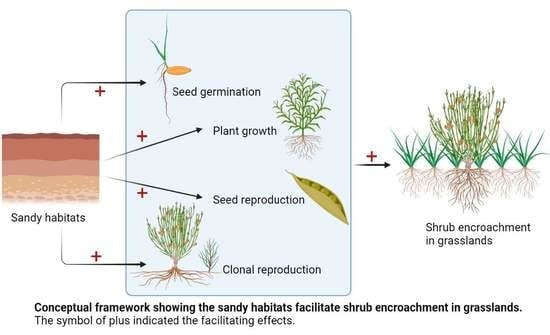
Sandy Habitats Play an Important Role in Shrub Encroachment in Grasslands
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Species and Study Site
2.2. Caragana Population Survey in Grassland and Sandy Land
2.3. Seed Preservation Experiment for Sand Burial Treatments
2.4. Seed Germination Experiment for Burial Treatments
2.5. Planting Experiment with Grassland Soil and Sandy Land Soil
2.6. Probability of Branches Forming Adventitious Roots under Buried or Not Buried Conditions
2.7. The Number of Adventitious Buds on Horizontal Roots under Wind Eroded and Non-Wind Eroded Conditions
2.8. Statistical Analyses
3. Results
3.1. Effect of Sand Burial on Seed Germination
3.2. Effects of Sandy Soil on Sapling Establishment and Plant Growth
3.3. Effect of Sandy Habitats on Seed Production and Seed Preservation
3.4. Effect of Sand Burial and Wind Erosion on Clonal Reproduction
3.5. Population Characteristics of Caragana in Grassland and Sandy Land
4. Discussion
4.1. Sandy Habitats Facilitate Seed Germination and Seedling Growth of Shrubs
4.2. Sandy Habitats Facilitate Plant Growth of Shrubs
4.3. Sandy Habitats Facilitate Seed Reproduction of Shrubs
4.4. Sandy Habitats Facilitate Clonal Reproduction of Shrubs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reynolds, J.F.; Smith, D.M.S.; Lambin, E.F.; Turner, B.L.; Mortimore, M.; Batterbury, S.P.; Downing, T.E.; Dowlatabadi, H.; Fernández, R.J.; Herrick, J.E.; et al. Global desertification: Building a science for dryland development. Science 2007, 316, 847–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Auken, O.W. Causes and consequences of woody plant encroachment into western North American grasslands. J. Environ. Manag. 2009, 90, 2931–2942. [Google Scholar] [CrossRef]
- Li, S.L.; Yu, F.H.; Werger, M.J.; Dong, M.; During, H.J.; Zuidema, P.A. Mobile dune fixation by a fast-growing clonal plant: A full life-cycle analysis. Sci. Rep. 2015, 5, 8935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, X.; Han, X.; Bai, Y.; Pan, Q. Increased distribution of Caragana microphylla in rangelands and its causes and consequences in Xilin River Basin. Acta Prataculturae Sin. 2003, 12, 57–62. [Google Scholar]
- Xiong, X.; Han, X.; Chen, Q.; Pan, Q. Increased abundance of woody plants in grasslands and savannas. Acta Ecol. Sin. 2003, 23, 2436–2443. [Google Scholar]
- Luo, W.; Zhao, W. Effects of wind erosion and sand burial on growth and reproduction of a clonal shrub. Flora 2015, 217, 164–169. [Google Scholar] [CrossRef]
- Wang, G.; Yu, K.; Gou, Q. Effects of sand burial disturbance on establishment of three desert shrub species in the margin of oasis in northwestern China. Ecol. Res. 2019, 34, 127–135. [Google Scholar] [CrossRef]
- Tracy, S.R.; Black, C.R.; Roberts, J.A.; Mooney, S.J. Exploring the interacting effect of soil texture and bulk density on root system development in tomato (Solanum lycopersicum L.). Environ. Exp. Bot. 2013, 91, 38–47. [Google Scholar] [CrossRef]
- White, R.E. Principles and Practice of Soil Science: The Soil as a Natural Resource, 4th ed.; Blackwell Publishing: Oxford, UK, 2005. [Google Scholar]
- Sladonja, B.; Krapac, M.; Ban, D.; Uzila, Z.; Dudas, S.; Dorcic, D. Effect of soil type on pyrethrum seed germination. J. Plant Prot. Res. 2014, 54, 421–425. [Google Scholar] [CrossRef]
- Li, X.R.; Zhang, Z.S.; Zhang, J.G.; Wang, X.P.; Jia, X.H. Association between vegetation patterns and soil properties in the southeastern Tengger Desert, China. Arid Land Res. Manag. 2004, 18, 369–383. [Google Scholar] [CrossRef]
- Medinski, T.V.; Mills, A.J.; Esler, K.J.; Schmiedel, U.; Jürgens, N. Do soil properties constrain species richness? Insights from boundary line analysis across several biomes in south western Africa. J. Arid Environ. 2010, 74, 1052–1060. [Google Scholar] [CrossRef]
- Zaller, J.G.; Frank, T.; Drapela, T. Soil sand content can alter effects of different taxa of mycorrhizal fungi on plant biomass production of grassland species. Eur. J. Soil Biol. 2011, 47, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Shi, X.; Wang, J.C.; Yin, L.K.; Huang, Z.Y.; Zhang, D.Y. Effects of sand burial, soil water content and distribution pattern of seeds in sand on seed germination and seedling survival of Eremosparton songoricum (Fabaceae), a rare species inhabiting the moving sand dunes of the Gurbantunggut Desert of China. Plant Soil 2011, 345, 69–87. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, X.; Baskin, C.C.; Baskin, J.M.; Dong, M.; Huang, Z. Effects of amount and frequency of precipitation and sand burial on seed germination, seedling emergence and survival of the dune grass Leymus secalinus in semiarid China. Plant Soil 2014, 374, 399–409. [Google Scholar] [CrossRef]
- Ye, X.; Li, L.; Baskin, C.C.; Baskin, J.M.; Du, J.; Huang, Z. Sand burial helps regulate timing of seed germination of a dominant herb in an inland dune ecosystem with a semiarid temperate climate. Sci. Total Environ. 2019, 680, 44–50. [Google Scholar] [CrossRef]
- Li, J.; Qu, H.; Zhao, H.; Zhou, R.; Yun, J.; Pan, C. Growth and physiological responses of Agriophyllum squarrosum to sand burial stress. J. Arid Land 2015, 7, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Zhang, Z.J.; Zhang, C.Y.; Zhang, J.Z. Effects of sand burial on survival, growth, gas exchange and biomass allocation of Ulmus pumila seedlings in the Hunshandak Sandland, China. Ann. Bot. 2004, 94, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Lai, L.; Jiang, L.; An, P.; Yu, Y.; Zheng, Y.; Shimizue, H.; Baskin, J.M.; Baskin, C.C. Moderate water supply and partial sand burial increase relative growth rate of two Artemisia species in an inland sandy land. J. Arid Environ. 2012, 8, 105–113. [Google Scholar] [CrossRef]
- Xu, L.; Huber, H.; During, H.J.; Dong, M.; Anten, N.P. Intraspecific variation of a desert shrub species in phenotypic plasticity in response to sand burial. New Phytol. 2013, 199, 991–1000. [Google Scholar] [CrossRef]
- Li, F.; Xie, Y.; Zhu, L.; Jiang, L.; Chen, X.; Pan, B.; Deng, Z. Changed clonal growth form induced by sand burial facilitates the acclimation of Carex brevicuspis to competition. PLoS ONE 2015, 10, e0121270. [Google Scholar] [CrossRef]
- Fan, B.; Zhao, C.; Zhang, X.; Sun, K. Impacts of sand burial and wind erosion on regeneration and growth of a desert clonal shrub. Front. Plant Sci. 2018, 9, 1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Zhao, W.; Zhuang, Y. Sand-burial and wind erosion promote oriented-growth and patchy distribution of a clonal shrub in dune ecosystems. Catena 2018, 167, 212–220. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, H.; Yang, W.; Qing, H.; Zhou, C.; Tang, J.; An, S. Variations in growth, clonal and sexual reproduction of Spartina alterniflora responding to changes in clonal integration and sand burial. CLEAN–Soil Air Water 2015, 43, 1100–1106. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Z.; Gao, S.; Cui, Q.; Liu, G.; Du, J.; Dong, M.; Huang, Z.; Cornelissen, J.H.C. Differential plant species responses to interactions of sand burial, precipitation enhancement and climatic variation promote co-existence in Chinese steppe vegetation. J. Veg. Sci. 2017, 28, 139–148. [Google Scholar] [CrossRef]
- Li, S.L.; Werger, M.J.; Zuidema, P.A.; Yu, F.H.; Dong, M. Seedlings of the semi-shrub Artemisia ordosica are resistant to moderate wind denudation and sand burial in Mu Us sandland, China. Trees 2010, 24, 515–521. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Wang, L.; Wang, Z. Responses of rhizomatous grass Phragmites communis to wind erosion: Effects on biomass allocation. Plant Soil 2014, 380, 389–398. [Google Scholar] [CrossRef]
- Yu, F.H.; Wang, N.; He, W.M.; Chu, Y.; Dong, M. Adaptation of rhizome connections in drylands: Increasing tolerance of clones to wind erosion. Ann. Bot. 2008, 102, 571–577. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Song, J.; Ru, J.; Zhou, Z.; Zhong, M.; Jiang, L.; Hui, D.F.; Wan, S. Effects of grazing, wind erosion, and dust deposition on plant community composition and structure in a temperate steppe. Ecosystems 2021, 24, 403–420. [Google Scholar] [CrossRef]
- Xie, L.N.; Ma, C.C.; Guo, H.Y.; Li, Q.F.; Gao, Y.B. Distribution pattern of Caragana species under the influence of climate gradient in the Inner Mongolia region, China. J. Arid Land 2014, 6, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.C.; Zhang, J.H.; Guo, H.Y.; Li, Q.F.; Xie, L.N.; Gao, Y.B. Alterations in canopy size and reproduction of Caragana stenophylla along a climate gradient on the Inner Mongolian Plateau. Flora 2013, 208, 97–103. [Google Scholar] [CrossRef]
- Wang, Z.W.; Xie, L.N.; Prather, M.C.; Guo, H.Y.; Han, G.D.; Ma, C.C. What drives the shift between sexual and clonal reproduction of Caragana stenophylla along a climatic aridity gradient? BMC Plant Biol. 2018, 18, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.Z.; Wang, Y.M.; Feng, Y.S.; Wang, J.H. Macro-scale survey and dynamic studies of sandy land in Ningxia by remote sensing. J. Desert Res. 2003, 23, 34–37. [Google Scholar]
- Reekie, E.G.; Bazzaz, F.A. Reproductive effort in plants. 1. Carbon allocation to reproduction. Am. Nat. 1987, 129, 876–896. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, M.; Huang, Z. Response of seed germination and seedling growth to sand burial of two dominant perennial grasses in Mu-Us sandy grassland, semiarid China. Rangel. Ecol. Manag. 2009, 62, 337–344. [Google Scholar] [CrossRef]
- Mao, P.L.; Mu, H.X.; Cao, B.H.; Liu, Y.H.; Fan, Z.F.; Wang, S.M. Effects of sand burial and overstory tree age on seedling establishment in coastal Pinus thunbergii forests in the northern Shandong Peninsula, China. For. Chron. 2016, 92, 357–365. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.L.; Liang, Z.L.; Zhu, X.W.; Mei, S.X.; Wang, H.Q.; Shen, Y.; Huang, Z.Y. Effects of sand burial and seed size on seed germination, seedling emergence and growth of Caragana korshinskii Kom (Fabaceae). Acta Ecol. Sin. 2012, 32, 7757–7763. [Google Scholar] [CrossRef] [Green Version]
- Hook, P.B.; Burke, I.C. Biogeochemistry in a shortgrass landscape: Control by topography, soil texture, and microclimate. Ecology 2000, 81, 2686–2703. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yu, F.H.; Dong, M. Effects of sand burial on the survival, growth, and biomass allocation in semi-shrub Hedysarum laeve seedlings. Acta Bot. Sin. 2002, 44, 337–343. [Google Scholar]
- Zhao, W.Z.; Li, Q.Y.; Fang, H.Y. Effects of sand burial disturbance on seedling growth of Nitraria sphaerocarpa. Plant Soil 2007, 295, 95–102. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Guan, D. Seedling growth variation in response to sand burial in four Artemisia species from different habitats in the semi-arid dune field. Trees 2008, 22, 41–47. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, J. Influence of seed predation on regeneration of three Caragana species. Biodivers. Sci. 2005, 13, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Ma, C.C.; Han, L.; Gao, Y.B. Nabkha morphology and sand-fixing capability of four dominant Caragana species in the desert region of the Inner Mongolia Plateau. Acta Ecol. Sin. 2012, 32, 3343–3351. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.N.; Guo, H.Y.; Gabler, C.A.; Li, Q.F.; Ma, C.C. Changes in Spatial Patterns of Caragana stenophylla along a Climatic Drought Gradient on the Inner Mongolian Plateau. PLoS ONE 2015, 10, e0121234. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, Z.; Lü, X.; Maestre, F.T.; Wang, L. Sand burial compensates for the negative effects of erosion on the dune-building shrub Artemisia wudanica. Plant Soil 2014, 374, 263–273. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Li, Y.; Guo, H.; Wang, C.; Chen, Q.; He, P.; Ma, C. Sandy Habitats Play an Important Role in Shrub Encroachment in Grasslands. Agronomy 2022, 12, 2858. https://doi.org/10.3390/agronomy12112858
Xie L, Li Y, Guo H, Wang C, Chen Q, He P, Ma C. Sandy Habitats Play an Important Role in Shrub Encroachment in Grasslands. Agronomy. 2022; 12(11):2858. https://doi.org/10.3390/agronomy12112858
Chicago/Turabian StyleXie, Lina, Yuchen Li, Hongyu Guo, Chunwen Wang, Qing Chen, Peng He, and Chengcang Ma. 2022. "Sandy Habitats Play an Important Role in Shrub Encroachment in Grasslands" Agronomy 12, no. 11: 2858. https://doi.org/10.3390/agronomy12112858
APA StyleXie, L., Li, Y., Guo, H., Wang, C., Chen, Q., He, P., & Ma, C. (2022). Sandy Habitats Play an Important Role in Shrub Encroachment in Grasslands. Agronomy, 12(11), 2858. https://doi.org/10.3390/agronomy12112858