Long-Term Effects of Biochar-Based Organic Amendments on Soil Microbial Parameters
Abstract
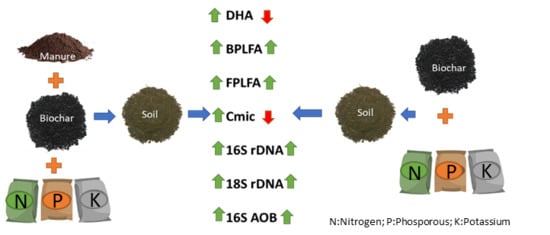
:1. Introduction
2. Materials and Methods
2.1. Study Site and Rationale behind the Field Scale Experiment
2.2. Soil Sampling and Preparation
2.3. Dehydrogenase Activities
2.4. Quantification of Microbial Biomass
2.5. Microbial Biomass Carbon
2.6. DNA Extraction and Real-Time qPCR
2.7. Statistics Analysis
3. Results and Discussion
3.1. Dehydrogenase
3.2. Soil Phospholipid Fatty Acid Analysis
3.3. Microbial Biomass Carbon
3.4. DNA Extraction and Real-time qPCR
3.4.1. 18S rDNA
3.4.2. 16S rDNA
3.4.3. 16S rDNA (AOB)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Singh, B.; Ryan, J. Managing fertilizers to enhance soil health. Int. Fertil. Ind. Assoc. Paris Fr. 2015, 1–24. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. In Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2011; Volume 2, pp. 761–786. [Google Scholar]
- Zimmer, D.; Kruse, J.; Siebers, N.; Panten, K.; Oelschläger, C.; Warkentin, M.; Hu, Y.; Zuin, L.; Leinweber, P. Bone char vs. S-enriched bone char: Multi-method characterization of bone chars and their transformation in soil. Sci. Total Environ. 2018, 643, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Lee, X.; Tang, Y.; Zhang, Q. Long-term effects of biochar amendment on rhizosphere and bulk soil microbial communities in a karst region, southwest China. Appl. Soil Ecol. 2019, 140, 126–134. [Google Scholar] [CrossRef]
- Liu, X.; Ye, Y.; Liu, Y.; Zhang, A.; Zhang, X.; Li, L.; Pan, G.; Kibue, G.W.; Zheng, J.; Zheng, J. Sustainable biochar effects for low carbon crop production: A 5-crop season field experiment on a low fertility soil from Central China. Agric. Syst. 2014, 129, 22–29. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Chi, Z.; Zheng, J.; Li, L.; Zhang, X.; Zheng, J.; Cheng, K.; Bian, R.; Pan, G. Biochar provided limited benefits for rice yield and greenhouse gas mitigation six years following an amendment in a fertile rice paddy. Catena 2019, 179, 20–28. [Google Scholar] [CrossRef]
- Rasmussen, P.E.; Goulding, K.W.; Brown, J.R.; Grace, P.R.; Janzen, H.H.; Körschens, M. Long-term agroecosystem experiments: Assessing agricultural sustainability and global change. Science 1998, 282, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Patra, A.K.; Singh, D.; Swarup, A.; Masto, R.E. Effect of long-term application of manure and fertilizer on biological and biochemical activities in soil during crop development stages. Bioresour. Technol. 2007, 98, 3585–3592. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.D.; Mullen, M.D.; Ringelberg, D.B.; Tyler, D.D.; Hedrick, D.B.; Gale, P.M.; White, D.C. Soil microbial community responses to dairy manure or ammonium nitrate applications. Soil Biol. Biochem. 2001, 33, 1011–1019. [Google Scholar] [CrossRef]
- Sumner, M.E. Handbook of Soil Science; CRC Press: Boca Raton, FL, USA, 2000; ISBN 0-8493-3136-6. [Google Scholar]
- Van-camp, L.; Bujarrabal, B.; Gentile, A.R.; Jones, R.J.; Montanarella, L.; Olazabal, C.; Selvaradjou, S. Technical Working Groups Established under the Thematic Strategy for Soil Protection; Office for Official Publications of the European Communities: Brussels, Belgium, 2004. [Google Scholar]
- Shindo, H.; Hirahara, O.; Yoshida, M.; Yamamoto, A. Effect of continuous compost application on humus composition and nitrogen fertility of soils in a field subjected to double cropping. Biol. Fertil. Soils 2006, 42, 437–442. [Google Scholar] [CrossRef]
- Nardi, S.; Morari, F.; Berti, A.; Tosoni, M.; Giardini, L. Soil organic matter properties after 40 years of different use of organic and mineral fertilisers. Eur. J. Agron. 2004, 21, 357–367. [Google Scholar] [CrossRef]
- Ros, M.; Klammer, S.; Knapp, B.; Aichberger, K.; Insam, H. Long-term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag. 2006, 22, 209–218. [Google Scholar] [CrossRef]
- Poulain, S.; Sergeant, C.; Simonoff, M.; Le Marrec, C.; Altmann, S. Microbial Investigations in Opalinus Clay, an Argillaceous Formation under Evaluation as a Potential Host Rock for a Radioactive Waste Repository. Geomicrobiol. J. 2008, 25, 240–249. [Google Scholar] [CrossRef]
- Lehmann, J.; da Silva, J.P., Jr.; Rondon, M.; Cravo, M.; da Silva, G.M.; Greenwood, J.; Nehls, T.; Steiner, C.; Glaser, B. Slash-and-char-a feasible alternative for soil fertility management in the central Amazon. In Proceedings of the 17th World Congress of Soil Science, Bangkok, Thailand, 14–21 August 2002; pp. 1–12. [Google Scholar]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar application to soil: Agronomic and environmental benefits and unintended consequences. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2011; Volume 112, pp. 103–143. ISBN 0065-2113. [Google Scholar]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; DeLuca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Lehmann, J. Biochar for Environmental Management; Routledge: Abingdon, UK, 2015. [Google Scholar]
- Azeem, M.; Hayat, R.; Hussain, Q.; Ahmed, M.; Imran, M.; Crowley, D.E. Effect of biochar amendment on soil microbial biomass, abundance and enzyme activity in the mash bean field. J. Biodivers. Environ. Sci. 2016, 8, 2222–3045. [Google Scholar]
- Clough, T.; Condron, L.; Kammann, C.; Müller, C. A review of biochar and soil nitrogen dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Powell, S.J.; Prosser, J.I. Protection of Nitrosomonas europaea colonizing clay minerals from inhibition by nitrapyrin. J. Gen. Microbiol. 1991, 137, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Thies, J.E.; Rillig, M.C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management; Routledge: Abingdon, UK, 2012; pp. 117–138. [Google Scholar]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, climate change and soil: A review to guide future research. CSIRO Land Water Sci. Rep. 2009, 5, 17–31. [Google Scholar]
- Sánchez-Monedero, M.A.; Cayuela, M.L.; Sánchez-García, M.; Vandecasteele, B.; D’Hose, T.; López, G.; Martínez-Gaitán, C.; Kuikman, P.J.; Sinicco, T.; Mondini, C. Agronomic Evaluation of Biochar, Compost and Biochar-Blended Compost across Different Cropping Systems: Perspective from the European Project FERTIPLUS. Agronomy 2019, 9, 225. [Google Scholar] [CrossRef]
- Plaza, C.; Giannetta, B.; Fernández, J.M.; López-de-Sá, E.G.; Polo, A.; Gascó, G.; Méndez, A.; Zaccone, C. Response of different soil organic matter pools to biochar and organic fertilizers. Agric. Ecosyst. Environ. 2016, 225, 150–159. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, R.; Nielsen, S.; Joseph, S.D.; Huang, D.; Thomas, T. A Combination of Biochar–Mineral Complexes and Compost Improves Soil Bacterial Processes, Soil Quality, and Plant Properties. Front. Microbiol. 2016, 7, 372. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Joseph, S.; Schmidt, H.-P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef] [PubMed]
- Seehausen, M.; Gale, N.; Dranga, S.; Hudson, V.; Liu, N.; Michener, J.; Thurston, E.; Williams, C.; Smith, S.; Thomas, S. Is There a Positive Synergistic Effect of Biochar and Compost Soil Amendments on Plant Growth and Physiological Performance? Agronomy 2017, 7, 13. [Google Scholar] [CrossRef]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Cely, P.; Tarquis, A.M.; Paz-Ferreiro, J.; Méndez, A.; Gascó, G. Factors driving the carbon mineralization priming effect in a sandy loam soil amended with different types of biochar. Solid Earth 2014, 5, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, S.; Batool, A.; Ghufran, M.A.; Khalid, A. Effect of dairy manure derived biochar on microbial biomass carbon, soil carbon and Vitis vinifera under water stress conditions. Pak. J. Bot 2018, 50, 1713–1718. [Google Scholar]
- Nguyen, B.T.; Trinh, N.N.; Le, C.M.T.; Nguyen, T.T.; Tran, T.V.; Thai, B.V.; Le, T.V. The interactive effects of biochar and cow manure on rice growth and selected properties of salt-affected soil. Arch. Agron. Soil Sci. 2018, 64, 1744–1758. [Google Scholar] [CrossRef]
- Dodor, D.E.; Amanor, Y.J.; Attor, F.T.; Adjadeh, T.A.; Neina, D.; Miyittah, M. Co-application of biochar and cattle manure counteract positive priming of carbon mineralization in a sandy soil. Environ. Syst. Res. 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Chen, L.; Liu, Z.; Jin, Y.; He, M.; Zhao, Z.; Liu, C.; Niyungeko, C.; Arai, Y. Composition of microbial community in pig manure biochar-amended soils and the linkage to the heavy metals accumulation in rice at harvest. Land Degrad. Dev. 2018, 29, 2189–2198. [Google Scholar] [CrossRef]
- Bamminger, C.; Poll, C.; Sixt, C.; Högy, P.; Wüst, D.; Kandeler, E.; Marhan, S. Short-term response of soil microorganisms to biochar addition in a temperate agroecosystem under soil warming. Agric. Ecosyst. Environ. 2016, 233, 308–317. [Google Scholar] [CrossRef]
- Hüppi, R.; Neftel, A.; Lehmann, M.F.; Krauss, M.; Six, J.; Leifeld, J. N use efficiencies and N2O emissions in two contrasting, biochar amended soils under winter wheat—Cover crop—Sorghum Rotation. Environ. Res. Lett. 2016, 11, 084013. [Google Scholar] [CrossRef]
- Horák, J.; Kondrlová, E.; Igaz, D.; Šimanský, V.; Felber, R.; Lukac, M.; Balashov, E.V.; Buchkina, N.P.; Rizhiya, E.Y.; Jankowski, M. Biochar and biochar with N-fertilizer affect soil N2O emission in Haplic Luvisol. Biologia (Bratisl.) 2017, 72. [Google Scholar] [CrossRef]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Coal fly ash and farmyard manure amendments in dry-land paddy agriculture field: Effect on N-dynamics and paddy productivity. Appl. Soil Ecol. 2011, 47, 133–140. [Google Scholar] [CrossRef]
- Pereira, R.C.; Kaal, J.; Arbestain, M.C.; Lorenzo, R.P.; Aitkenhead, W.; Hedley, M.; Macias, F.; Hindmarsh, J.; Macia-Agullo, J.A. Contribution to characterisation of biochar to estimate the labile fraction of carbon. Org. Geochem. 2011, 42, 1331–1342. [Google Scholar] [CrossRef]
- Datta, R.; Vranová, V.; Pavelka, M.; Rejšek, K.; Formánek, P. Effect of soil sieving on respiration induced by low-molecular-weight substrates. Int. Agrophysics 2014, 28, 119–124. [Google Scholar] [CrossRef] [Green Version]
- Tabatabai, M.A. Soil enzymes. In Methods Soil Anal. Part. 2—Microbiological Biochem. Prop; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Oravecz, O.; Elhottová, D.; Krištůfek, V.; Šustr, V.; Frouz, J.; Tříska, J.; Marialigeti, K. Application of ARDRA and PLFA analysis in characterizing the bacterial communities of the food, gut and excrement of saprophagous larvae ofPenthetria holosericea (Diptera: Bibionidae): A pilot study. Folia Microbiol. (Praha) 2004, 49, 83. [Google Scholar] [CrossRef] [PubMed]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Vainio, E.J.; Hantula, J. Direct analysis of wood-inhabiting fungi using denaturing gradient gel electrophoresis of amplified ribosomal DNA. Mycol. Res. 2000, 104, 927–936. [Google Scholar] [CrossRef]
- Hermansson, A.; Lindgren, P.-E. Quantification of ammonia-oxidizing bacteria in arable soil by real-time PCR. Appl. Env. Microbiol 2001, 67, 972–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balba, M.T.; Al-Awadhi, N.; Al-Daher, R. Bioremediation of oil-contaminated soil: Microbiological methods for feasibility assessment and field evaluation. J. Microbiol. Method. 1998, 32, 155–164. [Google Scholar] [CrossRef]
- Ukaoma, A.A.; Ukaoma, V.O.; Opara, F.N.; Osuala, F.O.U. Inhibition of Dehydrogenase Activity in Pathogenic Bacteria Isolates by Aqueous Extract of Curcuma Longa (Turmeric) Rhizome. J. Phytopharma. 2013, 2, 9–17. [Google Scholar]
- Walls-Thumma, D. Dehydrogenase Activity in Soil Bacteria. Dehydrogenase Act. Soil Bact. Gard. Pub 2000, 130633. [Google Scholar]
- Kumar, S.; Masto, R.E.; Ram, L.C.; Sarkar, P.; George, J.; Selvi, V.A. Biochar preparation from Parthenium hysterophorus and its potential use in soil application. Ecol. Eng. 2013, 55, 67–72. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant. Soil 2011, 348, 439. [Google Scholar] [CrossRef]
- Wu, F.; Jia, Z.; Wang, S.; Chang, S.X.; Startsev, A. Contrasting effects of wheat straw and its biochar on greenhouse gas emissions and enzyme activities in a Chernozemic soil. Biol. Fertil. Soils 2013, 49, 555–565. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Gutiérrez, B.; Méndez, A. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Case, S.D.C.; Alberti, G.; McNamara, N.P.; Zavalloni, C.; Vervisch, B.; delle Vedove, G.; De Neve, S. C mineralization and microbial activity in four biochar field experiments several years after incorporation. Soil Biol. Biochem. 2014, 78, 195–203. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton Jr, H. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Lammirato, C.; Miltner, A.; Kaestner, M. Effects of wood char and activated carbon on the hydrolysis of cellobiose by β-glucosidase from Aspergillus niger. Soil Biol. Biochem. 2011, 43, 1936–1942. [Google Scholar] [CrossRef]
- Czimczik, C.I.; Masiello, C.A. Controls on black carbon storage in soils. Glob. Biogeochem. Cycles 2007, 21. [Google Scholar] [CrossRef]
- Ameloot, N.; De Neve, S.; Jegajeevagan, K.; Yildiz, G.; Buchan, D.; Funkuin, Y.N.; Prins, W.; Bouckaert, L.; Sleutel, S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013, 57, 401–410. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, B.; Zhang, M.; Inyang, M.; Zimmerman, A.R. Effect of biochar amendment on sorption and leaching of nitrate, ammonium, and phosphate in a sandy soil. Chemosphere 2012, 89, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, N.; Kammann, C.I.; Schmidt, H.-P.; Kappler, A.; Behrens, S. Nitrate capture and slow release in biochar amended compost and soil. PLoS ONE 2017, 12, e0171214. [Google Scholar] [CrossRef] [PubMed]
- Kammann, C.I.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S. Erratum: Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 12378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Huang, C.; Xie, H.; Bi, Y. Study on the release property of nitrogen in sustained-release fertilizer with carrier of bentonite. In Proceedings of the 2013 Third International Conference on Intelligent System Design and Engineering Applications, Hong Kong, China, 16–18 January 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 1364–1367. [Google Scholar]
- Lu, Q.; Feng, X.; Sun, K.; Liao, Z. Study on the use of polymer/bentonite composites for controlled release. Plant. Nutr. Fertil. Sci. 2005, 2. [Google Scholar]
- Yuan, H.; Lu, T.; Wang, Y.; Chen, Y.; Lei, T. Sewage sludge biochar: Nutrient composition and its effect on the leaching of soil nutrients. Geoderma 2016, 267, 17–23. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’neill, B.; Skjemstad, J.O.; Thies, J.; Luizao, F.J.; Petersen, J. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef] [Green Version]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant. Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Elliott, G.N.; Graham, K.J.; Scow, K.M. Comparison of phospholipid fatty acid (PLFA) and total soil fatty acid methyl esters (TSFAME) for characterizing soil microbial communities. Soil Biol. Biochem. 2004, 36, 1793–1800. [Google Scholar] [CrossRef]
- Skjemstad, J.O.; Reicosky, D.C.; Wilts, A.R.; McGowan, J.A. Charcoal carbon in US agricultural soils. Soil Sci. Soc. Am. J. 2002, 66, 1249–1255. [Google Scholar] [CrossRef] [Green Version]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar-root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Bruun, E.W.; Hauggaard-Nielsen, H.; Ibrahim, N.; Egsgaard, H.; Ambus, P.; Jensen, P.A.; Dam-Johansen, K. Influence of fast pyrolysis temperature on biochar labile fraction and short-term carbon loss in a loamy soil. Biomass Bioenergy 2011, 35, 1182–1189. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Bogomolova, I.; Glaser, B. Biochar stability in soil: Decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil Biol. Biochem. 2014, 70, 229–236. [Google Scholar] [CrossRef]
- Hamer, U.; Marschner, B.; Brodowski, S.; Amelung, W. Interactive priming of black carbon and glucose mineralisation. Org. Geochem. 2004, 35, 823–830. [Google Scholar] [CrossRef]
- Hilscher, A.; Heister, K.; Siewert, C.; Knicker, H. Mineralisation and structural changes during the initial phase of microbial degradation of pyrogenic plant residues in soil. Org. Geochem. 2009, 40, 332–342. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Ascough, P.L.; Sturrock, C.J.; Bird, M.I. Investigation of growth responses in saprophytic fungi to charred biomass. Isotopes Environ. Health Stud. 2010, 46, 64–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, E.C.; Balogh-Brunstad, Z.; Jakobsen, I.; Olsson, P.A.; Stipp, S.L.S.; Rillig, M.C. A mycorrhizal fungus grows on biochar and captures phosphorus from its surfaces. Soil Biol. Biochem. 2014, 77, 252–260. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota–a review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S. Different approaches to evaluating soil quality using biochemical properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Harris, J.A. Measurements of the soil microbial community for estimating the success of restoration. Eur. J. Soil Sci. 2003, 54, 801–808. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.; Gleeson, D.; Solaiman, Z.M.; Jones, D.; Murphy, D. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant. Soil 2012, 354, 311–324. [Google Scholar] [CrossRef]
- Li, Q.; Lei, Z.; Song, X.; Zhang, Z.; Ying, Y.; Peng, C. Biochar amendment decreases soil microbial biomass and increases bacterial diversity in Moso bamboo (Phyllostachys edulis) plantations under simulated nitrogen deposition. Environ. Res. Lett. 2018, 13, 044029. [Google Scholar] [CrossRef]
- Zhang, H.; Voroney, R.P.; Price, G.W. Effects of Biochar Amendments on Soil Microbial Biomass and Activity. J. Environ. Qual. 2014, 43, 2104. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Gu, J.-D. Alteration of extracellular enzyme activity and microbial abundance by biochar addition: Implication for carbon sequestration in subtropical mangrove sediment. J. Environ. Manag. 2016, 182, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, S.; Liang, C.; Xu, Q.; Li, Y.; Qin, H.; Fuhrmann, J.J. Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: Effect of particle size and addition rate. Sci. Total Environ. 2017, 574, 24–33. [Google Scholar] [CrossRef] [PubMed]
- De Tender, C.A.; Debode, J.; Vandecasteele, B.; D’Hose, T.; Cremelie, P.; Haegeman, A.; Ruttink, T.; Dawyndt, P.; Maes, M. Biological, physicochemical and plant health responses in lettuce and strawberry in soil or peat amended with biochar. Appl. Soil Ecol. 2016, 107, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhai, L.; CaiJi, Z.; Liu, J.; Wang, H.; Ren, T.; Gai, X.; Xi, B.; Liu, H. Short-term effects of maize residue biochar on phosphorus availability in two soils with different phosphorus sorption capacities. Biol. Fertil. Soils 2014, 51, 113–122. [Google Scholar] [CrossRef]
- Ogawa, M.; Yambe, Y. Effects of charcoal on VA mycorrhiza and root nodule formations of soybean: Studies on nodule formation and nitrogen fixation in legume crops. Bull. Green Energy Program. Group II 1986, 8, 108–134. [Google Scholar]
- Saito, M. Charcoal as a micro-habitat for VA mycorrhizal fungi, and its practical implication. Agric. Ecosyst. Environ. 1990, 29, 341–344. [Google Scholar] [CrossRef]
- Gaur, A.; Adholeya, A. Effects of the particle size of soil-less substrates upon AM fungus inoculum production. Mycorrhiza 2000, 10, 43–48. [Google Scholar] [CrossRef]
- Ezawa, T.; Yamamoto, K.; Yoshida, S. Enhancement of the effectiveness of indigenous arbuscular mycorrhizal fungi by inorganic soil amendments. Soil Sci. Plant. Nutr. 2002, 48, 897–900. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Microscopy Observations of Habitable Space in Biochar for Colonization by Fungal Hyphae From Soil. J. Integr. Agric. 2014, 13, 483–490. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Kiikkilä, O.; Fritze, H. Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar] [CrossRef]
- Saito, M.; Marumoto, T. Inoculation with arbuscular mycorrhizal fungi: The status quo in Japan and the future prospects. In Diversity and Integration in Mycorrhizas; Springer: Berlin/Heidelberg, Germany, 2002; pp. 273–279. [Google Scholar]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil–concepts and mechanisms. Plant. Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lashari, M.S.; Liu, X.; Ji, H.; Li, L.; Zheng, J.; Kibue, G.W.; Joseph, S.; Pan, G. Changes in soil microbial community structure and enzyme activity with amendment of biochar-manure compost and pyroligneous solution in a saline soil from Central China. Eur. J. Soil Biol. 2015, 70, 67–76. [Google Scholar] [CrossRef]
- Elbl, J.; Záhora, J. The comparison of microbial activity in rhizosphere and non-rhizosphere soil stressed by drought. Proc. Mendel Net 2014, 234–240. [Google Scholar]
- Elbl, J.; Vaverková, M.; Adamcová, D.; Plošek, L.; Kintl, A.; LošÁk, T.; Hynšt, J.; Kotovicová, J. Influence of fertilization on microbial activities, soil hydrophobicity and mineral nitrogen leaching. Ecol. Chem. Eng. S 2014, 21, 661–675. [Google Scholar] [CrossRef] [Green Version]
- Mia, S.; Van Groenigen, J.W.; Van de Voorde, T.F.J.; Oram, N.J.; Bezemer, T.M.; Mommer, L.; Jeffery, S. Biochar application rate affects biological nitrogen fixation in red clover conditional on potassium availability. Agric. Ecosyst. Environ. 2014, 191, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zheng, H.; Luo, Y.; Deng, X.; Herbert, S.; Xing, B. Characterization and influence of biochars on nitrous oxide emission from agricultural soil. Environ. Pollut. 2013, 174, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-J.; Wang, X.-H.; Li, H.; Yao, H.-Y.; Su, J.-Q.; Zhu, Y.-G. Biochar impacts soil microbial community composition and nitrogen cycling in an acidic soil planted with rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef] [PubMed]
- Clough, T.J.; Bertram, J.E.; Ray, J.L.; Condron, L.M.; O’Callaghan, M.; Sherlock, R.R.; Wells, N.S. Unweathered wood biochar impact on nitrous oxide emissions from a bovine-urine-amended pasture soil. Soil Sci. Soc. Am. J. 2010, 74, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zong, H.; Zheng, H.; Liu, G.; Chen, L.; Xing, B. Reduced nitrification and abundance of ammonia-oxidizing bacteria in acidic soil amended with biochar. Chemosphere 2015, 138, 576–583. [Google Scholar] [CrossRef] [PubMed]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brtnicky, M.; Dokulilova, T.; Holatko, J.; Pecina, V.; Kintl, A.; Latal, O.; Vyhnanek, T.; Prichystalova, J.; Datta, R. Long-Term Effects of Biochar-Based Organic Amendments on Soil Microbial Parameters. Agronomy 2019, 9, 747. https://doi.org/10.3390/agronomy9110747
Brtnicky M, Dokulilova T, Holatko J, Pecina V, Kintl A, Latal O, Vyhnanek T, Prichystalova J, Datta R. Long-Term Effects of Biochar-Based Organic Amendments on Soil Microbial Parameters. Agronomy. 2019; 9(11):747. https://doi.org/10.3390/agronomy9110747
Chicago/Turabian StyleBrtnicky, Martin, Tereza Dokulilova, Jiri Holatko, Vaclav Pecina, Antonin Kintl, Oldrich Latal, Tomas Vyhnanek, Jitka Prichystalova, and Rahul Datta. 2019. "Long-Term Effects of Biochar-Based Organic Amendments on Soil Microbial Parameters" Agronomy 9, no. 11: 747. https://doi.org/10.3390/agronomy9110747
APA StyleBrtnicky, M., Dokulilova, T., Holatko, J., Pecina, V., Kintl, A., Latal, O., Vyhnanek, T., Prichystalova, J., & Datta, R. (2019). Long-Term Effects of Biochar-Based Organic Amendments on Soil Microbial Parameters. Agronomy, 9(11), 747. https://doi.org/10.3390/agronomy9110747