Effects of Previous Crop Management, Fertilization Regime and Water Supply on Potato Tuber Proteome and Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
| Restricted watering | Optimum watering | |
|---|---|---|
| Conventional previous crop management | ||
| Control | No fertilization | No fertilization * |
| Composted cattle manure | Low input | Low input |
| High input | High input * | |
| Composted chicken manure pellets | Low input | Low input |
| High input | High input * | |
| Organic previous crop management | ||
| Control | No fertilization | No fertilization * |
| Composted cattle manure | Low input | Low input * |
| High input | High input * | |
| Composted chicken manure pellets | Low input | Low input * |
| High input | High input * | |
| Composted cattle manure | Composted chicken manure pellets | |||
|---|---|---|---|---|
| Low input | High input | Low input | High input | |
| C | 931 | 1863 | 745.7 | 1491.4 |
| Total N | 85 | 170 | 85 | 170 |
| NH4+ | 1.0 | 2.0 | 20.2 | 40.4 |
| NO3− | 1.4 | 2.8 | 0.35 | 0.70 |
| Organic N | 82.6 | 165.2 | 64.5 | 129.0 |
| P | 27.2 | 54.4 | 24.3 | 48.6 |
| P2O5 | 62.6 | 125.2 | 57.4 | 114.8 |
| K | 79.7 | 159.4 | 48.7 | 97.3 |
| K2O | 95.6 | 191.2 | 57.4 | 114.8 |
2.2. Chlorophyll Content
2.3. Yield Assessments
2.4. Protein Profiling
2.4.1. Protein Extraction and 2-DE
2.4.2. Image Analysis
2.5. Statistical Analysis
2.6. Protein Inference by Gel Matching
3. Results
3.1. Effects of Previous Crop Management, Fertilization Regime, and Watering Supply on Potato Agronomic Traits
3.1.1. Chlorophyll Content of Potato Leaves
3.1.2. Potato Tuber Yield
| Factor | Tuber number plant−1 | Tuber fresh weight (g) plant−1 | N concentration (%) | Dry matter (%) |
|---|---|---|---|---|
| Means (± SE) | ||||
| WR | ||||
| Restricted | 13.2 ± 0.4 | 575.3 ± 10.9 | 1.16 ± 0.02 | 23.8 ± 0.2 |
| Optimized | 14.1 ± 0.3 | 733.4 ± 12.0 | 1.03 ± 0.02 | 23.1 ± 0.3 |
| PCM | ||||
| Conventional soil | 15.2 ± 0.4 | 691.5 ± 14.8 | 1.09 ± 0.03 | 22.7 ± 0.2 |
| Organic soil | 12.1 ± 0.3 | 617.1 ± 12.8 | 1.10 ± 0.02 | 24.1 ± 0.2 |
| FR | ||||
| Control | 12.3 ± 0.4 c | 563.9 ± 17.6 d | 1.02 ± 0.03 | 23.5 ± 0.4 ab |
| Composted cattle manure, 85 kg N ha−1 | 12.9 ± 0.6 bc | 620.0 ± 16.4 c | 1.09 ± 0.04 | 23.4 ± 0.4 ab |
| Composted chicken manure pellets, 85 kg N ha−1 | 13.2 ± 0.7 bc | 667.7 ± 21.8 b | 1.09 ± 0.04 | 23.6 ± 0.4 ab |
| Composted cattle manure, 170 kg N ha−1 | 14.1 ± 0.5 b | 654.6 ± 19.7 bc | 1.14 ± 0.03 | 22.8 ± 0.5 b |
| Composted chicken manure pellets, 170 kg N ha−1 | 15.7 ± 0.6 a | 765.4 ± 22.9 a | 1.14 ± 0.04 | 23.9 ± 0.3 a |
| ANOVA | ||||
| Main effects | ||||
| WR | ns | <0.0001 | 0.0024 | 0.0615 |
| PCM | <0.0001 | <0.0001 | ns | <0.0001 |
| FM | <0.0001 | <0.0001 | 0.0607 | ns |
| Interaction effects | ||||
| WR × PCM | ns | ns | 0.0062 | 0.0777 |
| WR × FR | ns | 0.0711 | ns | ns |
| PCM × FR | ns | ns | 0.0452 | 0.0687 |
| WR × PCM × FR | ns | ns | ns | ns |
3.1.3. Nitrogen Content of Potato Tubers
3.1.4. Associations between Agronomic Factors and Tuber Yield and Yield Components


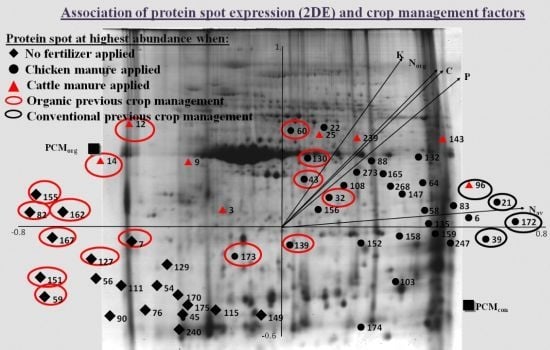
3.2. Effects of Previous Crop Management, Fertilization Regime, and Watering Supply on Potato Tuber Proteome

3.2.1. Associations between Agronomic Factors and Tuber Protein Profiles

3.2.2. Associations between Fertilization (Types and Input Levels) and Tuber Protein Profiles
3.2.3. Protein Inference by Gel Matching
| Protein spot | Protein inference by gel matching | Function | Treatment with greatest protein spot abundance 1 | Reference protein spot 2 |
|---|---|---|---|---|
| A. Protein spots significantly affected by fertilization treatments (and PCM) | ||||
| 176 | Actin-54 (N. tabacum) Actin | ATP binding | Cattle (high) | 4602 [31] |
| 38 [32] | ||||
| 4 | 14-3-3 protein | Disease/defence | Cattle (high) | 1315 [31] |
| 23 | Enolase | Energy-Glycolysis | Cattle (high) | 4707 [31], |
| 66 [32] | ||||
| 169 | Enolase | Energy-Glycolysis | Cattle (high) | 64 [32], |
| 5711 [31] | ||||
| 2 | Putative nascent polypeptide associated complex a-chain/expressed protein | Protein destination and storage | Cattle (high) | 40 [32] |
| 55 | ATP binding/hydrolase/nucleosidetriphosphatase/nucleotide binding (A. thaliana); | Protein synthesis, storage and turnover | Cattle (high) | 3827 [14] |
| Protein of AAA family (C. annuum) | ||||
| 177 | Enolase | Energy-Glycolysis | Cattle (high), PCMorg | 14 [32] |
| 158 | Malate dehydrogenase, cytosolic; | Energy-Glycolysis | Cattle (low) | 7 [32] |
| Glyceraldehyde 3-phosphate dehydrogenase | ||||
| 98 | Enolase-like | Energy-Glycolysis | Cattle (low) | 4710 [31] |
| 165 | Enolase | Energy-Glycolysis | Cattle (low) | 20 [32] |
| 4710 [31] | ||||
| 60 | UTP-Glc-1-P uridylyltransferase | Metabolism | Cattle (low), PCMorg | 45 [32] |
| 6 | Kunitz-type enzyme inhibitor/S9C11 | Disease/defence | Chicken (high) | 17 [32], |
| 1201 [31] | ||||
| 156 | Ascorbate peroxidase | Disease/defence | Chicken (high) | 42 [32], |
| 6301 [31] | ||||
| 171 | Proteasome subunit | Disease/defence | Chicken (high) | 5209 [31] |
| 91 | Fructokinase | Metabolism | Chicken (high) | 31 [32], |
| 4423 [31] | ||||
| 147 | Chaperonin 21 precursor | Protein destination and storage | Chicken (high) | 76 [32] |
| 163 | Cys proteinase precursor | Protein destination and storage | Chicken (high) | 7205 [31] |
| 34 [32] | ||||
| 18 | EST (similar to small heat shock proteins) | Stress response | Chicken (high) | 4208 [31] |
| 152 | Pathogenesis related protein 10 | Stress response | Chicken (high) | 6101 [31] |
| 178 | Hsp20.1 protein (L. peruvianum) | Stress response | Chicken (high) | 4102 [32] |
| 17 | Ascorbate peroxidase | Disease/defence | Chicken (high), PCMcon | 6304 [31] |
| 21 | Ascorbate peroxidase | Disease/defence | Chicken (high), PCMcon | 5308 [31], |
| 67 [32] | ||||
| 39 | Triosephosphate isomerase, cytosolic isoform (S. chacoense) | Energy-Glycolysis | Chicken (high), PCMcon | 6206 [31] |
| 6 [32] | ||||
| 173 | Glyceraldehyde 3-phosphate dehydrogenase | Energy-Glycolysis | Chicken (high), PCMorg | 7506 [31] |
| 7511 [14] | ||||
| 43 | UTP-Glc-1-P uridylyltransferase | Metabolism | Chicken (high), PCMorg | 37 [32] |
| 64 | ATP synthase b-chain precursor, mitochondrial | ATP synthesis | Chicken (low) | 36 [32] |
| 76 | Patatin precursor | Protein destination and storage | Control | 2402 [14] |
| 149 | Patatin precursor | Protein destination and storage | Control | 2603 [31] |
| 170 | Patatin protein 03 | Protein destination and storage | Control | 4504 [31] |
| 29 [32] | ||||
| Patatin | ||||
| 45 | PRCI (N. tabacum) Proteasome subunit alpha type-6 | ubiquitin-dependent protein catabolic process | Control | 7209 [31] |
| 20 | EST (peptide sequences LGSHFVSENQDVSIK VAYSIVGPTHSPLR FSTSSSSTK YETGRPHSYK YETGRPHSYKLR IEKYETGRPHSYKLR) | Unclassified | Control | 60 [32] |
| 175 | Patatin precursor; | Disease/defence | Control | 2610 [31] |
| Patatin protein | Lipid degradation and metabolism, | |||
| 59 | Superoxide dismutase [Cu-Zn] | Disease/defence | Control, PCMorg | 5 [32] |
| 167 | Patatin | Protein destination and storage | Control, PCMorg | 26 [32] |
| Patatin protein 03 | 3506 [31] | |||
| 155 | Hsp19.9 protein (L. peruvianum) | Stress response | Control, PCMorg | 5108 [31] |
| B. Protein spots significantly affected by PCM (not fertilization) | ||||
| 225 | Kunitz-type enzyme inhibitor S0C11 | Disease/defence | PCMcon | 3 [32] |
| 164 | Glyceraldehyde 3-phosphate dehydrogenase | Energy-Glycolysis | PCMcon | 8403 [14] |
| 8508 [31] | ||||
| 1 [32] | ||||
| 276 | Phosphoglycerate mutase | Energy-Glycolysis | PCMorg | 4809 [31] |
| 146 | Phosphoglycerate mutase | Energy-Glycolysis | PCMorg | 4813 [31] |
| C. Protein spots significantly affected by fertilization × PCM interaction effect | ||||
| 1 | Transporter | Protein transport | PCMcon: cattle (high) | 3209 [31] |
| PCMorg: chicken (low) | ||||
| 24 | Putative proteasome 20S beta1 subunit (B. napus) | Proteolysis | PCMcon: cattle (high) | 7202 [31] |
| PCMorg: control | ||||
| 174 | Pathogenesis-related protein 10 (S. virginianum); | Disease/defence | PCMcon: control | 3107 [31] |
| PCMorg: chicken (high) | ||||
| 82 | Patatin; Phosphoenolpyruvate carboxykinase | Protein destination and storage; | PCMcon: control | 28 [32], |
| signal transduction | PCMorg: cattle (high) | 4501 [31] | ||
| 162 | Patatin; | Protein destination and storage; | PCMcon: control | 27 [32] |
| Phosphoenolpyruvate carboxykinase | Signal transduction | |||
| PCMorg: cattle (high) | 3509 [31] | |||
| Patatin protein 07 | Protein destination and storage | |||
| 114 | Aspartic proteinase inhibitor 11 (AllergenSola t 2) | Stress response | PCMcon: control | 4105 [31] |
| PCMorg: cattle (high) | ||||
| 129 | Pathogenesis related protein 10 | Stress response | PCMcon: control | 5103 [31] |
| Pathogenesis-related protein STH-2 | Disease/defence | PCMorg: cattle (high) | 2 [32] | |

4. Discussion
4.1. Effects of Previous Crop Management, Fertilization Regime, and Watering Supply on Potato Traits
4.2. Effects of Previous Crop Management and Fertilization Regime on the Potato Tuber Proteome
Acknowledgments
Supplementary Files
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Flynn, H.C.; Smith, P. Greenhouse Gas Budgets of Crop Production—Current and Likely Future Trends; International Fertilizer Industry Association: Paris, France, 2010. [Google Scholar]
- Robertson, G.P.; Vitousek, P.M. Nitrogen in agriculture: Balancing the cost of an essential resource. Ann. Rev. Environ. Resour. 2009, 34, 97–125. [Google Scholar] [CrossRef]
- Cordell, D.; Dangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Fantel, R.J.; Peterson, G.R.; Stowasser, W.F. The worldwide availability of phosphate rock. Nat. Resour. Forum 1985, 9, 5–24. [Google Scholar] [CrossRef]
- Trehan, S.P.; Sharma, R.C. Differences in phosphorus use efficiency in potato genotypes. Adv. Hortic. Sci. 2005, 19, 13–20. [Google Scholar]
- Hepperly, P.; Lotter, D.; Ziegler, C.; Seidel, R.; Reider, C. Compost, manure and synthetic fertilizer influences crop yields, soil properties, nitrate leaching and crop nutrient content. Compost Sci. Util. 2009, 17, 117–126. [Google Scholar]
- Herencia, J.F.; Ruiz-Porras, J.C.; Melero, S.; Garcia-Galavis, P.A.; Morillo, E.; Maqueda, C. Comparison between organic and mineral fertilization for soil fertility levels, crop macronutrient concentrations, and yield. Agron. J. 2007, 99, 973–983. [Google Scholar] [CrossRef]
- Warman, P.R.; Burnham, J.C.; Eaton, L.J. Effects of repeated applications of municipal solid waste compost and fertilizers to three lowbush blueberry fields. Sci. Hortic. 2009, 122, 393–398. [Google Scholar]
- Eyre, M.D.; Sanderson, R.A.; Shotton, P.N.; Leifert, C. Investigating the effects of crop type, fertility management and crop protection on the activity of beneficial invertebrates in an extensive farm management comparison trial. Ann. Appl. Biol. 2009, 155, 267–276. [Google Scholar] [CrossRef]
- Bulluck, L.R.; Brosius, M.; Evanylo, G.K.; Ristaino, J.B. Organic and synthetic fertility amendments influence soil microbial, physical and chemical properties on organic and conventional farms. Appl. Soil Ecol. 2002, 19, 147–160. [Google Scholar] [CrossRef]
- The Council Of The European Communities. Council Directive 91/676/EEC of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. Eur. Union 1991, 91/676/EEC, 1–8.
- Van Dijk, J.P.; Cankar, K.; Hendriksen, P.J.M.; Beenen, H.G.; Zhu, M.; Scheffer, S.; Shepherd, L.V.T.; Stewart, D.; Davies, H.V.; Leifert, C.; Wilkockson, S.J.; Gruden, K.; Kok, E.J. The assessment of differences in the transcriptomes of organically and conventionally grown potato tubers. J. Agric. Food Chem. 2012, 60, 2090–2101. [Google Scholar]
- Lehesranta, S.J.; Koistinen, K.M.; Massat, N.; Davies, H.V.; Shepherd, L.V.T.; McNicol, J.W.; Cakmak, I.; Cooper, J.; Luck, L.; Karenlampi, S.O.; Leifert, C. Effects of agricultural production systems and their components on protein profiles of potato tubers. Proteomics 2007, 7, 597–604. [Google Scholar] [CrossRef]
- Mäder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil fertility and biodiversity in organic farming. Science 2002, 296, 1694–1697. [Google Scholar]
- Chang, E.H.; Chung, R.S.; Wang, F.N. Effect of different types of organic fertilizers on the chemical properties and enzymatic activities of an Oxisol under intensive cultivation of vegetables for 4 years. Soil Sci. Plant Nutr. 2008, 54, 587–599. [Google Scholar] [CrossRef]
- Ceylan, S.; Mordogan, N.; Akdemir, H.; Cakici, H. Effect of organic fertilizers on some agronomic and chemical properties of potato (Solanum tuberosum L.). Asian J. Chem. 2006, 18, 1223–1230. [Google Scholar]
- Gunapala, N.; Scow, K.M. Dynamics of soil microbial biomass and activity in conventional and organic farming systems. Soil Biol. Biochem. 1998, 30, 805–816. [Google Scholar] [CrossRef]
- Shannon, D.; Sen, A.M.; Johnson, D.B. A comparative study of the microbiology of soils managed under organic and conventional regimes. Soil Use Manag. 2002, 18, 274–283. [Google Scholar] [CrossRef]
- Rempelos, L.; Cooper, J.; Wilcockson, S.; Eyre, M.; Shotton, P.; Volakakis, N.; Orr, C.H.; Leifert, C.; Gatehouse, A.M.R.; Tétard-Jones, C. Quantitative proteomics to study the response of potato to contrasting fertilisation regimes. Mol. Breed. 2012, in press.. [Google Scholar]
- Tétard-Jones, C.; Shotton, P.N.; Rempelos, L.; Cooper, J.; Eyre, M.; Orr, C.H.; Leifert, C.; Gatehouse, A.M.R. Quantitative proteomics to study the response of wheat to contrasting fertilisation regimes. Mol. Breed. 2012, in press.. [Google Scholar]
- Varshney, R.K.; Graner, A.; Sorrells, M.E. Genomics-assisted breeding for crop improvement. Trends Plant Sci. 2005, 10, 621–630. [Google Scholar]
- Hack, H.; Gall, H.; Klemke, T.H.; Klose, R.; Meier, U.; Stauss, R.; Witzen-Berger, A. Phänologische Entwicklungsstadien der Kartoffel (Solanum tuberosum L). Codirung und Beschreibung nach der erweiterten BBCH-Skala mit Abbildungen. Nachrichtenbl. Deut. Pflanzenschutzd. 1993, 45, 11–19. [Google Scholar]
- Hurkman, W.J.; Tanaka, C.K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986, 81, 802–806. [Google Scholar] [CrossRef]
- Healthcare, G.E. 2-D Electrophoresis Principles and Methods; General Electric Company: Uppsala, Sweden, 2010. [Google Scholar]
- Pinheiro, J.C.; Bates, D.M. Mixed-Effects Models in S and S-PLUS; Springer: New York, NY, USA, 2000. [Google Scholar]
- Crawley, M.J. The R Book; John Wiley and Son: Chichester, UK, 2007. [Google Scholar]
- Team, R.D.C. R: A language and environment for statistical computing. R Foundation for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2009. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination, 4th ed; Centre for Biometry: Wageningen, The Netherlands, 1998. [Google Scholar]
- Chibani, K.; Ali-Rachedi, S.; Job, C.; Job, D.; Jullien, M.; Grappin, P. Proteomic analysis of seed dormancy in arabidopsis. Plant Physiol. 2006, 142, 1493–1510. [Google Scholar] [CrossRef]
- Lehesranta, S.J.; Davies, H.V.; Shepherd, L.V.T.; Koistinen, K.M.; Massat, N.; Nunan, N.; McNicol, J.W.; Kärenlampi, S.O. Proteomic analysis of the potato tuber life cycle. Proteomics 2006, 6, 6042–6052. [Google Scholar]
- Lehesranta, S.J.; Davies, H.V.; Shepherd, L.V.T.; Nunan, N.; McNicol, J.W.; Auriola, S.; Koistinen, K.M.; Suomalainen, S.; Kokko, H.I.; Kärenlampi, S.O. Comparison of tuber proteomes of potato varieties, landraces, and genetically modified lines. Plant Physiol. 2005, 138, 1690–1699. [Google Scholar] [CrossRef]
- Van Dijk, J.P.; Cankar, K.; Scheffer, S.J.; Beenen, H.G.; Shepherd, L.V.T.; Stewart, D.; Davies, H.V.; Wilkockson, S.J.; Lelfert, C.; Gruden, K.; Kok, E.J. Transcriptome analysis of potato tubers-effects of different agricultural practices. J. Agric. Food Chem. 2009, 57, 1612–1623. [Google Scholar]
- Olesinski, A.A.; Wolf, S.; Rudich, J.; Marani, A. The effect of nitrogen fertilization and irrigation frequency on photosynthesis of potatoes (Solarium tuberosum). Ann. Bot. 1989, 64, 651–657. [Google Scholar]
- Griffiths, B.S.; Ritz, K.; Wheatley, R.E. Nematodes as indicators of enhanced microbiological activity in a Scottish organic farming system. Soil Use Manag. 1994, 10, 20–24. [Google Scholar] [CrossRef]
- Vos, J.; Groenwold, J. Water relations of potato leaves, I. Diurnal changes, gradients in the canopy, and effects of leaf-insertion number, cultivar and drought. Ann. Bot. 1988, 62, 363–371. [Google Scholar]
- Songsri, P.; Jogloy, S.; Holbrook, C.C.; Kesmala, T.; Vorasoot, N.; Akkasaeng, C.; Patanothai, A. Association of root, specific leaf area and SPAD chlorophyll meter reading to water use efficiency of peanut under different available soil water. Agric. Water Manag. 2009, 96, 790–798. [Google Scholar] [CrossRef]
- Barraclough, P.B.; Kyte, J. Effect of water stress on chlorophyll meter readings in winter wheat. In Plant Nutrition—Food Security and Sustainability of Agro-ecosystems; Horst, W.J., Schenk, M.K., Burkert, A., Claassen, N., Flessa, H., Frommer, W.B., Goldbach, H., Olfs, H.W., Romheld, V., Sattelmacher, B., Schmidhalter, U., Schubert, S., Wiren, N., Wittenmayer, L., Eds.; Springer: Dordrecht, The Netherlands, 2001; Volume 92, pp. 722–723, Developments in Plant and Soil Sciences. [Google Scholar]
- Nawrocki, A.; Thorup-Kristensen, K.; Jensen, O.N. Quantitative proteomics by 2DE and MALDI MS/MS uncover the effects of organic and conventional cropping methods on vegetable products. J. Proteomics 2011, 74, 2810–2825. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tétard-Jones, C.; Edwards, M.G.; Rempelos, L.; Gatehouse, A.M.R.; Eyre, M.; Wilcockson, S.J.; Leifert, C. Effects of Previous Crop Management, Fertilization Regime and Water Supply on Potato Tuber Proteome and Yield. Agronomy 2013, 3, 59-85. https://doi.org/10.3390/agronomy3010059
Tétard-Jones C, Edwards MG, Rempelos L, Gatehouse AMR, Eyre M, Wilcockson SJ, Leifert C. Effects of Previous Crop Management, Fertilization Regime and Water Supply on Potato Tuber Proteome and Yield. Agronomy. 2013; 3(1):59-85. https://doi.org/10.3390/agronomy3010059
Chicago/Turabian StyleTétard-Jones, Catherine, Martin G. Edwards, Leonidas Rempelos, Angharad M.R. Gatehouse, Mick Eyre, Stephen J. Wilcockson, and Carlo Leifert. 2013. "Effects of Previous Crop Management, Fertilization Regime and Water Supply on Potato Tuber Proteome and Yield" Agronomy 3, no. 1: 59-85. https://doi.org/10.3390/agronomy3010059
APA StyleTétard-Jones, C., Edwards, M. G., Rempelos, L., Gatehouse, A. M. R., Eyre, M., Wilcockson, S. J., & Leifert, C. (2013). Effects of Previous Crop Management, Fertilization Regime and Water Supply on Potato Tuber Proteome and Yield. Agronomy, 3(1), 59-85. https://doi.org/10.3390/agronomy3010059