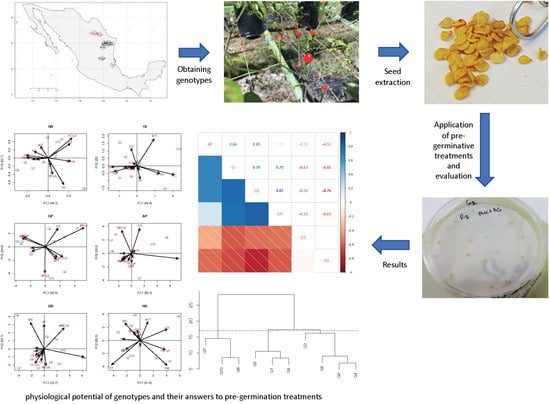
Seed Physiological Potential of Capsicum annuum var. glabriusculum Genotypes and Their Answers to Pre-Germination Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location of the Experimental Site
2.2. Plant Material
2.3. Treatment of Seeds
2.4. Assessed Seed Germination Traits (Variables)
2.5. Statistical Analysis
3. Results
3.1. Effects of Pre-Germination Treatments
3.2. Physiological Response of Genotypes
3.3. Specific Response of Treatment by Genotype
3.4. Association between Physiological Variables
3.5. Similarity of Genotypes
4. Discussion
4.1. Treatment Effect on Seed Physiology
4.2. Variability among Genotypes
4.3. Interaction between Genotypes and Treatments
4.4. Relationship among Physiological Variables Related to Germination
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Medina-Martínez, T.; Villalón-Mendoza, H.; Pérez-Hernández, J.M.; Sánchez-Ramos, G.; Salinas-Hernández, S. Avances y perspectivas de investigación del chile piquín en Tamaulipas, México. CienciaUAT 2010, 4, 16–21. [Google Scholar]
- González-Jara, P.; Moreno-Letelier, A.; Fraile, A.; Piñero, D.; García-Arenal, F. Impact of human management on the genetic variation of wild pepper, Capsicum annuum var. glabriusculum. PLoS ONE 2011, 6, e28715. [Google Scholar]
- Cano-Vázquez, A.; López-Peralta, M.C.; Zavaleta-Mancera, H.A.; Cruz-Huerta, N.; Ramírez-Ramírez, I.; Gardea-Béjar, A.; González-Hernández, V.A. Variation in seed dormancy among accessions of chile piquín (Capsicum annuum var. glabriusculum). Bot. Sci. 2015, 93, 175–184. [Google Scholar]
- Rueda-Puente, E.O.; Murillo-Amador, B.; Castellanos-Cervantes, T.; García-Hernández, J.L.; Tarazòn-Herrera, M.A.; Medina, S.M.; Barrera, L.E.G. Effects of plant growth promoting bacteria and mycorrhizal on Capsicum annuum L. var. aviculare ([Dierbach] D’Arcy and Eshbaugh) germination under stressing abiotic conditions. Plant Physiol. Biochem. 2010, 48, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Montes, H.S.; Ramírez, M.M.; Villalón, M.H.; Medina, M.T.; Morales, C.A.; Heredia, G.E.; Soto, R.J.M.; López, L.R.; Cardona, E.A.; Martínez, T.H.L. Conservación y aprovechamiento sostenible de chile silvestre (Capsicum spp. Solanaceae) en México. In Avances Investigacion la Red Hortalizas del SINAREFI; Libro Científico; INIFAP-CIR-CENTRO: Guanajuato, Mexico, 2006; pp. 71–134. [Google Scholar]
- Pedraza, R.L.C.; Gómez, G.A.A. Análisis exploratorio del mercado y la comercialización de chile piquín (C. annuum, var. aviculare Dierb.) en México. Tecsistecatl 2008, 1, 5. [Google Scholar]
- De la Rosa, M. Germination of simojovel pepper seeds (Capsicum annuum L.) previously exposed to NaCl and gibberellic acid. Phyton (Buenos Aires) 2012, 81, 165–168. [Google Scholar]
- Votava, E.J.; Nabhan, G.P.; Bosland, P.W. Genetic diversity and similarity revealed via molecular analysis among and within an in situ population and ex situ accessions of chiltepín (Capsicum annuum var. glabriusculum). Conserv. Genet. 2002, 3, 123–129. [Google Scholar] [CrossRef]
- Eshbaugh, W.H. Genetic and biochemical systematic studies of chili peppers (Capsicum-Solanaceae). Bull. Torrey Bot. Club 1975, 102, 396–403. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef]
- Doll, U.; Fredes, V.M.; Soto, V.C. Effect of different pre-germination treatments on germination of six native species from the mediterranean area of Chile. IDESIA (Arica) 2013, 31, 71–76. [Google Scholar] [CrossRef]
- Prado-Urbina, G.; Lagunes-Espinoza, L.; García-López, E.; Bautista-Muñoz, C.; Camacho-Chiu, W.; Mirafuentes, G.F.; Aguilar-Rincón, V.H. Seed germination of wild chili peppers in response to pre-germination treatments. Ecosistemas Recur. Agropecu. 2015, 2, 139–149. [Google Scholar]
- Félix-Herrán, J.A.; Sañudo-Torres, R.R.; Martínez-Ruiz, R.; Rojo-Martínez, G.E. Optimización del proceso germinativo de semillas de chile chiltepín [Capsicum annuum L. var. glabriusculum (Dunal) Heiser & Pickersgill]. Juyyaania 2013, 1, 57–69. [Google Scholar]
- Quintero, C.M.F.; Castillo, O.G.; Sánchez, P.D.; Marín-Sánchez, J.; Guzmán, A.I.; Sánchez, A.; Guzmán, J.M. Relieving dormancy and improving germination of Piquín chili pepper (Capsicum annuum var. glabriusculum) by priming techniques. Cogent Food Agric. 2018, 4, 1550275. [Google Scholar] [CrossRef]
- García, F.A.; Montes, H.S.; Rangel, L.J.A.; García, M.E.; Mendoza, E.M. Physiological response of chili piquin [Capsicum annuum var. glabriusculum (Dunal) Heiser & Pickersgill] seeds to gibberlic acid and hot water. Rev. Mex. Cienc. Agrícolas 2010, 1, 203–216. [Google Scholar]
- Asgari, A.; Moghaddam, P.R.; Koocheki, A. Methods for breaking Chinese lantern (Physalis alkekengi L.) seed dormancy. Laboratory and greenhouse studies. Rev. Fac. Agron. LUZ 2018, 35, 127–151. [Google Scholar]
- González-Cortés, N.; Jiménez, V.R.; Guerra, B.E.C.; Silos, E.H.; de la Payro, C.E. Germination of amashito Chili (Capsicum annuum L. var. Glabriusculum) in southeastern Mexico. Rev. Mex. Cienc. Agrícolas 2015, 6, 2211–2218. [Google Scholar]
- Koornneef, M.; Bentsink, L.; Hilhorst, H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002, 5, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Née, G.; Xiang, Y.; Soppe, W.J. The release of dormancy, a wake-up call for seeds to germinate. Curr. Opin. Plant Biol. 2017, 35, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Hayano-Kanashiro, C.; Gámez-Meza, N.; Medina-Juárez, L.Á. Wild pepper Capsicum annuum L. var. glabriusculum: Taxonomy, plant morphology, distribution, genetic diversity, genome sequencing, and phytochemical compounds. Crop Sci. 2016, 56, 1–11. [Google Scholar] [CrossRef]
- Hernandez-Verdugo, S.; Guevara-González, R.G.; Rivera-Bustamante, R.F.; Oyama, K. Screening wild plants of Capsicum annuum for resistance to pepper huasteco virus (PHV): Presence of viral DNA and differentiation among populations. Euphytica 2001, 122, 31–36. [Google Scholar] [CrossRef]
- Meyer, S.E.; Allen, P.S.; Beckstead, J. Seed germination regulation in Bromus tectorum (Poaceae) and its ecological significance. Oikos 1997, 78, 475–485. [Google Scholar] [CrossRef]
- Hernández-Verdugo, S.; López-España, R.G.; Porras, F.; Parra-Terraza, S.; Villarreal-Romero, M.; Osuna-Enciso, T. Variation in germination among populations and plants of wild chili pepper. Agrociencia 2010, 44, 667–677. [Google Scholar]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef] [Green Version]
- Kendal, E.; Sener, O. Examination of genotype × environment interactions by GGE biplot analysis in spring durum wheat. Indian J. Genet. 2015, 75, 341–348. [Google Scholar] [CrossRef]
- Letta, T.; D’Egidio, M.G.; Abinasa, M. Stability analysis of quality traits in durum wheat (Triticum durum Desf.) varieties under south Eastern Ethiopian conditions. World J. Agric. Sci. 2008, 4, 53–57. [Google Scholar]
- Muthoni, J.; Shimelis, H.; Melis, R. Genotype x environment interaction and stability of potato tuber yield and bacterial wilt resistance in Kenya. Am. J. Potato Res. 2015, 92, 367–378. [Google Scholar] [CrossRef]
- Haider, Z.; Akhter, M.; Mahmood, A.; Khan, R.A.R. Comparison of GGE biplot and AMMI analysis of multi-environment trial (MET) data to assess adaptability and stability of rice genotypes. Afr. J. Agric. Res. 2017, 12, 3542–3548. [Google Scholar] [Green Version]
- Hagos, H.G.; Abay, F. AMMI and GGE biplot analysis of bread wheat genotypes in the northern part of Ethiopia. J. Plant Breed. Genet. 2013, 1, 12–18. [Google Scholar]
- Gedif, M.; Yigzaw, D. Genotype by environment interaction analysis for tuber yield of potato (Solanum tuberosum L.) using a GGE biplot method in Amhara region, Ethiopia. Agric. Sci. 2014, 5, 239–249. [Google Scholar] [CrossRef]
- Baraki, F.; Tsehaye, Y.; Abay, F. Analysis of genotype x environment interaction and seed yield stability of sesame in Northern Ethiopia. J. Plant Breed. Crop Sci. 2016, 8, 240–249. [Google Scholar] [Green Version]
- Araya, E.; Gómez, L.; Hidalgo, N.; Valverde, R. Efecto de la Luz y del ácido gibérelico sobre la germinación in vitro de Jaul (Alnus acuminata). Agron. Costarric. 2000, 24, 75–80. [Google Scholar]
- Karimpour, S.; Davarynejad, G.H.; Rouhbakhsh, H.; Ardakani, E. Data on scarification and stratification treatments on germination and seedling growth of Ziziphus Jujuba seeds. Adv. Environ. Biol. 2013, 7, 501–505. [Google Scholar]
- Fredrick, C.; Muthuri, C.; Ngamau, K.; Sinclair, F. Provenance and pretreatment effect on seed germination of six provenances of Faidherbia albida (Delile) A. Chev. Agrofor. Syst. 2017, 91, 1007–1017. [Google Scholar] [CrossRef]
- Batlla, D.; Benech-Arnold, R.L. A framework for the interpretation of temperature effects on dormancy and germination in seed populations showing dormancy. Seed Sci. Res. 2015, 25, 147–158. [Google Scholar] [CrossRef]
- Nguyen, T.C.T.; Obermeier, C.; Friedt, W.; Abrams, S.R.; Snowdon, R.J. Disruption of germination and seedling development in Brassica napus by mutations causing severe seed hormonal imbalance. Front. Plant Sci. 2016, 7, 322. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, L.; Tanveer, M.; Song, J. Seed heteromorphism: An important adaptation of halophytes for habitat heterogeneity. Front. Plant Sci. 2018, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Rolston, M.P. Water impermeable seed dormancy. Bot. Rev. 1978, 44, 365–396. [Google Scholar] [CrossRef]
- Rodríguez, L.Y.; Depestre, M.T.L.; Palloix, A. Behavior of new pepper (Capsicum annuum L.) F1 hybrid and varieties with multirresistence to virus in open field conditions. Cultiv. Trop. 2014, 35, 51–59. [Google Scholar]



| ID | Locality | Municipality | State | Geographical Coordinates |
|---|---|---|---|---|
| G1 | Estación Álamo | Villaldama | Nuevo León | 26°23′51″ N, 100°23′42″ W |
| G2 | Ej. Potrero de Zamora | Aramberri | Nuevo León | 24°02′20″ N, 99°55′15″ W |
| G3 | Ej. Lázaro Cárdenas | Burgos | Tamaulipas | 24°56′56″ N, 98°47′59″ W |
| G4 | Barranco Azul | San Carlos | Tamaulipas | 24°24′01″ N, 99°06′50″ W |
| G5 | Palo Blanco | Castaños | Coahuila | 26°46′02″ N, 101°30′01″ W |
| G6 | Colatlán | Ixhuatlán de Madero | Veracruz | 20°49′04″ N, 98°05′45″ W |
| G7 | Tiopancahuatl | Ixhuatlán de Madero | Veracruz | 20°41′06″ N, 98°00′44″ W |
| G8 | Colatlán (Nursery) | Ixhuatlán de Madero | Veracruz | 20°49′04″ N, 98°05′45″ W |
| G9 | El Rincón | Linares | Nuevo León | 24°53′40″ N, 99°28′13″ W |
| G10 | La Laborcilla | Rioverde | San Luis Potosí | 21°51′37″ N, 99°54′40″ W |
| ID | Treatment | Application | Time |
|---|---|---|---|
| HP | Hydrogen peroxide | 3% | 24 h |
| GA | Gibberellic acid | 5000 ppm | 24 h |
| AV | Agromil-V® | 2% v/v | 24 h |
| HC | Hydrochloric acid | 10% | 30 min |
| KN | Potassium nitrate | 3% | 168 h |
| HW | Hot water | At 83 °C let cool naturally | 24 h |
| MS | Mechanical scarification | Sanding softly with 1500 grit sandpaper | 30 s |
| HWGA | Hot water + Gibberellic acid | 24 h per tratament | |
| HPGA | Hydrogen peroxide + Gibberellic acid | 24 h per tratament | |
| HCGA | Hydrochloric acid + Gibberellic acid | 1st tratament 30 min and 2nd tratament 24 h | |
| MSGA | Mechanical scarification + Gibberellic acid | 2nd tratament 24 h | |
| WIT | Witness | Direct seeding | |
| SV | DF | GS | GI | GP | AP | DS | HS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treat | 11 | 1.285 | ** | 9.590 | ** | 0.129 | ** | 0.079 | ** | 0.045 | ** | 0.150 | ** |
| Error (a) | 24 | 0.004 | 0.134 | 0.002 | 0.002 | 0.004 | 0.004 | ||||||
| Gen | 9 | 0.331 | ** | 5.149 | ** | 0.062 | ** | 0.018 | ** | 0.074 | ** | 0.029 | ** |
| Treat × Gen | 99 | 0.030 | ** | 0.442 | ** | 0.007 | ** | 0.005 | ** | 0.007 | ** | 0.009 | ** |
| Error (b) | 216 | 0.006 | 0.115 | 0.002 | 0.002 | 0.002 | 0.003 | ||||||
| CV (a) | 4.89 | 18.68 | 4.34 | 4.49 | 5.13 | 5.200 | |||||||
| CV (b) | 6.12 | 17.35 | 4.27 | 3.83 | 4.14 | 4.500 | |||||||
| Treat | GS | GI | GP | AP | DS | HS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AV | 0.41 | c | 3.35 | cd | 15.17 | bc | 9.83 | cde | 30.67 | cde | 44.33 | abc |
| GA | 1.52 | a | 6.19 | a | 40.00 | a | 26.67 | ab | 21.83 | e | 11.17 | d |
| HC | 0.46 | c | 4.54 | bc | 0.00 | e | 30.67 | a | 34.83 | bcde | 34.5 | c |
| HCGA | 1.22 | b | 5.79 | ab | 37.33 | a | 19.67 | bc | 26.5 | de | 16.5 | d |
| HP | 0.11 | de | 1.53 | ef | 5.17 | de | 4.00 | de | 39.83 | bc | 51.00 | ab |
| HPGA | 0.40 | c | 3.61 | cd | 13.83 | bcd | 11.83 | cd | 36.17 | bcd | 38.17 | bc |
| HW | 0.02 | e | 0.20 | fg | 1.00 | e | 0.33 | e | 53.67 | a | 45.00 | abc |
| HWGA | 0.00 | e | 0.00 | g | 0.00 | e | 0.00 | e | 45.00 | ab | 55.33 | a |
| KN | 0.18 | d | 2.48 | de | 8.67 | cde | 5.17 | de | 31.5 | cde | 54.67 | a |
| MS | 0.19 | d | 2.50 | de | 9.67 | cde | 4.33 | de | 34.33 | bcde | 51.67 | ab |
| MSGA | 1.46 | a | 6.01 | ab | 34.17 | a | 29.5 | ab | 24.67 | de | 11.67 | d |
| WIT | 0.38 | c | 5.19 | ab | 23.33 | b | 6.67 | de | 26.33 | de | 43.67 | abc |
| Genotype | GS | GI | GP | AP | DS | HS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | 0.29 | e | 1.73 | fg | 5.14 | de | 9.72 | c | 38.06 | abc | 47.08 | a |
| G2 | 0.59 | cd | 3.57 | cd | 18.19 | b | 11.67 | bc | 30.83 | cde | 39.03 | abc |
| G3 | 0.54 | d | 3.34 | cde | 14.31 | bc | 13.19 | bc | 45.14 | a | 27.36 | c |
| G4 | 0.50 | d | 3.01 | def | 12.36 | bcd | 13.06 | bc | 33.47 | bcd | 41.11 | a |
| G5 | 0.28 | e | 2.19 | efg | 6.11 | cde | 10.42 | c | 44.72 | ab | 38.75 | abc |
| G6 | 0.47 | d | 3.16 | de | 15.97 | b | 9.31 | c | 38.75 | abc | 35.97 | abc |
| G7 | 0.67 | c | 4.63 | bc | 27.08 | a | 9.58 | c | 23.61 | def | 39.72 | ab |
| G8 | 1.01 | a | 5.46 | ab | 27.64 | a | 22.36 | a | 21.11 | ef | 29.17 | bc |
| G9 | 0.15 | f | 1.05 | g | 3.06 | e | 5.14 | c | 44.86 | ab | 46.94 | a |
| G10 | 0.80 | b | 6.35 | a | 27.08 | a | 19.44 | ab | 17.22 | f | 36.25 | abc |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcalá-Rico, J.S.G.J.; López-Benítez, A.; Vázquez-Badillo, M.E.; Sánchez-Aspeytia, D.; Rodríguez-Herrera, S.A.; Pérez-Rodríguez, M.Á.; Ramírez-Godina, F. Seed Physiological Potential of Capsicum annuum var. glabriusculum Genotypes and Their Answers to Pre-Germination Treatments. Agronomy 2019, 9, 325. https://doi.org/10.3390/agronomy9060325
Alcalá-Rico JSGJ, López-Benítez A, Vázquez-Badillo ME, Sánchez-Aspeytia D, Rodríguez-Herrera SA, Pérez-Rodríguez MÁ, Ramírez-Godina F. Seed Physiological Potential of Capsicum annuum var. glabriusculum Genotypes and Their Answers to Pre-Germination Treatments. Agronomy. 2019; 9(6):325. https://doi.org/10.3390/agronomy9060325
Chicago/Turabian StyleAlcalá-Rico, Juan Samuel Guadalupe Jesús, Alfonso López-Benítez, Mario Ernesto Vázquez-Badillo, David Sánchez-Aspeytia, Sergio Alfredo Rodríguez-Herrera, Miguel Ángel Pérez-Rodríguez, and Francisca Ramírez-Godina. 2019. "Seed Physiological Potential of Capsicum annuum var. glabriusculum Genotypes and Their Answers to Pre-Germination Treatments" Agronomy 9, no. 6: 325. https://doi.org/10.3390/agronomy9060325
APA StyleAlcalá-Rico, J. S. G. J., López-Benítez, A., Vázquez-Badillo, M. E., Sánchez-Aspeytia, D., Rodríguez-Herrera, S. A., Pérez-Rodríguez, M. Á., & Ramírez-Godina, F. (2019). Seed Physiological Potential of Capsicum annuum var. glabriusculum Genotypes and Their Answers to Pre-Germination Treatments. Agronomy, 9(6), 325. https://doi.org/10.3390/agronomy9060325