Using HPLC–MS/MS to Assess the Quality of Beet, Mizuna, Lettuce and Corn Salad after Juglone and Walnut Leaf Extract Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Growing Conditions
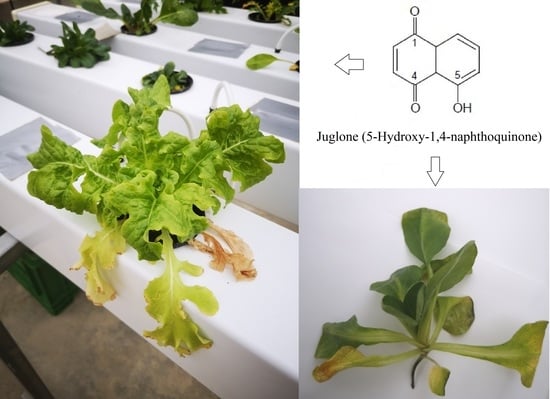
2.3. Chemicals and Plant Material
2.4. Sampling of the Plants
2.5. Extraction of the Phenolic Compounds
2.6. Preparation of J. regia Leaf Extract
2.7. HPLC–Mass Spectrometry Analysis of Individual Phenolic Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. Identification of Individual Phenolic Compounds in the Crop Vegetables
3.2. Effects of the Juglone Treatments on the Crop Vegetable Yields
3.3. Effects of the Juglone Treatments on the Crop Vegetable Quality
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.; Shanks, C.B. Quality of Vegetables Based on Total Phenolic Concentration Is Lower in More Rural Consumer Food Environments in a Rural American State. Int. J. Environ. Res. Public Health 2017, 14, 924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. Int. Rev. J. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Medic, A.; Jakopic, J.; Solar, A.; Hudina, M.; Veberic, R. Walnut (J. regia) Agro-Residues as a Rich Source of Phenolic Compounds. Biology 2021, 10, 535. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, K.L.; Kang, X.; He, X.; Dong, M.; Zhang, Q.; Liu, R.H. Cellular Antioxidant Activity of Common Fruits. J. Agric. Food Chem. 2008, 56, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Di Noia, J. Defining Powerhouse Fruits and Vegetables: A Nutrient Density Approach. Prev. Chronic Dis. 2014, 11, E95. [Google Scholar] [CrossRef] [Green Version]
- Medic, A.; Zamljen, T.; Hudina, M.; Veberic, R. Identification and Quantification of Naphthoquinones and Other Phenolic Compounds in Leaves, Petioles, Bark, Roots, and Buds of Juglans regia L., Using HPLC–MS/MS. Horticulturae 2021, 7, 326. [Google Scholar] [CrossRef]
- Ahmed, S.; Stepp, J.R. Beyond yields: Climate change effects on specialty crop quality and agroecological management. Elem. Sci. Anthr. 2016, 4, 92. [Google Scholar] [CrossRef] [Green Version]
- Medic, A.; Solar, A.; Hudina, M.; Veberic, R. Phenolic Response to Walnut Anthracnose (Ophiognomonia leptostyla) Infection in Different Parts of Juglans regia Husks, Using HPLC–MS/MS. Agriculture 2021, 11, 659. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Introduction: Biochemistry, Role and Biotechnology of Secondary Metabolites. Annu. Plant Rev. Online 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Zubay, P.; Kunzelmann, J.; Ittzés, A.; Zámboriné, É.N.; Szabó, K. Allelopathic effects of leachates of Juglans regia L., Populus tremula L. and juglone on germination of temperate zone cultivated medicinal and aromatic plants. Agrofor. Syst. 2021, 95, 431–442. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Widhalm, J.R. Agricultural Uses of Juglone: Opportunities and Challenges. Agronomy 2020, 10, 1500. [Google Scholar] [CrossRef]
- Medic, A.; Zamljen, T.; Slatnar, A.; Hudina, M.; Veberic, R. Is Juglone the Only Naphthoquinone in Juglans regia L. with Allelopathic Effects? Agriculture 2021, 11, 784. [Google Scholar] [CrossRef]
- Aliskan, I.K.; Terzi, I. Allelopathic effects of walnut leaf extracts and juglone on seed germination and seedling growth. J. Hortic. Sci. Biotechnol. 2001, 76, 436–440. [Google Scholar] [CrossRef]
- de Scisciolo, B.; Leopold, D.J.; Walton, D.C. Seasonal patterns of juglone in soil beneath Juglans nigra (black walnut) and influence of J. nigra on understory vegetation. J. Chem. Ecol. 1990, 16, 1111–1130. [Google Scholar] [CrossRef] [PubMed]
- Maršić, N.K.; Mikulič-Petkovšek, M.; Hudina, M.; Veberič, R.; Slatnar, A. Leafy Asian vegetables cultivated on a floating hydroponic system and a substrate culture, during the autumn period in greenhouse. Acta Hortic. 2021, 1320, 413–420. [Google Scholar] [CrossRef]
- Medic, A.; Jakopic, J.; Hudina, M.; Solar, A.; Veberic, R. Identification and quantification of the major phenolic constituents in Juglans regia L. peeled kernels and pellicles, using HPLC–MS/MS. Food Chem. 2021, 352, 129404. [Google Scholar] [CrossRef]
- Liu, P.; Li, L.; Song, L.; Sun, X.; Yan, S.; Huang, W. Characterisation of phenolics in fruit septum of Juglans regia Linn. by ultra performance liquid chromatography coupled with Orbitrap mass spectrometer. Food Chem. 2019, 286, 669–677. [Google Scholar] [CrossRef]
- Hernández, V.; Botella, M.; Hellín, P.; Cava, J.; Fenoll, J.; Mestre, T.; Martínez, V.; Flores, P. Phenolic and Carotenoid Profile of Lamb’s Lettuce and Improvement of the Bioactive Content by Preharvest Conditions. Foods 2021, 10, 188. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Contreras, M.D.M.; Arraez-Roman, D.; Carretero, A.S.; Fernández-Gutiérrez, A. Reversed-phase ultra-high-performance liquid chromatography coupled to electrospray ionization-quadrupole-time-of-flight mass spectrometry as a powerful tool for metabolic profiling of vegetables: Lactuca sativa as an example of its application. J. Chromatogr. A 2013, 1313, 212–227. [Google Scholar] [CrossRef]
- Harbaum, B.; Hubbermann, E.M.; Wolff, C.; Herges, R.; Zhu, Z.; Schwarz, K. Identification of Flavonoids and Hydroxycinnamic Acids in Pak Choi Varieties (Brassica campestris L. ssp. chinensis var. communis) by HPLC–ESI-MSnand NMR and Their Quantification by HPLC–DAD. J. Agric. Food Chem. 2007, 55, 8251–8260. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Pereira, C.; Pires, T.C.; Calhelha, R.C.; Alves, M.J.; Ferreira, O.; Barros, L.; Ferreira, I.C. Phenolic profile, antioxidant and antibacterial properties of Juglans regia L. (walnut) leaves from the Northeast of Portugal. Ind. Crop. Prod. 2019, 134, 347–355. [Google Scholar] [CrossRef]
- Vissers, A.; Kiskini, A.; Hilgers, R.; Marinea, M.; Wierenga, P.A.; Gruppen, H.; Vincken, J.-P. Enzymatic Browning in Sugar Beet Leaves (Beta vulgaris L.): Influence of Caffeic Acid Derivatives, Oxidative Coupling, and Coupled Oxidation. J. Agric. Food Chem. 2017, 65, 4911–4920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ghffar, E.A.A.; Hegazi, N.M.; Saad, H.H.; Soliman, M.M.; El-Raey, M.A.; Shehata, S.M.; Barakat, A.; Yasri, A.; Sobeh, M. HPLC–ESI–MS/MS analysis of beet (Beta vulgaris) leaves and its beneficial properties in type 1 diabetic rats. Biomed. Pharmacother. 2019, 120, 109541. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.; Aaby, K.; Borge, G.I.A. Characterization and Quantification of Flavonoids and Hydroxycinnamic Acids in Curly Kale (Brassica oleracea L. Convar. acephala Var. sabellica) by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2009, 57, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Duroux, L.; Delmotte, F.M.; Lancelin, J.-M.; Kéravis, G.; Jay-Allemand, C. Insight into naphthoquinone metabolism: β-glucosidase-catalysed hydrolysis of hydrojuglone β-d-glucopyranoside. Biochem. J. 1998, 333, 275–283. [Google Scholar] [CrossRef]
- Ercisli, S.; Turkkal, C. Allelopathic effects of juglone and walnut leaf extracts on growth, fruit yield and plant tissue composition in strawberry cvs. ‘Camarosa’ and ‘Sweet Charlie’. J. Hortic. Sci. Biotechnol. 2005, 80, 39–42. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; de Bruijn, W.J.; Vincken, J.-P. A comparison of the phenolic composition of old and young tea leaves reveals a decrease in flavanols and phenolic acids and an increase in flavonols upon tea leaf maturation. J. Food Compos. Anal. 2020, 86, 103385. [Google Scholar] [CrossRef]
- Dadáková, K.; Heinrichová, T.; Lochman, J.; Kašparovský, T. Production of Defense Phenolics in Tomato Leaves of Different Age. Molecules 2020, 25, 4952. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Ribas-Agustí, A.; Gratacós-Cubarsí, M.; Sárraga, C.; García-Regueiro, J.-A.; Castellari, M. Analysis of Eleven Phenolic Compounds Including Novel p-Coumaroyl Derivatives in Lettuce (Lactuca sativa L.) by Ultra-high-performance Liquid Chromatography with Photodiode Array and Mass Spectrometry Detection. Phytochem. Anal. 2011, 22, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Oliveira, M.; Ibáñez, E.; Herrero, M. Phenolic profile evolution of different ready-to-eat baby-leaf vegetables during storage. J. Chromatogr. A 2014, 1327, 118–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Source | Compound | Rt (min) | [M-H]− (m/z) | MS2 (m/z) | MS3 (m/z) | Expressed as |
|---|---|---|---|---|---|---|
| Beta vulgaris L. | p-Coumaroylcaffeic acid | 11.09 | 337 | 119 (100), 179 (42), 163 (12) | p-Coumaric acid | |
| p-Coumaric acid hexoside | 12.17 | 325 | 163 (100), 145 (83), 119 (8), 235 (8) | p-Coumaric acid | ||
| Ferulic acid hexoside | 13.12 | 355 | 193 (100), 217 (54), 175 (29) | Ferulic acid | ||
| Vitexin hexoside | 14.85 | 593 | 311 (100), 341 (25) | 283 (100), 191 (3) | Apigenin-7-glucoside | |
| Ferulic acid | 15.13 | 193 | 149 (100), 178 (72), 134 (48) | Ferulic acid | ||
| Ferulic acid derivative 1 | 17.48 | 443 | 267 (100), 193 (9) | 249 (100), 113 (72), 175 (14) | Ferulic acid | |
| Ferulic acid derivative 2 | 17.79 | 639 | 443 (100) | 267 (100), 193 (10) | Ferulic acid | |
| Vitexin hexoside | 18.23 | 577 | 413 (100) | 293 (100) | Apigenin-7-glucoside | |
| Luteolin dihexoside | 18.89 | 609 | 285 (100), 257 (4) | 257 (100), 241 (45), 229 (37), 151 (32) 213 (26) | Luteolin-7-glucoside | |
| Vitexin pentoside | 18.89 | 563 | 413 (100) | 293 (100) | Apigenin-7-glucoside | |
| Vitexin (apigenin-C-hexoside isomer) | 19.47 | 431 | 311 (100) | 283 (100), 191 (3) | Apigenin-7-glucoside | |
| Isorhamnetin dihexoside | 19.93 | 639 | 315 (100), 300 (17) | 300 (100), 287 (4) | Isorhamnetin-3-glucoside | |
| Vitexin hexoside derivative | 20.19 | 651 | 607 (100) | 457 (100), 293 (3) | Apigenin-7-glucoside | |
| Isorhamnetin rutinoside | 20.68 | 609 | 315 (100), 300 (13) | 300 (100), 287 (5) | Isorhamnetin-3-glucoside | |
| Malonyl pentosylvitexin | 21.59 | 649 | 605 (100) | 455 (100), 293 (5) | Apigenin-7-glucoside | |
| Brassica rapa L. var. japonica | Neochlorogenic acid (3-caffeoylquinic acid) | 9.27 | 353 | 191 (100), 179 (46), 135 (7) | Neochlorogenic acid | |
| Kaempferol-3-O-diglucoside-7-O-glucoside | 10.34 | 771 | 609 (100) | 285 (100), 284 (100), 429 (98), 257 (10), 179 (3) | Kaempferol-3-glucoside | |
| Gluconapin | 11.57 | 372 | 259 (100), 275 (29), 194 (20), 130 (7) | 139 (100), 97 (39), 199 (13), 241 (7) | Gluconapin | |
| Kaempferol-3-O-caffeoyldiglucoside-7-O-glucoside | 12.19 | 933 | 771 (100) | 609 (100) | Kaempferol-3-glucoside | |
| Kaempferol-3-O-sinapoyldiglucoside-7-O-glucoside | 13.42 | 977 | 815 (100) | 609 (100) | Kaempferol-3-glucoside | |
| Sinapoyl glycoside | 13.42 | 385 | 223 (100), 247 (48), 205 (40) | 164 (100), 208 (44), 179 (35) | Sinapic acid | |
| Kaempferol diglucoside | 13.57 | 609 | 447 (100), 285 (13), 284 (2) | 284 (100), 285 (46), 151 (5) | Kaempferol-3-glucoside | |
| Kaempferol-3-O-feruoylglucoside-7-O-glucoside | 14.07 | 947 | 785 (100) | 623 (100), 609 (92), 591 (43) | Kaempferol-3-glucoside | |
| Isorhamnetin-3-O-glucoside-7-O-glucoside | 14.07 | 639 | 477 (100), 315 (9), 300 (1) | Isorhamnetin-3-glucoside | ||
| Caffeoyl malate | 17.50 | 295 | 179 (100), 133 (1) | Caffeic acid | ||
| Hydroxyferuoyl malate | 17.89 | 325 | 209 (100), 133 (23), 165 (5) | Ferulic acid | ||
| Kaempferol hexoside derivative | 19.92 | 567 | 447 (100) | 285 (100), 284 (31) | Kaempferol-3-glucoside | |
| Coumaroyl malate | 21.41 | 279 | 163 (100), 133 (19) | p-Coumaric acid | ||
| Sinapoyl malate | 22.06 | 339 | 223 (100) | 164 (100), 208 (79), 179 (53) | Sinapic acid | |
| Feruloyl malate | 22.24 | 309 | 193 (100), 133 (7) | Ferulic acid | ||
| Lactuca sativa L. | Dihydroxybenzoic acid hexoside | 8.69 | 315 | 153 (100), 108 (19) | Gallic acid | |
| Esculetin glucoside | 9.87 | 339 | 177 (100) | Gallic acid | ||
| Chlorogenic acid (5-caffeoylquinic acid) | 12.47 | 353 | 191 (100), 179 (4), 135 (1) | Chlorogenic acid | ||
| Galloyl hexoside | 13.13 | 331 | 313 (100), 168 (61), 125 (19), 169 (24) | Gallic acid | ||
| Cryptochlorogenic acid (4-caffeoylquinic acid) | 14.21 | 353 | 191 (100), 179 (3), 135 (1) | Cryptochlorogenic acid | ||
| Sinapoyl hexoside derivative | 15.38 | 431 | 385 (100) | 223 (100), 179 (41), 208 (27) | Sinapic acid | |
| cis 5-O-p-Coumaroylquinic acid | 15.91 | 337 | 191 (100), 163 (8) | p-Coumaric acid | ||
| trans 5-O-p-Coumaroylquinic acid | 17.24 | 337 | 191 (100), 163 (7) | p-Coumaric acid | ||
| Caffeoyl malate | 17.60 | 295 | 179 (100), 133 (53) | Caffeic acid | ||
| Quercetin-3-O-galactoside | 19.34 | 463 | 301 (100), 300 (3) | Quercetin-3-O-galactoside | ||
| Quercetin-3-O-glucoside | 20.68 | 463 | 301 (100), 300 (19) | Quercetin-3-O-glucoside | ||
| Kaempferol-3-O-glucuronide | 21.42 | 463 | 285 (100), 284 (41) | Kaempferol-3-glucoside | ||
| Quercetin-3-O-glucuronide | 21.96 | 477 | 301 (100), 300 (4) | Quercetin-3-O-glucoside | ||
| Quercetin 3-(6″-malonylglucoside) | 22.55 | 549 | 505 (100) | 301 (100), 300 (57) | Quercetin-3-O-glucoside | |
| Quercetin-3-(6″-acetylglucoside) | 23.70 | 505 | 301 (100), 300 (64) | Quercetin-3-O-glucoside | ||
| Caffeoyltartaric acid hexoside 1 | 26.63 | 473 | 293 (100), 311 (99) | Caffeic acid | ||
| Caffeoyltartaric acid hexoside 2 | 29.21 | 473 | 311 (100), 293 (87) | Caffeic acid | ||
| Valerianella locusta Laterr. | 4-Hydroxyphenylaoyl glucoside derivative | 7.16 | 359 | 313 (100) | 151 (100), 269 (2), 185 (1) | Gallic acid |
| Chlorogenic acid (5-caffeoylquinic acid) | 12.37 | 353 | 191 (100), 179 (4), 135 (1) | Chlorogenic acid | ||
| Cryptochlorogenic acid (4-caffeoylquinic acid) | 14.10 | 353 | 191 (100), 179 (3), 135 (1) | Cryptochlorogenic acid | ||
| cis 5-O-p-Coumaroylquinic acid | 15.89 | 337 | 191 (100), 163 (8) | p-Coumaric acid | ||
| cis 5-O-Feruoylquinic acid | 16.88 | 367 | 191 (100), 173 (3) | Ferulic acid | ||
| trans 5-O-p-Coumaroylquinic acid | 17.22 | 337 | 191 (100), 163 (7) | p-Coumaric acid | ||
| trans 5-O-Feruoylquinic acid | 18.02 | 367 | 191 (100), 173 (2) | Ferulic acid | ||
| Luteolin-7-rutinoside | 19.62 | 593 | 285 (100) | 285 (100), 241 (24), 217 (14), 199 (16), 175 (17), 151 (6) | Luteolin-7-glucoside | |
| Diosmetin apiosylglucoside | 21.74 | 593 | 299 (100), 284 (17) | 284 (100) | Luteolin-7-glucoside | |
| Diosmin (diosmetin-7-O-rutinoside) | 22.07 | 607 | 299 (100), 284 (24) | Luteolin-7-glucoside | ||
| Dicaffeoylquinic acid | 22.58 | 515 | 353 (100), 179 (2) | Caffeic acid | ||
| Apigenin-rutinoside | 24.75 | 577 | 531 (100), 269 (98) | Apigenin-7-glucoside | ||
| Caffeic acid hexoside derivative | 25.48 | 637 | 535 (100), 341 (23) | 161 (100), 179 (57), 341 (57) | Caffeic acid |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medic, A.; Zamljen, T.; Grohar, M.C.; Slatnar, A.; Hudina, M.; Veberic, R. Using HPLC–MS/MS to Assess the Quality of Beet, Mizuna, Lettuce and Corn Salad after Juglone and Walnut Leaf Extract Treatments. Agronomy 2022, 12, 347. https://doi.org/10.3390/agronomy12020347
Medic A, Zamljen T, Grohar MC, Slatnar A, Hudina M, Veberic R. Using HPLC–MS/MS to Assess the Quality of Beet, Mizuna, Lettuce and Corn Salad after Juglone and Walnut Leaf Extract Treatments. Agronomy. 2022; 12(2):347. https://doi.org/10.3390/agronomy12020347
Chicago/Turabian StyleMedic, Aljaz, Tilen Zamljen, Mariana Cecilia Grohar, Ana Slatnar, Metka Hudina, and Robert Veberic. 2022. "Using HPLC–MS/MS to Assess the Quality of Beet, Mizuna, Lettuce and Corn Salad after Juglone and Walnut Leaf Extract Treatments" Agronomy 12, no. 2: 347. https://doi.org/10.3390/agronomy12020347
APA StyleMedic, A., Zamljen, T., Grohar, M. C., Slatnar, A., Hudina, M., & Veberic, R. (2022). Using HPLC–MS/MS to Assess the Quality of Beet, Mizuna, Lettuce and Corn Salad after Juglone and Walnut Leaf Extract Treatments. Agronomy, 12(2), 347. https://doi.org/10.3390/agronomy12020347