Para el Control Biológico de la Langosta Voladora Schistocerca cancellata (Orthoptera: Acrididae) en el I.I.A. “El Vallecito”, Santa Cruz, Bolivia, 2017. Tesis de Grado, UAGRM, Presente en la Biblioteca Rafael Peña de la Facultad de Ciencias Agrícolas Con N° de Registro T-2570, 2018; p. 63.
- Greathead, D.J. Natural enemies of tropical locusts and grasshoppers: Their impact and potential as biological control agents. In Biological Control of Locusts and Grasshoppers; Lomer, C.J., Prior, C., Eds.; CABI: Wallingford, UK, 1992; pp. 105–121. [Google Scholar]
- Mullié, W.C.; Keith, J.O. The Effects of Aerially Applied Fenitrothion and Chlorpyrifos on Birds in the Savannah of Northern Senegal. J. Appl. Ecol. 1993, 30, 536. [Google Scholar] [CrossRef]
- D’Hérelle, F. Sur la propagation, dans la République Argentine, de l´épizootie des sauterelles du Mexique. C. R. Acad. Sci. Paris Ser. D 1912, 154, 623–625. [Google Scholar]
- Deveson, E.; Martinez, A. Locusts in Southern Settler Societies: Argentine and Australian Experience and Responses, 1880–1940. In Environmental History in the Making: Volume I: Explaining; Vaz, E., Joanaz de Melo, C., Costa Pinto, L.M., Eds.; Environmental History; Springer International Publishing: Cham, Switzerland, 2017; pp. 259–286. ISBN 978-3-319-41085-2. [Google Scholar]
- Lange, C.E.; Wittenstein, E. Susceptibilidad de la langosta Schistocerca cancellata (Orthoptera: Acrididae) a diferentes entomo-patógenos. Rev. Soc. Entomol. Argent. 1998, 57, 19–22. [Google Scholar]
- Plischuk, S.; Pocco, M.E.; Quintana, S.; de Wysiecki, M.L.; Lange, C.E. A new symbiont associated with the South American locust Schistocerca cancellata. In Proceedings of the 13th International Congress of Orthopterology, Agadir, Morocco, 24–28 March 2019. [Google Scholar]
- Pelizza, S.A.; Medina, H.E.; Elíades, L.A.; Pocco, M.E.; Stenglein, S.A.; Lange, C.E. Virulence and enzymatic activity of three new isolates of Beauveria bassiana (Ascomycota: Hypocreales) from the South American locust Schistocerca cancellata (Orthop-tera: Acrididae). J. King Saud Univ.-Sci. 2020, 32, 44–47. [Google Scholar] [CrossRef]
- Pelizza, S.A.; Ferreri, N.A.; Elíades, L.A.; Galarza, B.; Cabello, M.N.; Russo, M.L.; Vianna, F.; Scorsetti, A.C.; Lange, C.E. Enzymatic activity and virulence of Cordyceps locustiphila (Hypocreales: Cordycipitaceae) on the South American locust Schistocerca cancellata (Orthoptera: Acrididae). J. King Saud Univ.-Sci. 2021, 33, 101411. [Google Scholar] [CrossRef]
- Zhang, L.; Hunter, D. Management of locusts and grasshoppers in China. J. Orthoptera Res. 2017, 26, 155–159. [Google Scholar] [CrossRef]
- Lange, C.E.; Mariottini, Y.; Plischuk, S.; Cigliano, M.M. Naturalized, newly-associated microsporidium continues causing epizootics and expanding its host range. Protistology 2020, 14, 32–37. [Google Scholar] [CrossRef]
- Pocco, M.E.; De Wysiecki, M.L.; Lange, C.E. Infectivity of Paranosema locustae (Microsporidia) against gregarious-phase South American locust (Orthoptera) when treated en masse. J. Invertebr. Pathol. 2020, 177, 107504. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.J.; Hunter, D.M.; Shi, Y.P. Effect of Paranosema (Nosema) locustae (Microsporidia) on morphological phase transfor-mation of Locusta migratoria manilensis (Orthoptera: Acrididae). Biocontrol Sci. Technol. 2010, 20, 683–693. [Google Scholar] [CrossRef]
- Li, A.-M.; Yue, Y.; Zhang, Y.-X.; Zhang, L.; Zhang, K.Q.; Shen, J.; Shen, S.Q.; Shi, Y.P. Effects of Paranosema locustae (Micro-sporidia) on the development and morphological phase transformation of Locusta migratoria (Orthoptera: Acrididae) through modulation of the neurotransmitter taurine. J. Integr. Agric. 2020, 19, 204–210. [Google Scholar] [CrossRef]
- Tranchini, E.M. Politicas Agrarias y Comportamientos Sociales: El caso de la Plaga de Langosta en la Región Pampeana; Facultad de Humanidades y Ciencias de la Educación, Uni-versidad Nacional de La Plata: La Plata, Argentina, 1995; p. 97. [Google Scholar]
- Libonati, V.J. La Langosta: Su Historia en la Argentina (Síntesis) Buenos Aires: Casartelli y Fiol. 1928; p. 159.
- Lizer y Trelles, C.C. La Lucha Moderna Contra la Langosta en el País; Academia Nacional de Agronomía y Veterinaria: Buenos Aires, Argentina; Universidad Nacional de La Plata: La Plata, Argentina, 1940; p. 33. [Google Scholar]
- Uvarov, B.P. A Revision of the Genus Locusta, L. (= Pachytylus, Fieb.), with a New Theory as to the Periodicity and Migrations of Locusts. Bull. Entomol. Res. 1921, 12, 135–163. [Google Scholar] [CrossRef] [Green Version]
- Predtechensky, S.A. The annual cycle of the Desert locust (Schistocerca gregaria Forsk.), its migrations and periodicity in Persia and adjacent countries of tropical and subtropical Asia. Bull. Plant Prot. Entomol. 1935, 12, 5–135. [Google Scholar]
- Waloff, Z. Seasonal breeding and migration of the Desert locust (Schistocerca gregaria Forskål) in Eastern Africa. Anti-Locust Mem. 1946, 1, 1–74. [Google Scholar]
- Kennedy, J.S. The migration of the Desert locust (Schistocerca gregaria Forsk.). I. The behaviour of swarms. II. A theory of long-range migrations. Biol. Sciences Rev. 1951, 235, 163–290. [Google Scholar]
- Piou, C.; Bacar, M.E.H.J.; Ebbe, M.A.O.B.; Chihrane, J.; Ghaout, S.; Cisse, S.; Lecoq, M.; Halima, T.B. Mapping the spatiotemporal distributions of the Desert Locust in Mauritania and Morocco to improve preventive management. Basic Appl. Ecol. 2017, 25, 37–47. [Google Scholar] [CrossRef]
- Deveson, E.D.; Drake, V.A.; Hunter, D.M.; Walker, P.W.; Wang, H.K. Evidence from traditional and new technologies for northward migrations of Australian plague locusts (Chortoicetes terminifera) (Walker) (Orthoptera: Acrididae) to western Queensland. Austral Ecol. 2005, 30, 920–935. [Google Scholar] [CrossRef]
- Köhler, P. Informe de la Comisión Investigadora del Oeste. Memoria. Comn. Cent. Invest. Langosta Corresp. Al Año 1936, 1936, 17–106. [Google Scholar]
- Archibald, E.D. Locusts and Sun-Spots. Nature 1878, 19, 145–146. [Google Scholar] [CrossRef]
- Swinton, A.H. Locust and sunspots. Science 1881, 2, 255. [Google Scholar] [CrossRef]
- Uichanco, L.B. Secular Trends of Locust Outbreaks in the Philippines and their apparent Relation with Sunspot Cycles. Philip-Pine Agric. 1936, 25, 321–354. [Google Scholar]
- Cheke, R.A.; Young, S.; Wang, X.; Tratalos, J.A.; Tang, S.; Cressman, K. Evidence for a Causal Relationship between the Solar Cycle and Locust Abundance. Agronomy 2020, 11, 69. [Google Scholar] [CrossRef]
- Maldonado-Bruzzone, R. Informe de la primera comisión exploradora. In Memoria de la Comisión Central de Investigaciones Sobre la Langosta; Ministerio de Agricultura de la Nación: Buenos Aires, Argentina, 1936; pp. 13–68. [Google Scholar]
- Hunter, D.M. Locusts in the World. In Olfactory Concepts of Insect Control-Alternative to Insecticides; Picimbon, J.F., Ed.; Springer: Cham, Switzerland, 2019; Volume 1, pp. 30–48. [Google Scholar]
- Bruch, C. Investigaciones sobre la langosta, experimentos en cautividad. Mems. Comn. Cent. Invest. Langosta 1936, 1936, 143–190. [Google Scholar]
- CIPA, Comité Interamericano Permanente Antiacridiano, Memoria de la Conferencia Internacional de Lucha Antiacridiana realizada en Santa Cruz de la Sierra del 30 de enero al 1o de febrero, Ministerio de Agricultura, Ganadería y Colonización, Di-rección General de Agricultura Departamento de Sanidad Vegetal “Entomología”. La Paz Bolivia. 1949, 38.
- Maldonado-Bruzzone, R. La formación de mangas de Schistocerca cancellata (Serville), Informe sobre una gira al oriente boli-viano, Año IV, Serie A, N° 35, Ministerio de Agricultura de la Nación, Buenos Aires. Argentina 1948, 22. [Google Scholar]
- Decreto Supremo No. 3081, 2017, Bolivia. Available online: https://www.lexivox.org/norms/BO-DS-N3081.html (accessed on 2 August 2021).
- GICSV. Informe Regional Langosta Sudamericana Octubre. 2020. Available online: http://apps.iica.int/GICSV/programas/SanidadVegetal/archivos/Langosta/_Informe%20langostas%20GICSV%2010_2020.pdf (accessed on 2 August 2021).
- Metsul “Guerra aos Gafanhotos” Que “Vai Ser Dura a Parada”. 2020. Available online: https://metsul.com/guerra-aos-gafanhotos-que-a-parada-vai-ser-dura/ (accessed on 25 April 2021).
- Lorier, E.; Zorbino, M.S. La langosta voladora Schistocerca cancellata (SERVILLE, 1838) (ORTHOPTERA, ACRIDIDAE, CYR-TACANTHACRIDINAE) en Uruguay. Bol. Soc. Zool. Urug. 2020, 29, 52–65. [Google Scholar]
- Gunn, D.L. Nomad encompassed. The development of preventive control of the red locust (Nomadacris sepermfaciata (Serville) by International Red Locust Control Service. J. Ent. Soc. South Afr. 1960, 23, 65–125. [Google Scholar]
- Therville, C.; Anderies, J.; Lecoq, M.; Cease, A. Locusts and People: Integrating the Social Sciences in Sustainable Locust Management. Agronomy 2021, 11, 951. [Google Scholar] [CrossRef]
- Daguerre, J.B. Estado actual de la langosta voladora. Revta Soc. Ent. Argent. 1970, 32, 115–116. [Google Scholar]
- Therville, C.; Anderies, J.M.; Medina, H.E.; Overson, R.; Trumper, E.V.; Cease, A.J. Synthesis of the Governance Workshop on the South American Locust; Technical Report; Arizona State University: Tempe, AZ, USA, 2020; pp. 1–18. [Google Scholar]
- Lecoq, M. Le Criquet pèlerin. Enseignements de la dernière invasion et perspectives offertes par la biomodélisation. In La Lutte Anti-Acridienne; Essaid, A., Ed.; AUPELF-UREF: Montreal, QC, Canada; John Libbey Eurotext: Paris, France, 1991; pp. 71–98. [Google Scholar]
- Gay, P.-E.; Lecoq, M.; Piou, C. Improving preventive locust management: Insights from a multi-agent model. Pest Manag. Sci. 2017, 74, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Gay, P.; Lecoq, M.; Piou, C. The limitations of locust preventive management faced with spatial uncertainty: Exploration with a multi-agent model. Pest Manag. Sci. 2019, 76, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Medina, H.E. Langosta. Explosión Demográfica de la Plaga Que Nunca se fue. XII Encuentro de Monitoreo y Manejo de Plagas, Enfermedades y Malezas, Ciudad de Córdoba, 2016. Expuesto en Forma Oral, Publicado en Resumen de la Jornada. Available online: https://www.researchgate.net/publication/308892241_Langosta_Explosion_demografica_de_la_plaga_que_nunca_se_fue (accessed on 17 December 2021).
- Medina, H.E. Emergencia Langostas 2020-2021-SENASA Argentina. 2020. Available online: https://geonode.senasa.gob.ar/maps/1806 (accessed on 10 August 2020).
- Medina, H.E.; Massola, M. Informe de Gestión. Programa Nacional de Langostas y Tucuras. 2020. Available online: https://www.argentina.gob.ar/sites/default/files/anuario_2020_programa_nacional_de_langostas_y_tucuras_v_1.2.pdf (accessed on 10 August 2020).
- Morello, J.; Pengue, W.; Rodríguez, A. Un Siglo de Cambios de Diseño del Paisaje: El Chaco Argentino. Primeras Jornadas Argentinas de Ecología del Paisaje. 2005, pp. 1–31. Available online: https://repositorio.cepal.org/bitstream/handle/11362/22011/S81102169_es.pdf?sequence=1 (accessed on 17 December 2021).
- Volante, J.; Alcaraz-Segura, D.; Mosciaro, M.; Viglizzo, E.; Paruelo, J.M. Ecosystem functional changes associated with land clearing in NW Argentina. Agric. Ecosyst. Environ. 2012, 154, 12–22. [Google Scholar] [CrossRef]
- Vallejos, M.; Volante, J.N.; Mosciaro, M.J.; Vale, L.M.; Bustamante, M.L.; Paruelo, J.M. Transformation dynamics of the natural cover in the Dry Chaco ecoregion: A plot level geo-database from 1976 to 2012. J. Arid. Environ. 2015, 123, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Piou, C.; Gay, P.-E.; Benahi, A.S.; Ebbe, M.A.O.B.; Chihrane, J.; Ghaout, S.; Cisse, S.; Diakite, F.; Lazar, M.; Cressman, K.; et al. Soil moisture from remote sensing to forecast desert locust presence. J. Appl. Ecol. 2018, 56, 966–975. [Google Scholar] [CrossRef]
- Cressman, K. Climate Change and Locusts in the WANA Region. In Climate Change and Food Security in West Africa and North Africa; Sivakumar, M.V.K., Selvaraju, R.L.R., Hamdan, I., Eds.; Springer: Cham, Switzerland, 2013; Chapter 7; pp. 131–143. [Google Scholar]
- Lecoq, M. Desert locust management: From ecology to anthropology. J. Orthoptera Res. 2005, 14, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Cisse, S.; Ghaout, S.; Mazih, A.; Babah Ebbe, M.A.O.; Benahi, A.S.; Piou, C. Effect of vegetation on density thresholds of adult desert locust gregarization from survey data in Mauritania. Èntomol. Exp. Appl. 2013, 149, 159–165. [Google Scholar] [CrossRef]
- Cisse, S.; Ghaout, S.; Mazih, A.; Ebbe, M.A.O.B.; Piou, C. Estimation of density threshold of gregarization of desert locust hoppers from field sampling in Mauritania. Èntomol. Exp. Appl. 2015, 156, 136–148. [Google Scholar] [CrossRef]
- Lazar, M.; Piou, C.; Doumandji-Mitiche, B.; Lecoq, M. Importance of solitarious desert locust population dynamics: Lessons from historical survey data in Algeria. Èntomol. Exp. Appl. 2016, 161, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Kayalto, M.; Hassani, M.I.; Lecoq, M.; Gay, P.-E.; Piou, C. Cartographie des zones de reproduction et de grégarisation du criquet pèlerin au Tchad. Cah. Agric. 2020, 29, 14. [Google Scholar] [CrossRef]
- Hunter, D.M. Advances in the control of locusts (Orthoptera: Acrididae) in eastern Australia: From crop protection to preventive control. Aust. J. Entomol. 2004, 43, 293–303. [Google Scholar] [CrossRef]
- Williams, T.; Arredondo-Bernal, H.C.; Rodríguez-Del-Bosque, L.A. Biological Pest Control in Mexico. Annu. Rev. Èntomol. 2013, 58, 119–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization of the United Nations. Red Locust disaster in Eastern Africa prevented. In Biopesticides being Used on a Large Scale; FAO: Rome, Italy, 2009; Available online: http://www.fao.org/news/story/en/item/21084/icode/ (accessed on 14 August 2021).
- Stokstad, E. In Somalia, an unprecedented effort to kill massive locust swarms with biocontrol. Science 2020. [Google Scholar] [CrossRef]


| Schistocerca cancellata | |||||
|---|---|---|---|---|---|
| Solitarious Phase | Gregarious Phase | ||||
| Traits | Nymphs | Adults | Nymphs | Adults | References |
| Size | Final instar: males smaller; females larger | Males smaller; females similar size, narrower head | Final instar: males larger; females smaller | Males larger; females similar size, wider head | [9] |
| Coloration | Green, varying from light brown to light green, | General brownish, contrasting pattern of stripes in pronotum | Pattern of striking yellow, red, or reddish orange & black | General brownish; pattern of stripes in pronotum | [9,11] |
| with small black dots | faintly evident In nature: reddish (immature) | ||||
| pale yellow (mature) | |||||
| Behavior | Sedentary, disperse in the vegetation and develop in isolation | Sedentary, disperse in the vegetation and develop in isolation | Active; dense groups (marching bands) | Active; dense groups (swarms) | [9,11] |
| Femur hairs (%) | Higher | Lower | [9] | ||
| F/C ratio | Higher | Lower | [9] | ||
| Life cycle: | |||||
| N° instars | 6 (in lab.) | 6 (in lab.); 5 (in nature) | [9,17] | ||
| Mean duration of stage | 47.9 days | 87 days | 35.6 days | 58 days | [9,34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trumper, E.V.; Cease, A.J.; Cigliano, M.M.; Copa Bazán, F.; Lange, C.E.; Medina, H.E.; Overson, R.P.; Therville, C.; Pocco, M.E.; Piou, C.; et al. A Review of the Biology, Ecology, and Management of the South American Locust, Schistocerca cancellata (Serville, 1838), and Future Prospects. Agronomy 2022, 12, 135. https://doi.org/10.3390/agronomy12010135
Trumper EV, Cease AJ, Cigliano MM, Copa Bazán F, Lange CE, Medina HE, Overson RP, Therville C, Pocco ME, Piou C, et al. A Review of the Biology, Ecology, and Management of the South American Locust, Schistocerca cancellata (Serville, 1838), and Future Prospects. Agronomy. 2022; 12(1):135. https://doi.org/10.3390/agronomy12010135
Chicago/Turabian StyleTrumper, Eduardo V., Arianne J. Cease, María Marta Cigliano, Fernando Copa Bazán, Carlos E. Lange, Héctor E. Medina, Rick P. Overson, Clara Therville, Martina E. Pocco, Cyril Piou, and et al. 2022. "A Review of the Biology, Ecology, and Management of the South American Locust, Schistocerca cancellata (Serville, 1838), and Future Prospects" Agronomy 12, no. 1: 135. https://doi.org/10.3390/agronomy12010135
APA StyleTrumper, E. V., Cease, A. J., Cigliano, M. M., Copa Bazán, F., Lange, C. E., Medina, H. E., Overson, R. P., Therville, C., Pocco, M. E., Piou, C., Zagaglia, G., & Hunter, D. (2022). A Review of the Biology, Ecology, and Management of the South American Locust, Schistocerca cancellata (Serville, 1838), and Future Prospects. Agronomy, 12(1), 135. https://doi.org/10.3390/agronomy12010135