Salvia Species: Biotechnological Strategies Applied to In Vitro Cultures for the Controlled Production of Bioactive Diterpenoids
Abstract
:1. Introduction
2. Search Strategy
3. Structure and Biological Activity of the Main Diterpenoids Obtained from In Vitro Cultures of Salvia Species
3.1. Tanshinones: Tanshinone I, Tanshinone IIA, Cryptotanshinone, Dihydrotanshinone I
3.2. Abietane Diterpenoids: Aethiopinone, Salvipisone, Ferruginol, and 1-Oxoaethiopinone
3.3. Abietane Diterpenoids: Carnosic Acid and Carnosol
3.4. Abietane Diterpenoids: Taxodone, Taxodione, and 15-Deoxyfuerstione
3.5. Icetexane Diterpenoids: Demethylfruticuline A and Fruticuline A
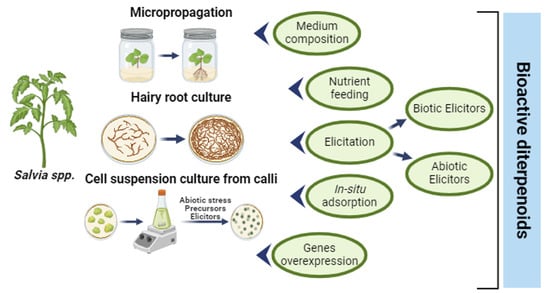
4. Strategies to Increase the In Vitro Production of Diterpenoids
4.1. Medium Composition and Effect of Nutrients (Mineral, Hormone, and Sucrose Composition)
4.1.1. Effect of Salts
4.1.2. Effect of Hormones
4.1.3. Effect of Sucrose
4.1.4. Effect of Environment Factors
4.2. Elicitation
4.2.1. Biotic Elicitors
Microorganism—Roots Interaction
Microorganism Extracts and Constituents
4.2.2. Abiotic Elicitors
Heavy Metals
Plant Signal Compounds
Other Elicitors
Effect of Light Irradiation
4.2.3. Combination or Synergic Effect of Elicitors
4.2.4. Elicitation and Nutrient or Medium Feeding or Renewal
4.3. Production with In Situ Adsorption
4.4. Gene Overexpression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Will, M.; Claßen-Bockhoff, R. Time to split Salvia s.l. (Lamiaceae)—New insights from Old World Salvia phylogeny. Mol. Phylogen. Evol. 2017, 109, 33–58. [Google Scholar] [CrossRef]
- Hu, G.-X.; Takano, A.; Drew, B.T.; Liu, E.-D.; Soltis, D.E.; Soltis, P.S.; Peng, H.; Xiang, C.-L. Phylogeny and staminal evolution of Salvia (Lamiaceae, Nepetoideae) in East Asia. Ann. Bot. 2018, 122, 649–668. [Google Scholar] [CrossRef]
- Jenks, A.A.; Walker, J.B.; Kim, S.C. Phylogeny of New World Salvia subgenus Calosphace (Lamiaceae) based on cpDNA (psbA-trnH) and nrDNA (ITS) sequence data. J. Plant Res. 2013, 126, 483–496. [Google Scholar] [CrossRef]
- Drew, B.T.; González-Gallegos, J.G.; Xiang, C.-L.; Kriebel, R.; Drummond, C.P.; Walker, J.B.; Sytsma, K.J. Salvia united: The greatest good for the greatest number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef]
- Hardman, R. SAGE—The Genus Salvia; Kintzios, S.E., Ed.; OPA (Overseas Publishers Association) N.V.; Harwood Academic Publishers, Part of the Gordon and Breach Publishing Group: New York, NY, USA, 2000. [Google Scholar]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef]
- Bisio, A.; Pedrelli, F.; D’Ambola, M.; Labanca, F.; Schito, A.M.; Govaerts, R.; De Tommasi, N.; Milella, L. Quinone diterpenes from Salvia species: Chemistry, botany, and biological activity. Phytochem. Rev. 2019, 18, 665–842. [Google Scholar] [CrossRef]
- Wu, Y.-B.; Ni, Z.-Y.; Shi, Q.-W.; Dong, M.; Kiyota, H.; Gu, Y.-C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef]
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef]
- Iobbi, V.; Brun, P.; Bernabé, G.; Dougué Kentsop, R.A.; Donadio, G.; Ruffoni, B.; Fossa, P.; Bisio, A.; De Tommasi, N. Labdane diterpenoids from Salvia tingitana Etl. Synergize with clindamycin against methicillin-resistant Staphylococcus aureus. Molecules 2021, 26, 6681. [Google Scholar] [CrossRef]
- Lubbe, A.; Verpoorte, R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crop. Prod. 2011, 34, 785–801. [Google Scholar] [CrossRef]
- FDA. Title 21—Food and Drugs. Chapter I—Food and Drug Administration. Department of Health and Human Services. Subchapter B—Food for Human Consumption (Continued). PART 182—Substances Generally Recognized as Safe. Subpart A—General Provisions. Sec. 182.10 Spices and Other Natural Seasonings and Flavorings; FDA: Washington, DC, USA, 2023. [Google Scholar]
- Bisio, A.; Schito, A.M.; Pedrelli, F.; Danton, O.; Reinhardt, J.K.; Poli, G.; Tuccinardi, T.; Bürgi, T.; De Riccardis, F.; Giacomini, M.; et al. Antibacterial and ATP synthesis modulating compounds from Salvia tingitana. J. Nat. Prod. 2020, 83, 1027–1042. [Google Scholar] [CrossRef]
- Topçu, G. Bioactive triterpenoids from Salvia species. J. Nat. Prod. 2006, 69, 482–487. [Google Scholar] [CrossRef]
- Topçu, G.; Ozturk, M.; Kusman, T.; Barla Demirkoz, A.A.; Kolak, U.; Ulubelen, A. Terpenoids, essential oil composition, fatty acid profile, and biological activities of Anatolian Salvia fruticosa Mill. Turk. J. Chem. 2013, 37, 619–632. [Google Scholar] [CrossRef]
- Rodríguez-Hahn, L.; Esquivel, B.; Cárdenas, J.; Ramamoorthy, T.P. The distribution of diterpenoids in Salvia. In Advances in Labiatae Science; Harley, R.M., Reynolds, T., Eds.; The Royal Botanic Gardens: Richmond, UK, 1992. [Google Scholar]
- Lu, Y.R.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Li, M.-H.; Chen, J.-M.; Peng, Y.; Xiao, P.-G. Distribution of phenolic acids in Chinese Salvia plants. WST 2008, 10, 46–52. [Google Scholar]
- Valant-Vetschera, K.M.; Roitman, J.N.; Wollenweber, E. Chemodiversity of exudate flavonoids in some members of the Lamiaceae. Biochem. Syst. Ecol. 2003, 31, 1279–1289. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Wollenweber, E. Flavonoid aglycones from the leaf surfaces of some Labiatae species. Plant Syst. Evol. 1990, 173, 109–118. [Google Scholar] [CrossRef]
- Kabouche, A.; Kabouche, Z. Bioactive Diterpenoids of Salvia Species. Stud. Nat. Prod. Chem. 2008, 35, 753–833. [Google Scholar]
- Bisio, A.; Fontana, N.; Romussi, G.; De Tommasi, N. Diterpenes and triterpenes from Salvia aurea L.—Part-2—Constituents of Labiatae. Pharmazie 1998, 53, 210–211. [Google Scholar]
- Rodriguez-Hahn, L.; Esquivel, B.; Sanchez, A.; Sanchez, C.; Cardenas, J.; Ramamoorthy, T. Diterpenos abietánicos de Salvias mexicanas. Rev. Latinoamer. Quím. 1989, 20, 105–110. [Google Scholar]
- Hu, J.; Wang, F.; Liang, F.; Wu, Z.; Jiang, R.; Li, J.; Chen, J.; Qiu, S.; Wang, J.; Zhang, Y.; et al. Identification of abietane-type diterpenoids and phenolic acids biosynthesis genes in Salvia apiana Jepson through full-length transcriptomic and metabolomic profiling. Front. Plant Sci. 2022, 13, 919025. [Google Scholar] [CrossRef]
- Rodriguez-Hahn, L.; Esquivel, B.; Càrdenas, J. Clerodane diterpenes in Labiatae. In Progress in the Chemistry of Organic Natural Products; Herz, W., Kirby, G.W., Moore, R.E., Steglich, W., Tamm, C., Eds.; Springer: Vienna, Austria, 1994; Volume 63, pp. 107–196. [Google Scholar]
- Bisio, A.; Damonte, G.; Fraternale, D.; Giacomelli, E.; Sails, A.; Romussi, G.; Cafaggi, S.; Ricci, D.; De Tommasi, N. Phytotoxic clerodane diterpenes from Salvia miniata Fernald (Lamiaceae). Phytochemistry 2011, 72, 265–275. [Google Scholar] [CrossRef]
- Bisio, A.; Mieri, M.D.; Milella, L.; Schito, A.M.; Parricchi, A.; Russo, D.; Alfei, S.; Lapillo, M.; Tuccinardi, T.; Hamburger, M. Antibacterial and hypoglycemic diterpenoids from Salvia chamaedryoides. J. Nat. Prod. 2017, 80, 503–514. [Google Scholar] [CrossRef]
- Bisio, A.; Fontana, N.; Romussi, G.; Ciarallo, G.; De Tommasi, N.; Pizza, C.; Mugnoli, A. Clerodane diterpenoids from Salvia blepharophylla. Phytochemistry 1999, 52, 1535–1540. [Google Scholar] [CrossRef]
- Capasso, R.; Izzo, A.; Capasso, F.; Romussi, G.; Bisio, A.; Mascolo, N. A diterpenoid from Salvia cinnabarina inhibits mouse intestinal motility in vivo. Planta Medica 2004, 70, 375–377. [Google Scholar] [CrossRef]
- Bisio, A.; Romussi, G.; Russo, E.; Cafaggi, S.; Schito, A.M.; Repetto, B.; De Tommasi, N. Antimicrobial activity of the ornamental species Salvia corrugata, a potential new crop for extractive purposes. J. Agric. Food Chem. 2008, 56, 10468–10472. [Google Scholar] [CrossRef]
- Giacomelli, E.; Bertrand, S.; Nievergelt, A.; Zwick, V.; Simoes-Pires, C.; Marcourt, L.; Rivara-Minten, E.; Cuendet, M.; Bisio, A.; Wolfender, J.-L. Cancer chemopreventive diterpenes from Salvia corrugata. Phytochemistry 2013, 96, 257–264. [Google Scholar] [CrossRef]
- Simmons, E.M.; Sarpong, R. Structure, biosynthetic relationships and chemical synthesis of the icetexane diterpenoids. Nat. Prod. Rep. 2009, 26, 1195–1217. [Google Scholar] [CrossRef]
- Marchev, A.; Haas, C.; Schulz, S.; Georgiev, V.; Steingroewer, J.; Bley, T.; Pavlov, A. Sage in vitro cultures: A promising tool for the production of bioactive terpenes and phenolic substances. Biotechnol. Lett. 2014, 36, 211–221. [Google Scholar] [CrossRef]
- Karalija, E.; Dahija, S.; Tarkowski, P.; Zeljković, S. Influence of climate-related environmental stresses on economically important essential oils of Mediterranean Salvia spp. Front. Plant Sci. 2022, 13, 864807. [Google Scholar] [CrossRef]
- Mohaddab, M.; El Goumi, Y.; Gallo, M.; Montesano, D.; Zengin, G.; Bouyahya, A.; Fakiri, M. Biotechnology and in vitro culture as an alternative system for secondary metabolite production. Molecules 2022, 27, 8093. [Google Scholar] [CrossRef]
- Ozyigit, I.; Dogan, I.; Hocaoglu-Ozyigit, A.; Yalcin, B.; Erdogan, A.; Yalcin, I.E.; Cabi, E.; Kaya, Y. Production of secondary metabolites using tissue culture-based biotechnological applications. Front. Plant Sci. 2023, 14, 1132555. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Bilichowski, I.; Mikiciuk-Olasik, E.; Wysokinska, H. In vitro cultures of Salvia officinalis L. as a source of antioxidant compounds. Acta Soc. Bot. Pol. 2005, 74, 17–21. [Google Scholar] [CrossRef]
- Bisio, A.; Fraternale, D.; Schito, A.M.; Parricchi, A.; Dal Piaz, F.; Ricci, D.; Giacomini, M.; Ruffoni, B.; De Tommasi, N. Establishment and analysis of in vitro biomass from Salvia corrugata Vahl. and evaluation of antimicrobial activity. Phytochemistry 2016, 122, 276–285. [Google Scholar] [CrossRef]
- Ruffoni, B.; Raffi, D.; Rizzo, A.M.; Oleszek, W.; Giardi, M.T.; Bertoli, A.; Pistelli, L. Establisment of in vitro Salvia cell biomass for the controlled production of antioxidant metabolites. Acta Hortic. 2010, 829, 423–427. [Google Scholar] [CrossRef]
- Martini, A.N.; Vlachou, G.; Papafotiou, M. Effect of explant origin and medium plant growth regulators on in vitro shoot proliferation and rooting of Salvia tomentosa, a native sage of the Northeastern Mediterranean basin. Agronomy 2022, 12, 1889. [Google Scholar] [CrossRef]
- Georgiev, V.; Pavlov, A. Salvia Biotechnology; Springer: New York, NY, USA, 2017; pp. 1–439. [Google Scholar]
- Kintzios, S.; Nikolaou, A.; Skoula, M. Somatic embryogenesis and in vitro rosmarinic acid accumulation in Salvia officinalis and S. fruticosa leaf callus cultures. Plant Cell Rep. 1999, 18, 462–466. [Google Scholar] [CrossRef]
- Bassolino, L.; Giacomelli, E.; Giovanelli, S.; Pistelli, L.; Cassetti, A.; Damonte, G.; Bisio, A.; Ruffoni, B. Tissue culture and aromatic profile in Salvia dolomitica Codd. Plant Cell Tissue Organ Cult. 2014, 121, 83–95. [Google Scholar] [CrossRef]
- Fraternale, D.; Bisio, A.; Ricci, D. Salvia x jamensis J. Compton: In vitro regeneration of shoots through TDZ and BA. Plant Biosyst. 2013, 147, 713–716. [Google Scholar] [CrossRef]
- Dougué Kentsop, R.A. Biotechnology Applied to Aromatic Plants for the Controlled Production of Bioactive Compounds; University of Genoa: Genoa, Italy, 2020. [Google Scholar]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1986, 3, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1987, 4, 399–413. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1990, 7, 149–164. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1992, 9, 1–16. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1994, 11, 265–277. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 1996, 13, 59–71. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2002, 19, 125–132. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2003, 20, 70–78. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2004, 21, 785–793. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2005, 22, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2006, 23, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2009, 26, 1156–1171. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2011, 28, 1755–1772. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2015, 32, 1654–1663. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2016, 33, 1227–1238. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2017, 34, 1233–1243. [Google Scholar] [CrossRef]
- Yang, M.H.; Blunden, G.; Xu, Y.X.; Nagy, G.; Mathe, I. Diterpenoids from Salvia species. Pharm. Pharmacol. Commun. 1996, 2, 69–71. [Google Scholar]
- Zhi, B.H.; Alfermann, A. Diterpenoid production in hairy root cultures of Salvia miltiorrhiza. Phytochemistry 1993, 32, 699–703. [Google Scholar] [CrossRef]
- Chen, H.; Yuan, J.-P.; Chen, F.; Zhang, Y.-L.; Song, J.-Y. Tanshinone production in Ti-transformed Salvia miltiorrhiza cell suspension cultures. J. Biotechnol. 1997, 58, 147–156. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Li, H.; Peng, L.; Ru, M.; Liang, Z.; Yan, X.; Zhu, Y. Establishment of Salvia castanea Diels f. tomentosa Stib. hairy root cultures and the promotion of tanshinone accumulation and gene expression with Ag(+), methyl jasmonate, and yeast extract elicitation. Protoplasma 2016, 253, 87–100. [Google Scholar] [CrossRef]
- Vaccaro, M.; Malafronte, N.; Alfieri, M.; De Tommasi, N.; Leone, A. Enhanced biosynthesis of bioactive abietane diterpenes by overexpressing AtDXS or AtDXR genes in Salvia sclarea hairy roots. Plant Cell Tissue Organ Cult. 2014, 119, 65–77. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Seabra, R.M.; Andrade, P.B.; Fernandes-Ferreira, M. Determination of phenolic antioxidant compounds produced by calli and cell suspensions of sage (Salvia officinalis L.). J. Plant Physiol. 2003, 160, 1025–1032. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Kaiser, M.; Wysokińska, H. The production and antiprotozoal activity of abietane diterpenes in Salvia austriaca hairy roots grown in shake flasks and bioreactor. Prep. Biochem. Biotechnol. 2017, 47, 58–66. [Google Scholar] [CrossRef]
- LI, M.H.; Peng, Y.; Xiao, P.G. Distribution of tanshinones in the genus Salvia (family Lamiaceae) from China and its systematic significance. J. Syst. Evol. 2010, 48, 118–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, P.; Ye, M.; Kim, S.-H.; Jiang, C.; Lü, J. Tanshinones: Sources, Pharmacokinetics and Anti-Cancer Activities. Int. J. Mol. Sci. 2012, 13, 13621–13666. [Google Scholar] [CrossRef]
- Li, M.-H.; Li, Q.-Q.; Liu, Y.-Z.; Cui, Z.-H.; Zhang, N.; Huang, L.-Q.; Xiao, P.-G. Pharmacophylogenetic study on plants of genus Salvia L. from China. Chin. Herb. Med. 2013, 5, 164–181. [Google Scholar]
- Zhou, L.; Zuo, Z.; Chow, M.S.S. Danshen: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2005, 45, 1345–1359. [Google Scholar] [CrossRef]
- Li, H.-B.; Chen, F. Preparative isolation and purification of six diterpenoids from the Chinese medicinal plant Salvia miltiorrhiza by high-speed counter-current chromatography. J. Chromatogr. A 2001, 925, 109–114. [Google Scholar] [CrossRef]
- Li, M.-H.; Chen, J.-M.; Peng, Y.; Wu, Q.; Xiao, P.-G. Investigation of Danshen and related medicinal plants in China. J. Ethnopharmacol. 2008, 120, 419–426. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, Y.J.; Hillwig, M.L.; Shen, Y.; Yang, L.; Wang, Y.; Zhang, X.; Liu, W.; Peters, R.J.; Chen, X. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts. Proc. Natl. Acad. Sci. USA 2013, 110, 12108–12113. [Google Scholar] [CrossRef]
- Gao, W.; Hillwig, M.L.; Huang, L.; Cui, G.; Wang, X.; Kong, J.; Yang, B.; Peters, R.J. A functional genomics approach to tanshinone biosynthesis provides stereochemical insights. Org. Lett. 2009, 11, 5170–5173. [Google Scholar] [CrossRef]
- Ikeshiro, Y.; Mase, I.; Tomita, Y. Abietane type diterpenoids from Salvia miltiorrhiza. Phytochemistry 1989, 28, 3139–3141. [Google Scholar] [CrossRef]
- Lee, A.R.; Wu, W.L.; Chang, W.L.; Lin, H.C.; King, M.L. Isolation and bioactivity of new tanshinones. J. Nat. Prod. 1987, 50, 157–160. [Google Scholar] [CrossRef]
- Wang, X.; Morris-Natschke, S.L.; Lee, K.-H. New developments in the chemistry and biology of the bioactive constituents of tanshen. Med. Res. Rev. 2007, 27, 133–148. [Google Scholar] [CrossRef]
- Bo, L.; Fan-Di, N.; Zhong-Wen, L.; Hong-Jie, Z.; De-Zu, W.; Han-Dong, S. Diterpenoids from the roots of Salvia przewalskii. Phytochemistry 1991, 30, 3815–3817. [Google Scholar] [CrossRef]
- Liu, L.; Yang, D.; Xing, B.; Zhang, H.; Liang, Z. Salvia castanea hairy roots are more tolerant to phosphate deficiency than Salvia miltiorrhiza hairy roots based on the secondary metabolism and antioxidant defenses. Molecules 2018, 23, 1132. [Google Scholar] [CrossRef]
- Skała, E.; Mielicki, W.; Wysokińska, H. Tanshinones in culture of Salvia przewalskii Maxim in vitro. Acta Biol. Cracoviensia Ser. Bot. 2014, 56, 104–110. [Google Scholar] [CrossRef]
- Skała, E.; Wysokińska, H. Tanshinone production in roots of micropropagated Salvia przewalskii Maxim. Z. Naturforschung C 2005, 60, 583–586. [Google Scholar] [CrossRef]
- Sairafianpour, M.; Christensen, J.; Stærk, D.; Budnik, B.A.; Kharazmi, A.; Bagherzadeh, K.; Jaroszewski, J.W. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1,2-quinones from Perovskia abrotanoides: New source of tanshinones. J. Nat. Prod. 2001, 64, 1398–1403. [Google Scholar] [CrossRef]
- Zaker, A.; Sykora, C.; Gössnitzer, F.; Abrishamchi, P.; Asili, J.; Mousavi, S.H.; Wawrosch, C. Effects of some elicitors on tanshinone production in adventitious root cultures of Perovskia abrotanoides Karel. Ind. Crop. Prod. 2015, 67, 97–102. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, critical pharmacological components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef]
- Rodríguez, B.; Fernández-Gadea, F.; Savona, G. A rearranged abietane diterpenoid from the root of Salvia aethiopis. Phytochemistry 1984, 23, 1805–1806. [Google Scholar] [CrossRef]
- Ulubelen, A.; Tan, N. Terpenoids from Salvia recognita and Salvia aethiopis. Sci. Pharm. 1999, 67, 83–88. [Google Scholar]
- Boya, M.T.; Valverde, S. An orthoquinone isolated from Salvia aethiopis. Phytochemistry 1981, 20, 1367–1368. [Google Scholar] [CrossRef]
- Michavila, A.; María, C.; Rodríguez, B. 20-Nor-abietane and rearranged abietane diterpenoids from the root of Salvia argentea. Phytochemistry 1986, 25, 1935–1937. [Google Scholar] [CrossRef]
- Ulubelen, A.; Topcu, G.; Tan, N. Rearranged abietane diterpenes from Salvia candidissima. Phytochemistry 1992, 31, 3637–3638. [Google Scholar] [CrossRef]
- Goren, A.C.; Topcu, G.; Oksuz, S.; Kokdil, G.; Voelter, W.; Ulubelen, A. Diterpenoids from Salvia ceratophylla. Nat. Prod. Lett. 2002, 16, 47–52. [Google Scholar] [CrossRef]
- Gökdil, G.; Topcu, G.; Sönmez, U.; Ulubelen, A. Terpenoids and flavonoids from Salvia cyanescens. Phytochemistry 1997, 46, 799–800. [Google Scholar] [CrossRef]
- Ulubelen, A.; Sönmez, U.; Topcu, G. Diterpenoids from the roots of Salvia sclarea. Phytochemistry 1997, 44, 1297–1299. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Skrzypek, Z.; Wysokińska, H. Diterpenoids and triterpenoids in hairy roots of Salvia sclarea. Plant Cell Tissue Organ Cult. 2006, 84, 171–179. [Google Scholar] [CrossRef]
- Kuzma, Ł.; Bruchajze, E.; Wysokińska, H. Diterpenoid production in hairy root culture of Salvia sclarea L. Z. Naturforsch. Sect. C 2008, 63, 621–624. [Google Scholar] [CrossRef]
- Kentsop, R.A.D.; Iobbi, V.; Donadio, G.; Ruffoni, B.; De Tommasi, N.; Bisio, A. Abietane diterpenoids from the hairy roots of Salvia corrugata. Molecules 2021, 26, 5144. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Derda, M.; Hadaś, E.; Wysokińska, H. Abietane diterpenoids from Salvia sclarea transformed roots as growth inhibitors of pathogenic Acanthamoeba spp. Parasitol. Res. 2015, 114, 323–327. [Google Scholar] [CrossRef]
- Ebrahimi, S.N.; Zimmermann, S.; Zaugg, J.; Smiesko, M.; Brun, R.; Hamburger, M. Abietane diterpenoids from Salvia sahendicab—Antiprotozoal activity and determination of their absolute configurations. Planta Medica 2013, 29, 150–156. [Google Scholar]
- Hernández-Pérez, M.; Rabanal, R.M.; de la Torre, M.C.; Rodríguez, B. Analgesic, anti-inflammatory, antipyretic and haematological effects of aethiopinone, an o-naphthoquinone diterpenoid from Salvia aethiopis roots and two hemisynthetic derivatives. Planta Medica 1995, 61, 505–509. [Google Scholar] [CrossRef]
- Walencka, E.; Rozalska, S.; Wysokinska, H.; Rozalski, M.; Kuzma, L.; Rozalska, B. Salvipisone and aethiopinone from Salvia sclarea hairy roots modulate staphylococcal antibiotic resistance and express anti-biofilm activity. Planta Medica 2007, 73, 545–551. [Google Scholar] [CrossRef]
- Różalski, M.; Walencka, E.; Różalska, B.; Wysokińska, H. Antimicrobial activity of diterpenoids from hairy roots of Salvia sclarea L.: Salvipisone as a potential anti-biofilm agent active against antibiotic resistant Staphylococci. Phytomedicine 2007, 14, 31–35. [Google Scholar]
- Hernandez-Perez, M.; Rabanal, R.; Arias, A.; de La Torre, M.; Rodriguez, B. Aethiopinone, an antibacterial and cytotoxic agent from Salvia aethiopis roots. Pharm. Biol. 1999, 37, 17–21. [Google Scholar] [CrossRef]
- Różalski, M.; Kuźma, Ł.; Wysokińska, H.; Krajewska, U. Cytotoxic and proapoptotic activity of diterpenoids from in vitro cultivated Salvia sclarea roots. Studies on the leukemia cell lines. Z. Naturforschung C 2006, 61, 483–488. [Google Scholar] [CrossRef]
- Božić, D.; Papaefthimiou, D.; Brückner, K.; De Vos, R.C.H.; Tsoleridis, C.A.; Katsarou, D.; Papanikolaou, A.; Pateraki, I.; Chatzopoulou, F.M.; Dimitriadou, E. Towards elucidating carnosic acid biosynthesis in Lamiaceae: Functional characterization of the three first steps of the pathway in Salvia fruticosa and Rosmarinus officinalis. PLoS ONE 2015, 10, e0124106. [Google Scholar] [CrossRef]
- Birtić, S.; Dussort, P.; Pierre, F.-X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef]
- Lin, K.-I.; Lin, C.-C.; Kuo, S.-M.; Lai, J.-C.; Wang, Y.-Q.; You, H.-L.; Hsu, M.-L.; Chen, C.-H.; Shiu, L.-Y. Carnosic acid impedes cell growth and enhances anticancer effects of carmustine and lomustine in melanoma. Biosci. Rep. 2018, 38, BSR20180005. [Google Scholar] [CrossRef]
- Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Extraction of carnosic acid and carnosol from sage (Salvia officinalis L.) leaves by supercritical fluid extraction and their antioxidant and antibacterial activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef]
- Poeckel, D.; Greiner, C.; Verhoff, M.; Rau, O.; Tausch, L.; Hörnig, C.; Steinhilber, D.; Schubert-Zsilavecz, M.; Werz, O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem. Pharmacol. 2008, 76, 91–97. [Google Scholar] [CrossRef]
- Bauer, J.; Kuehnl, S.; Rollinger, J.M.; Scherer, O.; Northoff, H.; Stuppner, H.; Werz, O.; Koeberle, A. Carnosol and carnosic acids from Salvia officinalis inhibit microsomal prostaglandin E2 synthase-1. J. Pharmacol. Exp. Ther. 2012, 342, 169–176. [Google Scholar] [CrossRef]
- Ninomiya, K.; Matsuda, H.; Shimoda, H.; Nishida, N.; Kasajima, N.; Yoshino, T.; Morikawa, T.; Yoshikawa, M. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bioorganic Med. Chem. Lett. 2004, 14, 1943–1946. [Google Scholar] [CrossRef]
- Zunin, P.; Leardi, R.; Bisio, A.; Boggia, R.; Romussi, G. Oxidative stability of virgin olive oil enriched with carnosic acid. Food Res. Int. 2010, 43, 1511–1516. [Google Scholar] [CrossRef]
- Cortese, K.; Daga, A.; Monticone, M.; Tavella, S.; Stefanelli, A.; Aiello, C.; Bisio, A.; Bellese, G.; Castagnola, P. Carnosic acid induces proteasomal degradation of Cyclin B1, RB and SOX2 along with cell growth arrest and apoptosis in GBM cells. Phytomedicine 2016, 23, 679–685. [Google Scholar] [CrossRef]
- Maione, F.; Cantone, V.; Pace, S.; Chini, M.G.; Bisio, A.; Romussi, G.; Pieretti, S.; Werz, O.; Koeberle, A.; Mascolo, N. Anti-inflammatory and analgesic activity of carnosol and carnosic acid in vivo and in vitro and in silico analysis of their target interactions. Br. J. Pharmacol. 2017, 174, 1497–1508. [Google Scholar] [CrossRef]
- D’Alesio, C.; Bellese, G.; Gagliani, M.C.; Aiello, C.; Grasselli, E.; Marcocci, G.; Bisio, A.; Tavella, S.; Daniele, T.; Cortese, K.; et al. Cooperative antitumor activities of carnosic acid and Trastuzumab in ERBB2+ breast cancer cells. J. Exp. Clin. Cancer Res. 2017, 36, 154. [Google Scholar] [CrossRef]
- Gonzalez, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Abreu, M.E.; Müller, M.; Alegre, L.; Munné-Bosch, S. Phenolic diterpene and α-tocopherol contents in leaf extracts of 60 Salvia species. J. Sci. Food Agric. 2008, 88, 2648–2653. [Google Scholar] [CrossRef]
- Brückner, K.; Božić, D.; Manzano, D.; Papaefthimiou, D.; Pateraki, I.; Scheler, U.; Ferrer, A.; de Vos, R.C.H.; Kanellis, A.K.; Tissier, A. Characterization of two genes for the biosynthesis of abietane-type diterpenes in rosemary (Rosmarinus officinalis) glandular trichomes. Phytochemistry 2014, 101, 52–64. [Google Scholar] [CrossRef]
- Anwar, F.; Qadir, R. Carnosic acid and carnosol. In A Centum of Valuable Plant Bioactives; Mushtaq, M., Anwar, F., Eds.; Elsevier: Cambridge, MA, USA, 2021; pp. 261–274. [Google Scholar]
- Min, F.; Liu, X.; Li, Y.; Dong, M.; Qu, Y.; Liu, W. Carnosic acid suppresses the development of oral squamous cell carcinoma via mitochondrial-mediated apoptosis. Front. Oncol. 2021, 11, 760861. [Google Scholar] [CrossRef]
- Iobbi, V.; Parisi, V.; Bernabè, G.; De Tommasi, N.; Bisio, A.; Brun, P. Anti-biofilm activity of carnosic acid from Salvia rosmarinus against methicillin-resistant Staphylococcus aureus. Plants 2023, 12, 3679. [Google Scholar] [CrossRef]
- Iobbi, V.; Donadio, G.; Lanteri, A.P.; Maggi, N.; Kirchmair, J.; Parisi, V.; Minuto, G.; Copetta, A.; Giacomini, M.; Bisio, A.; et al. Targeted metabolite profiling of Salvia rosmarinus Italian local ecotypes and cultivars and inhibitory activity against Pectobacterium carotovorum subsp. carotovorum. Front. Plant Sci. 2024, 15, 1164859. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Hadavand Mirzaei, H.; Firuzi, O.; Pirhadi, S.; Asadollahi, M.; Chandran, J.N.; Schneider, B. Cytotoxic abietane-type diterpenoids from roots of Salvia spinosa and their in silico pharmacophore modeling. Nat. Prod. Res. 2022, 36, 3183–3188. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Wysokińska, H.; Sikora, J.; Olszewska, P.; Mikiciuk-Olasik, E.; Szymański, P. Taxodione and extracts from Salvia austriaca roots as human cholinesterase inhibitors. Phytother. Res. 2016, 30, 234–242. [Google Scholar] [CrossRef]
- Búfalo, J.; Cantrell, C.L.; Jacob, M.R.; Schrader, K.K.; Tekwani, B.L.; Kustova, T.S.; Ali, A.; Boaro, C.S. Antimicrobial and antileishmanial activities of diterpenoids isolated from the roots of Salvia deserta. Planta Medica 2015, 82, 131–137. [Google Scholar] [CrossRef]
- Shafaei-Bajestani, N.; Emami, S.A.; Asili, J.; Tayarani-Najaran, Z. Anti-apoptotic effect of taxodione on serum/glucose deprivation-induced PC12 cells death. Cell. Mol. Neurobiol. 2014, 34, 1103–1109. [Google Scholar] [CrossRef]
- Sadowska, B.; Kuźma, Ł.; Micota, B.; Budzyńska, A.; Wysokińska, H.; Kłys, A.; Więckowska-Szakiel, M.; Różalska, B. New biological potential of abietane diterpenoids isolated from Salvia austriaca against microbial virulence factors. Microb. Pathog. 2016, 98, 132–139. [Google Scholar] [CrossRef]
- Rodríguez-Hahn, L.; Esquivel, B.; Sánchez, C.; Estebanes, L.; Cárdenas, J.; Soriano-García, M.; Toscano, R.; Ramamoorthy, T. Abietane type diterpenoids from Salvia fruticulosa. A revision of the structure of fruticulin B. Phytochemistry 1989, 28, 567–570. [Google Scholar] [CrossRef]
- Erbano, M.; Ehrenfried, C.A.; Stefanello, M.É.A.; Dos Santos, É.P. Morphoanatomical and phytochemical studies of Salvia lachnostachys (Lamiaceae). Microsc. Res. Tech. 2012, 75, 1737–1744. [Google Scholar] [CrossRef]
- Bisio, A.; Romussi, G.; Russo, E.; De Tommasi, N.; Mascolo, N.; Alfieri, A.; Bonito, M.C.; Cicala, C. Platelet antiaggregating activity and chemical constituents of Salvia x jamensis J. Compton. Nat. Prod. Commun. 2008, 3, 881–884. [Google Scholar] [CrossRef]
- Schito, A.; Piatti, G.; Stauder, M.; Bisio, A.; Giacomelli, E.; Romussi, G.; Pruzzo, C. Effects of demethylfruticuline A and fruticuline A from Salvia corrugata Vahl. on biofilm production in vitro by multiresistant strains of Staphylococcus aureus, Staphylococcus epidermidis and Enterococcus faecalis. Int. J. Antimicrob. Agents 2011, 37, 129–134. [Google Scholar] [CrossRef]
- Santos Oliveira, C.; Alvarez, C.J.; Pereira Cabral, M.R.; Sarragiotto, M.H.; Salvador, M.J.; Alves Stefanello, M.É. Three new diterpenoids from the leaves of Salvia lachnostachys. Nat. Prod. Res. 2022, 36, 5600–5605. [Google Scholar] [CrossRef]
- Piccinelli, A.C.; Figueiredo de Santana Aquino, D.; Morato, P.N.; Kuraoka-Oliveira, Â.M.; Strapasson, R.L.B.; Dos Santos, E.P.; Stefanello, M.É.A.; Oliveira, R.J.; Kassuya, C.A.L. Anti-inflammatory and antihyperalgesic activities of ethanolic extract and fruticulin a from Salvia lachnostachys leaves in mice. Evid. Based Complement. Altern. Med. 2014, 2014, 835914. [Google Scholar] [CrossRef]
- Giannoni, P.; Narcisi, R.; De Totero, D.; Romussi, G.; Quarto, R.; Bisio, A. The administration of demethyl fruticulin A from Salvia corrugata to mammalian cells lines induces “anoikis”, a special form of apoptosis. Phytomedicine 2010, 17, 449–456. [Google Scholar] [CrossRef]
- Monticone, M.; Bisio, A.; Daga, A.; Giannoni, P.; Giaretti, W.; Maffei, M.; Pfeffer, U.; Romeo, F.; Quarto, R.; Romussi, G. Demethyl fruticulin A (SCO-1) causes apoptosis by inducing reactive oxygen species in mitochondria. J. Cell. Biochem. 2010, 111, 1149–1159. [Google Scholar] [CrossRef]
- Hadavand Mirzaei, H.; Hosseini, S.M. In-silico assessments of fruticulin-a and demethylfruticulin-a isolated from Salvia species against important anticancer targets. RJP 2023, 10, 31–41. [Google Scholar]
- Rhodes, M.J.C.; Parr, A.J.; Giulietti, A.; Aird, E.L.H. Influence of exogenous hormones on the growth and secondary metabolite formation in transformed root cultures. Plant Cell Tissue Organ Cult. 1994, 38, 143–151. [Google Scholar] [CrossRef]
- Nussbaumer, P.; Kapétanidis, I.; Christen, P. Hairy roots of Datura candida × D. aurea: Effect of culture medium composition on growth and alkaloid biosynthesis. Plant Cell Rep. 1998, 17, 405–409. [Google Scholar] [CrossRef]
- Poulev, A.; O’Neal, J.M.; Logendra, S.; Pouleva, R.B.; Timeva, V.; Garvey, A.S.; Gleba, D.; Jenkins, I.S.; Halpern, B.T.; Kneer, R. Elicitation, a new window into plant chemodiversity and phytochemical drug discovery. J. Med. Chem. 2003, 46, 2542–2547. [Google Scholar] [CrossRef]
- Smetanska, I. Production of secondary metabolites using plant cell cultures. Adv. Biochem. Eng./Biotechnol. 2008, 111, 187–228. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.-L.; Lin, B.; Rahman, K.; Zheng, C.-J.; Han, T.; Qin, L.-P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.C.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Veliky, I.A.; Martin, S.M. A fermenter for plant cell suspension cultures. Can. J. Microbiol. 1970, 16, 223–226. [Google Scholar] [CrossRef]
- Efferth, T. Biotechnology Applications of Plant Callus Cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Guo, X.; Gao, W.; Li, K. Tissue culture of Salvia miltiorrhiza adventitious roots (II) Effects of carbon, nitrogen, and phosphate sources on culture of Salvia miltiorrhiza adventitious roots. Chin. Tradit. Herb. Drugs 1994, wpr-576777. Available online: https://www.kczg.org.cn/scholar/paperDetail?uid=115791&id=52132373 (accessed on 15 February 2024).
- Gupta, S.K.; Liu, R.-B.; Liaw, S.-Y.; Chan, H.-S.; Tsay, H.-S. Enhanced tanshinone production in hairy roots of ‘Salvia miltiorrhiza Bunge’ under the influence of plant growth regulators in liquid culture. Bot. Stud. 2011, 52, 435–443. [Google Scholar]
- Rao, S.R.; Ravishankar, G. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar]
- Abbasi, B.H.; Tian, C.-L.; Murch, S.J.; Saxena, P.K.; Liu, C.-Z. Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep. 2007, 26, 1367–1372. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H. Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014, 140, 223–227. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Wang, Y. Artemisinin: Current state and perspectives for biotechnological production of an antimalarial drug. Appl. Microbiol. Biotechnol. 2006, 72, 11–20. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Tabata, M.; Mizukami, H.; Hiraoka, N.; Konoshima, M. Pigment formation in callus cultures of Lithospermum erythrorhizon. Phytochemistry 1974, 13, 927–932. [Google Scholar] [CrossRef]
- Chandra, S.; Chandra, R. Engineering secondary metabolite production in hairy roots. Phytochem. Rev. 2011, 10, 371–395. [Google Scholar] [CrossRef]
- Radman, R.; Saez, T.; Bucke, C.; Keshavarz, T. Elicitation of plants and microbial cell systems. Biotechnol. Appl. Biochem. 2003, 37, 91–102. [Google Scholar] [CrossRef]
- Soundarapandian, S.; Dhandayuthapani, K. Interaction of plant growth promoting rhizobacteria (PGPR) and endophytes with medicinal plants-new avenues for phytochemicals. J. Phytol. 2010, 2, 91–100. [Google Scholar]
- Nartop, P. Engineering of biomass accumulation and secondary metabolite production in plant cell and tissue cultures. In Plant Metabolites and Regulation under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 169–194. [Google Scholar]
- Ganapathi, B.; Kargi, F. Recent advances in indole alkaloid production by Catharanthus roseus (Periwinkle). J. Exp. Bot. 1990, 41, 259–267. [Google Scholar] [CrossRef]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and biotic elicitors-role in secondary metabolites production through in vitro culture of medicinal plants. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A.K., Shanker, C., Eds.; InTech: Vienna, Austria, 2016; pp. 247–277. [Google Scholar]
- Ferrari, S. Biological elicitors of plant secondary metabolites: Mode of action and use in the production of nutraceutics. Adv. Exp. Med. Biol. 2010, 698, 152–166. [Google Scholar] [CrossRef]
- Giauque, H.; Hawkes, C.V. Climate affects symbiotic fungal endophyte diversity and performance. Am. J. Bot. 2013, 100, 1435–1444. [Google Scholar] [CrossRef]
- Tyc, O.; Song, C.; Dickschat, J.S.; Vos, M.; Garbeva, P. The ecological role of volatile and soluble secondary metabolites produced by soil bacteria. Trends Microbiol. 2017, 25, 280–292. [Google Scholar] [CrossRef]
- Adegboye, M.F.; Babalola, O.O. Taxonomy and ecology of antibiotic producing actinomycetes. Afr. J. Agric. Res. 2012, 7, 2255–2261. [Google Scholar]
- Olanrewaju, O.S.; Babalola, O.O. Streptomyces: Implications and interactions in plant growth promotion. Appl. Microbiol. Biotechnol. 2019, 103, 1179–1188. [Google Scholar] [CrossRef]
- Shweta, S.; Gurumurthy, B.R.; Ravikanth, G.; Ramanan, U.S.; Shivanna, M.B. Endophytic fungi from Miquelia dentata Bedd., produce the anti-cancer alkaloid, camptothecine. Phytomedicine 2013, 20, 337–342. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, Q.-H.; Wang, L.-N.; Duan, C.-M.; Xue, L.; Mao, N. Antagonistic effect of multifunctional actinomycete strain Act12 on soil-borne pathogenic fungi and its identification. Chin. J. Eco-Agric. 2011, 19, 394–398. [Google Scholar] [CrossRef]
- de Jesus Sousa, J.A.; Olivares, F.L. Plant growth promotion by streptomycetes: Ecophysiology, mechanisms and applications. Chem. Biol. Technol. Agric. 2016, 3, 24. [Google Scholar]
- Yan, Y.; Zhang, S.; Yang, D.; Zhang, J.; Liang, Z. Effects of Streptomyces pactum Act12 on Salvia miltiorrhiza hairy root growth and tanshinone synthesis and its mechanisms. Appl. Biochem. Biotechnol. 2014, 173, 883–893. [Google Scholar] [CrossRef]
- Barea, J.-M.; Pozo, M.J.; Azcon, R.; Azcon-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef]
- Paterson, J.; Jahanshah, G.; Li, Y.; Wang, Q.; Mehnaz, S.; Gross, H. The contribution of genome mining strategies to the understanding of active principles of PGPR strains. FEMS Microbiol. Ecol. 2016, 93, 249. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.L.; Zhou, L.G.; Wu, J.Y. Promotion of Salvia miltiorrhiza hairy root growth and tanshinone production by polysaccharide–protein fractions of plant growth-promoting rhizobacterium Bacillus cereus. Process Biochem. 2010, 45, 1517–1522. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ng, J.; Shi, M.; Wu, S.-J. Enhanced secondary metabolite (tanshinone) production of Salvia miltiorrhiza hairy roots in a novel root–bacteria coculture process. Appl. Microbiol. Biotechnol. 2007, 77, 543–550. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, S.; Zhang, J.; Ma, P.; Duan, J.-L.; Liang, Z. Effect and mechanism of endophytic bacteria on growth and secondary metabolite synthesis in Salvia miltiorrhiza hairy roots. Acta Physiol. Plant. 2014, 36, 1095–1105. [Google Scholar] [CrossRef]
- Zhai, X.; Luo, D.; Li, X.; Han, T.; Jia, M.; Kong, Z.; Ji, J.; Rahman, K.; Qin, L.; Zheng, C. Endophyte Chaetomium globosum D38 promotes bioactive constituents accumulation and root production in Salvia miltiorrhiza. Front. Microbiol. 2018, 8, 2694. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43. [Google Scholar] [CrossRef]
- Ming, Q.; Han, T.; Li, W.; Zhang, Q.; Zhang, H.; Zheng, C.; Huang, F.; Rahman, K.; Qin, L. Tanshinone IIA and tanshinone I production by Trichoderma atroviride D16, an endophytic fungus in Salvia miltiorrhiza. Phytomedicine 2012, 19, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Ming, Q.; Su, C.; Zheng, C.; Jia, M.; Zhang, Q.; Zhang, H.; Rahman, K.; Han, T.; Qin, L. Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis. J. Exp. Bot. 2013, 64, 5687–5694. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.-T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Zhou, L.-G.; Wu, J.-Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures. Appl. Microbiol. Biotechnol. 2010, 87, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Yan, J.; Li, H.; Li, S.; Yao, R.; Deng, H.; Xie, Q.; Xie, D. The Arabidopsis F-box protein CORONATINE INSENSITIVE1 is stabilized by SCFCOI1 and degraded via the 26S proteasome pathway. Plant Cell 2013, 25, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Weiler, E.; Kutchan, T.; Gorba, T.; Brodschelm, W.; Niesel, U.; Bublitz, F. The Pseudomonas phytotoxin coronatine mimics octadecanoid signalling molecules of higher plants. FEBS Lett. 1994, 345, 9–13. [Google Scholar] [CrossRef]
- Vaccaro, M.C.; Mariaevelina, A.; Malafronte, N.; De Tommasi, N.; Leone, A. Increasing the synthesis of bioactive abietane diterpenes in Salvia sclarea hairy roots by elicited transcriptional reprogramming. Plant Cell Rep. 2017, 36, 375–386. [Google Scholar] [CrossRef]
- Li, G.-J.; Wang, S.-C.; Xia, K.; Zhou, X. Effect of yeast elicitor and salicylic acid on the fluctuation of phytohormone contents in Ti-transformed Salvia miltiorrhiza cell cultures. Plant Growth Regul. 2003, 39, 27–32. [Google Scholar] [CrossRef]
- Miyasaka, H.; Nasu, M.; Yoneda, K. Salvia miltiorrhiza: In vitro production of cryptotanshinone and ferruginol. In Medicinal and Aromatic Plants II; Springer: Berlin/Heidelberg, Germany, 1989; pp. 417–430. [Google Scholar]
- Chen, H.; Chen, F. Effects of methyl jasmonate and salicylic acid on cell growth and cryptotanshinone formation in Ti transformed Salvia miltiorrhiza cell suspension cultures. Biotechnol. Lett. 1999, 21, 803–807. [Google Scholar] [CrossRef]
- Shi, M.; Kwok, K.W.; Wu, J.Y. Enhancement of tanshinone production in Salvia miltiorrhiza Bunge (red or Chinese sage) hairy-root culture by hyperosmotic stress and yeast elicitor. Biotechnol. Appl. Biochem. 2007, 46, 191–196. [Google Scholar] [CrossRef]
- Ge, X.; Wu, J. Induction and potentiation of diterpenoid tanshinone accumulation in Salvia miltiorrhiza hairy roots by β-aminobutyric acid. Appl. Microbiol. Biotechnol. 2005, 68, 183–188. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Shi, M. Ultrahigh diterpenoid tanshinone production through repeated osmotic stress and elicitor stimulation in fed-batch culture of Salvia miltiorrhiza hairy roots. Appl. Microbiol. Biotechnol. 2008, 78, 441–448. [Google Scholar] [CrossRef]
- Yan, Q.; Hu, Z.; Tan, R.X.; Wu, J. Efficient production and recovery of diterpenoid tanshinones in Salvia miltiorrhiza hairy root cultures with in situ adsorption, elicitation and semi-continuous operation. J. Biotechnol. 2005, 119, 416–424. [Google Scholar] [CrossRef]
- Yang, D.; Fang, Y.; Xia, P.; Zhang, X.; Liang, Z. Diverse responses of tanshinone biosynthesis to biotic and abiotic elicitors in hairy root cultures of Salvia miltiorrhiza and Salvia castanea Diels f. tomentosa. Gene 2018, 643, 61–67. [Google Scholar] [CrossRef]
- Chen, H.; Chena, F.; Chiu, F.C.; Lo, C.M. The effect of yeast elicitor on the growth and secondary metabolism of hairy root cultures of Salvia miltiorrhiza. Enzyme Microb. Technol. 2001, 28, 100–105. [Google Scholar] [CrossRef]
- Owolabi, I.O.; Yupanqui, C.T.; Siripongvutikorn, S. Enhancing secondary metabolites (emphasis on phenolics and antioxidants) in plants through elicitation and metabolomics. Pak. J. Nutr. 2018, 17, 411–420. [Google Scholar]
- Bian, L.H.; Zou, L.; Zhou, B.Q.; Liu, W.; Zhou, J.; Wang, X. Effect of Lanthanum on accumulation of active constituent and key enzymes expression of Salvia miltiorrhiza hairy root. Zhongguo Zhong Yao Za Zhi 2016, 41, 4344–4349. [Google Scholar]
- Han, M.; Guo, W.; Liang, Z.; Yang, D.; Yan, X.; Zhu, Y.; Liu, Y. Effects of cerous nitrate on growth and tanshinone production in Salvia miltiorrhiza hairy roots. J. Rare Earths 2015, 33, 1228–1235. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, Q.; Cheuk, W.-K.; Wu, J. Enhancement of tanshinone production in Salvia miltiorrhiza hairy root culture by Ag+ elicitation and nutrient feeding. Planta Medica 2004, 70, 147–151. [Google Scholar]
- Yu, Y.; Wang, T.; Wu, Y.; Zhou, Y.; Jiang, Y.; Zhang, L. Effect of elicitors on the metabolites in the suspension cell culture of Salvia miltiorrhiza Bunge. Physiol. Mol. Biol. Plants 2019, 25, 229–242. [Google Scholar] [CrossRef]
- Rohwer, C.L.; Erwin, J.E. Horticultural applications of jasmonates. J. Hortic. Sci. Biotechnol. 2008, 83, 283–304. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- De Geyter, N.; Gholami, A.; Goormachtig, S.; Goossens, A. Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci. 2012, 17, 349–359. [Google Scholar] [CrossRef]
- Liang, Z.S.; Yang, D.F.; Liang, X.; Zhang, Y.J.; Liu, Y.; Liu, F.H. Roles of reactive oxygen species in methyl jasmonate and nitric oxide-induced tanshinone production in Salvia miltiorrhiza hairy roots. Plant Cell Rep. 2012, 31, 873–883. [Google Scholar] [CrossRef]
- Hao, X.; Shi, M.; Cui, L.; Xu, C.; Zhang, Y.; Kai, G. Effects of methyl jasmonate and salicylic acid on tanshinone production and biosynthetic gene expression in transgenic Salvia miltiorrhiza hairy roots. Biotechnol. Appl. Biochem. 2015, 62, 24–31. [Google Scholar] [CrossRef]
- Xing, B.; Liang, L.; Liu, L.; Hou, Z.; Yang, D.; Yan, K.; Zhang, X.; Liang, Z. Overexpression of SmbHLH148 induced biosynthesis of tanshinones as well as phenolic acids in Salvia miltiorrhiza hairy roots. Plant Cell Rep. 2018, 37, 1681–1692. [Google Scholar] [CrossRef]
- Wang, X.Y.; Cui, G.H.; Huang, L.Q.; Qiu, D.Y. Effects of methyl jasmonate on accumulation and release of tanshinones in suspension cultures of Salvia miltiorrhiza hairy root. Zhongguo Zhong Yao Za Zhi 2007, 32, 300–302. [Google Scholar]
- Kuźma, Ł.; Bruchajzer, E.; Wysokińska, H. Methyl jasmonate effect on diterpenoid accumulation in Salvia sclarea hairy root culture in shake flasks and sprinkle bioreactor. Enzym. Microb. Technol. 2009, 44, 406–410. [Google Scholar] [CrossRef]
- Kračun-Kolarević, M.; Dmitrović, S.; Filipović, B.; Perić, M.; Mišić, D.; Simonović, A.; Todorović, S. Influence of sodium salicylate on rosmarinic acid, carnosol and carnosic acid accumulation by Salvia officinalis L. shoots grown in vitro. Biotechnol. Lett. 2015, 37, 1693–1701. [Google Scholar] [CrossRef]
- Yang, D.; Sheng, D.; Duan, Q.; Liang, X.; Liang, Z.; Liu, Y. PEG and ABA trigger the burst of reactive oxygen species to increase tanshinone production in Salvia miltiorrhiza hairy roots. J. Plant Growth Regul. 2012, 31, 579–587. [Google Scholar] [CrossRef]
- Zhou, J.; Ran, Z.; Xu, Z.; Liu, Q.; Huang, M.; Fang, L.; Guo, L. Effects of smoke-water and smoke-isolated karrikinolide on tanshinones production in Salvia miltiorrhiza hairy roots. S. Afr. J. Bot. 2018, 119, 265–270. [Google Scholar] [CrossRef]
- Wang, C.H.; Zheng, L.P.; Tian, H.; Wang, J.W. Synergistic effects of ultraviolet-B and methyl jasmonate on tanshinone biosynthesis in Salvia miltiorrhiza hairy roots. J. Photochem. Photobiol. B 2016, 159, 93–100. [Google Scholar] [CrossRef]
- Chen, I.J.; Lee, M.S.; Lin, M.K.; Ko, C.Y.; Chang, W.T. Blue light decreases tanshinone IIA content in Salvia miltiorrhiza hairy roots via genes regulation. J. Photochem. Photobiol. B 2018, 183, 164–171. [Google Scholar] [CrossRef]
- Cheng, Q.; He, Y.; Li, G.; Liu, Y.; Gao, W.; Huang, L. Effects of combined elicitors on tanshinone metabolic profiling and SmCPS expression in Salvia miltiorrhiza hairy root cultures. Molecules 2013, 18, 7473–7485. [Google Scholar] [CrossRef]
- Kai, G.; Liao, P.; Zhang, T.; Zhou, W.; Wang, J.; Xu, H.; Liu, Y.; Zhang, L. Characterization, expression profiling, and functional identification of a gene encoding geranylgeranyl diphosphate synthase from Salvia miltiorrhiza. Biotechnol. Bioprocess Eng. 2010, 15, 236–245. [Google Scholar] [CrossRef]
- Hong, M.L.K.; Bhatt, A.; Ping, N.S.; Keng, C.L. Detection of elicitation effect on Hyoscyamus niger L. root cultures for the root growth and production of tropane alkaloids. Rom. Biotechnol. Lett. 2012, 17, 7340–7351. [Google Scholar]
- Ge, X.; Wu, J. Tanshinone production and isoprenoid pathways in Salvia miltiorrhiza hairy roots induced by Ag+ and yeast elicitor. Plant Sci. 2005, 168, 487–491. [Google Scholar] [CrossRef]
- Zhao, J.-L.; Zou, L.; Zhang, C.-Q.; Li, Y.-Y.; Peng, L.-X.; Xiang, D.-B.; Zhao, G. Efficient production of flavonoids in Fagopyrum tataricum hairy root cultures with yeast polysaccharide elicitation and medium renewal process. Pharmacogn. Mag. 2014, 10, 234. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Mei, X. Enhancement of taxol production and excretion in Taxus chinensis cell culture by fungal elicitation and medium renewal. Appl. Microbiol. Biotechnol. 2001, 55, 404–410. [Google Scholar] [CrossRef]
- Diwan, R.; Malpathak, N. Bioprocess optimization of furanocoumarin elicitation by medium renewal and re-elicitation: A perfusion-based approach. Appl. Biochem. Biotechnol. 2011, 163, 756–764. [Google Scholar] [CrossRef]
- Chiang, L.; Abdullah, M.A. Enhanced anthraquinones production from adsorbent-treated Morinda elliptica cell suspension cultures in production medium strategy. Process Biochem. 2007, 42, 757–763. [Google Scholar] [CrossRef]
- Yan, Q.; Wu, J.Y.; Liu, R. Modeling of tanshinone synthesis and phase distribution under the combined effect of elicitation and in situ adsorption in Salvia miltiorrhiza hairy root cultures. Biotechnol. Lett. 2011, 33, 813–819. [Google Scholar] [CrossRef]
- Miyasaka, H.; Nasu, M.; Yamamoto, T.; Endo, Y.; Yoneda, K. Production of cryptotanshinone and ferruginol by immobilized cultured cells of Salvia miltiorrhiza. Phytochemistry 1986, 25, 1621–1624. [Google Scholar] [CrossRef]
- Vuong, T.V.; Franco, C.; Zhang, W. Treatment strategies for high resveratrol induction in Vitis vinifera L. cell suspension culture. Biotechnol. Rep. 2014, 1, 15–21. [Google Scholar] [CrossRef]
- Wei, T.; Gao, Y.; Deng, K.; Zhang, L.; Yang, M.; Liu, X.; Qi, C.; Wang, C.; Song, W.; Zhang, Y. Enhancement of tanshinone production in Salvia miltiorrhiza hairy root cultures by metabolic engineering. Plant Methods 2019, 15, 53. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Shi, M.; Hao, X.; Zhao, W.; Wang, Y.; Ren, J.; Kai, G. Transcription factor SmWRKY1 positively promotes the biosynthesis of tanshinones in Salvia miltiorrhiza. Front. Plant Sci. 2018, 9, 554. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, L.; Zheng, X.; Zhang, J.; Yang, L.; Tan, R.; Zhao, S. Overexpression of SmMYB9b enhances tanshinone concentration in Salvia miltiorrhiza hairy roots. Plant Cell Rep. 2017, 36, 1297–1309. [Google Scholar] [CrossRef]
- Shi, M.; Luo, X.; Ju, G.; Li, L.; Huang, S.; Zhang, T.; Wang, H.; Kai, G. Enhanced diterpene tanshinone accumulation and bioactivity of transgenic Salvia miltiorrhiza hairy roots by pathway engineering. J. Agric. Food Chem. 2016, 64, 2523–2530. [Google Scholar] [CrossRef]
- Gu, X.-C.; Chen, J.-F.; Xiao, Y.; Di, P.; Xuan, H.-J.; Zhou, X.; Zhang, L.; Chen, W.-S. Overexpression of allene oxide cyclase promoted tanshinone/phenolic acid production in Salvia miltiorrhiza. Plant Cell Rep. 2012, 31, 2247–2259. [Google Scholar] [CrossRef]
- Bai, Z.; Li, W.; Jia, Y.; Yue, Z.; Jiao, J.; Huang, W.; Xia, P.; Liang, Z. The ethylene response factor SmERF6 co-regulates the transcription of SmCPS1 and SmKSL1 and is involved in tanshinone biosynthesis in Salvia miltiorrhiza hairy roots. Planta 2018, 248, 243–255. [Google Scholar] [CrossRef]
- Alfieri, M.; Vaccaro, M.C.; Cappetta, E.; Ambrosone, A.; De Tommasi, N.; Leone, A. Coactivation of MEP-biosynthetic genes and accumulation of abietane diterpenes in Salvia sclarea by heterologous expression of WRKY and MYC2 transcription factors. Sci. Rep. 2018, 8, 11009. [Google Scholar] [CrossRef]
- Vaccaro, M.; Bernal, V.O.; Malafronte, N.; De Tommasi, N.; Leone, A. High yield of bioactive abietane diterpenes in Salvia sclarea hairy roots by overexpressing cyanobacterial DXS or DXR genes. Planta Medica 2019, 85, 973–980. [Google Scholar] [CrossRef]

| Compound Number | Name | Salvia Species | In Vitro Culture | References |
|---|---|---|---|---|
| 1 | tanshinone I | S. miltiorrhiza | HR | [63] |
| CC | [64] | |||
| S. castanea | HR | [65] | ||
| 2 | tanshinone IIA | S. miltiorrhiza | HR | [63] |
| CC | [64] | |||
| S. castanea | HR | [65] | ||
| 3 | cryptotanshinone | S. miltiorrhiza | HR | [63] |
| CC | [64] | |||
| S. castanea | HR | [65] | ||
| 4 | dihydrotanshinone I | S. miltiorrhiza | HR | [63] |
| S. castanea | HR | [65] | ||
| 5 | salvipisone | S. sclarea | HR | [66] |
| 6 | aethiopinone | S. sclarea | HR | [66] |
| 7 | 1-oxoaethiopinone | S. sclarea | HR | [66] |
| 8 | ferruginol | S. sclarea | HR | [66] |
| S. miltiorrhiza | HR | [63] | ||
| 9 | carnosic acid | S. officinalis | C | [67] |
| CC | [67] | |||
| MP | [37] | |||
| 10 | carnosol | S. officinalis | C | [67] |
| CC | [67] | |||
| MP | [37] | |||
| 11 | taxodone | S. austriaca | HR | [68] |
| 12 | taxodione | S. austriaca | HR | [68] |
| 13 | 15-deoxy-fuerstione | S. austriaca | HR | [68] |
| 14 | demethylfruticuline A | S. corrugata | RS | [38] |
| MP | [38] | |||
| apex | [38] | |||
| leaves | [38] | |||
| 15 | fruticuline A | S. corrugata | RS | [38] |
| apex | [38] | |||
| leaves | [38] |
| Biotic Elicitors | Abiotic Elicitors | |||
|---|---|---|---|---|
| Physical | Chemical | Hormones | Plant Signal Compounds | |
| Polysaccharide | UV radiation | Heavy metals | ABA | Jasmonic acid |
| Yeast extract | Osmotic stress | Mineral salts | TDZ | Salicylic acid |
| Fungi | Salinity | Gaseous toxins | ||
| Bacteria | Drought | |||
| Thermal stress | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dougué Kentsop, R.A.; Devi, P.; Copetta, A.; Ruffoni, B.; Parisi, V.; Bisio, A.; Iobbi, V. Salvia Species: Biotechnological Strategies Applied to In Vitro Cultures for the Controlled Production of Bioactive Diterpenoids. Agronomy 2024, 14, 835. https://doi.org/10.3390/agronomy14040835
Dougué Kentsop RA, Devi P, Copetta A, Ruffoni B, Parisi V, Bisio A, Iobbi V. Salvia Species: Biotechnological Strategies Applied to In Vitro Cultures for the Controlled Production of Bioactive Diterpenoids. Agronomy. 2024; 14(4):835. https://doi.org/10.3390/agronomy14040835
Chicago/Turabian StyleDougué Kentsop, Roméo Arago, Poonam Devi, Andrea Copetta, Barbara Ruffoni, Valentina Parisi, Angela Bisio, and Valeria Iobbi. 2024. "Salvia Species: Biotechnological Strategies Applied to In Vitro Cultures for the Controlled Production of Bioactive Diterpenoids" Agronomy 14, no. 4: 835. https://doi.org/10.3390/agronomy14040835
APA StyleDougué Kentsop, R. A., Devi, P., Copetta, A., Ruffoni, B., Parisi, V., Bisio, A., & Iobbi, V. (2024). Salvia Species: Biotechnological Strategies Applied to In Vitro Cultures for the Controlled Production of Bioactive Diterpenoids. Agronomy, 14(4), 835. https://doi.org/10.3390/agronomy14040835