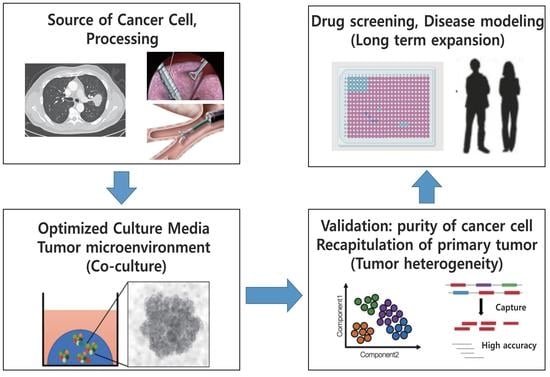
Scientific Validation and Clinical Application of Lung Cancer Organoids
Abstract
:1. Introduction
2. Lung Organoids
2.1. Lung Stem Cell, Development, and Mesenchymal Cell
2.2. Organoid Culture Media
2.3. Clinical Application of Lung Organoids
3. Lung Cancer Organoids
3.1. Accomplishments and Challenges of LCO
3.2. Purity of Cancer Cells in LCOs
3.3. Validation of LCOs
3.4. Tumor Microenvironment and Co-Culture
3.5. Single-Cell RNA Sequencing of LCOs
3.6. Disease Modeling and Clinical Application of LCOs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naylor, E.C.; Desani, J.K.; Chung, P.K. Targeted Therapy and Immunotherapy for Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Huang, L.L.; Chen, J.H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Therapy 2019, 4, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto ABLowy DRFeuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.F.; Ma, P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncology 2019, 12, 134. [Google Scholar] [CrossRef] [Green Version]
- Clevers, H.C. Organoids, Avatars for Personalized Medicine. Keio J. Med. 2019, 68, 95. [Google Scholar] [CrossRef] [Green Version]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.K.; Knoblich, J.A. Human organoids, Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef]
- Choi, J.; Iich, E.; Lee, J.H. Organogenesis of adult lung in a dish: Differentiation, disease and therapy. Dev. Biol. 2016, 420, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Pomerenke, A. Organotypic Models of Lung Cancer. Curr. Top. Microbiol. Immunol. 2021, 430, 161–181. [Google Scholar]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.J.; Chun, S.M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef]
- Hu, Y.; Sui, X.; Song, F.; Li, Y.; Li, K.; Chen, Z.; Yang, F.; Chen, X.; Zhang, Y.; Wang, X.; et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat. Commun. 2021, 12, 2581. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Radulovich, N.; Ng, C.; Liu, N.; Notsuda, H.; Cabanero, M.; Martins-Filho, S.N.; Raghavan, V.; Li, Q.; Mer, A.S.; et al. Organoid Cultures as Preclinical Models of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 1162–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salahudeen, A.A.; Choi, S.S.; Rustagi, A.; Zhu, J.; van Unen, V.; de la O, S.M.; Flynn, R.A.; Margalef-Català, M.; Santos, A.J.M.; Ju, J.; et al. Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 2020, 588, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Katsura, H.; Sontake, V.; Tata, A.; Kobayashi, Y.; Edwards, C.E.; Heaton, B.E.; Konkimalla, A.; Asakura, T.; Mikami, Y.; Fritch, E.J.; et al. Human Lung Stem Cell-Based Alveolospheres Provide Insights into SARS-CoV-2-Mediated Interferon Responses and Pneumocyte Dysfunction. Cell Stem Cell 2020, 27, 890–904. [Google Scholar] [CrossRef] [PubMed]
- Ebisudani, T.; Sugimoto, S.; Haga, K.; Mitsuishi, A.; Takai-Todaka, R.; Fujii, M.; Toshimitsu, K.; Hamamoto, J.; Sugihara, K.; Hishida, T.; et al. Direct derivation of human alveolospheres for SARS-CoV-2 infection modeling and drug screening. Cell Rep. 2021, 35, 109218. [Google Scholar] [CrossRef] [PubMed]
- Sachs, N.; Papaspyropoulos, A.; Zomer-van Ommen, D.D.; Heo, I.; Böttinger, L.; Klay, D.; Weeber, F.; Huelsz-Prince, G.; Iakobachvili, N.; Amatngalim, G.D.; et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019, 38, e100300. [Google Scholar] [CrossRef]
- Evans, K.V.; Lee, J.H. Alveolar wars: The rise of in vitro models to understand human lung alveolar maintenance, regeneration, and disease. Stem Cells Transl. Med. 2020, 9, 867–881. [Google Scholar] [CrossRef] [Green Version]
- Zepp, J.A.; Zacharias, W.J.; Frank, D.B.; Cavanaugh, C.A.; Zhou, S.; Morley, M.P.; Morrisey, E.E. Distinct Mesenchymal Lineages and Niches Promote Epithelial Self-Renewal and Myofibrogenesis in the Lung. Cell 2017, 170, 1134–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, E.A.; Sun, X. Tissue crosstalk in lung development. J. Cell. Biochem. 2014, 115, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Tammela, T.; Hofree, M.; Choi, J.; Marjanovic, N.D.; Han, S.; Canner, D.; Wu, K.; Paschini, M.; Bhang, D.H.; et al. Anatomically and Functionally Distinct Lung Mesenchymal Populations Marked by Lgr5 and Lgr6. Cell 2017, 170, 1149–1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Bhang, D.H.; Beede, A.; Huang, T.L.; Stripp, B.R.; Bloch, K.D.; Wagers, A.J.; Tseng, Y.H.; Ryeom, S.; Kim, C.F. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 2014, 156, 440–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolić, M.Z.; Caritg, O.; Jeng, Q.; Johnson, J.A.; Sun, D.; Howell, K.J.; Brady, J.L.; Laresgoiti, U.; Allen, G.; Butler, R.; et al. Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. ELife 2017, 6, e26575. [Google Scholar] [CrossRef] [PubMed]
- Youk, J.; Kim, T.; Evans, K.V.; Jeong, Y.I.; Hur, Y.; Hong, S.P.; Kim, J.H.; Yi, K.; Kim, S.Y.; Na, K.J.; et al. Three-Dimensional Human Alveolar Stem Cell Culture Models Reveal Infection Response to SARS-CoV-2. Cell Stem Cell 2020, 27, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Rawlins, E.L. Developmental mechanisms and adult stem cells for therapeutic lung regeneration. Dev. Biol. 2018, 433, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Volckaert, T.; De Langhe, S.P. Wnt and FGF mediated epithelial-mesenchymal crosstalk during lung development. Dev. Dyn. 2015, 244, 342–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; An, G.H.; Kim, J.Y.; Rasaei, R.; Kim, W.J.; Jin, X.; Woo, D.H.; Han, C.; Yang, S.R.; Kim, J.H.; et al. Human pluripotent stem-cell-derived alveolar organoids for modeling pulmonary fibrosis and drug testing. Cell Death Discov. 2021, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, G.; Luca, A.; Jose, S.S.; Antonini, M.; Teloni, I.; Fric, J.; Zelante, T. Using Lung Organoids to Investigate Epithelial Barrier Complexity and IL-17 Signaling During Respiratory Infection. Front. Immunol. 2019, 10, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleischer, A.; Vallejo-Díez, S.; Martín-Fernández, J.M.; Sánchez-Gilabert, A.; Castresana, M.; Del Pozo, A.; Esquisabel, A.; Ávila, S.; Castrillo, J.L.; Gaínza, E.; et al. iPSC-Derived Intestinal Organoids from Cystic Fibrosis Patients Acquire CFTR Activity upon TALEN-Mediated Repair of the p.F508del Mutation. Mol. Ther. Methods Clin. Deve. 2020, 17, 858–870. [Google Scholar] [CrossRef]
- Han, Y.; Duan, X.; Yang, L.; Nilsson-Payant, B.E.; Wang, P.; Duan, F.; Tang, X.; Yaron, T.M.; Zhang, T.; Uhl, S.; et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 2021, 589, 270–275. [Google Scholar] [CrossRef]
- Verheyden, J.M.; Sun, X. A transitional stem cell state in the lung. Nat. Cell Biol. 2020, 22, 1025–1026. [Google Scholar] [CrossRef]
- Choi, J.; Park, J.E.; Tsagkogeorga, G.; Yanagita, M.; Koo, B.K.; Han, N.; Lee, J.H. Inflammatory Signals Induce AT2 Cell-Derived Damage-Associated Transient Progenitors that Mediate Alveolar Regeneration. Cell Stem Cell 2020, 27, 366–382. [Google Scholar] [CrossRef]
- Strunz, M.; Simon, L.M.; Ansari, M.; Kathiriya, J.J.; Angelidis, I.; Mayr, C.H.; Tsidiridis, G.; Lange, M.; Mattner, L.F.; Yee, M.; et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat. Commun. 2020, 11, 3559. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Kaing, S.; de Groot, R.; Wolkers, M.C.; Clevers, H.; et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.Y.; Cho, Y.H.; Kim, D.S.; Ji, W.; Choi, C.M.; Lee, J.C.; Rho, J.K.; Jeong, G.S. Establishment and Long-Term Expansion of Small Cell Lung Cancer Patient-Derived Tumor Organoids. Int. J. Mol. Sci. 2021, 22, 1349. [Google Scholar] [CrossRef]
- Yokota, E.; Iwai, M.; Yukawa, T.; Yoshida, M.; Naomoto, Y.; Haisa, M.; Monobe, Y.; Takigawa, N.; Guo, M.; Maeda, Y.; et al. Clinical application of a lung cancer organoid (tumoroid) culture system. NPJ Precis. Oncol. 2021, 5, 29. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Monkhorst, K.; Schipper, L.J.; Hartemink, K.J.; Smit, E.F.; Kaing, S.; de Groot, R.; Wolkers, M.C.; Clevers, H.; Cuppen, E.; et al. Challenges in Establishing Pure Lung Cancer Organoids Limit Their Utility for Personalized Medicine. Cell Rep. 2020, 31, 107588. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Sugano, M.; Miyashita, T.; Hashimoto, H.; Ochiai, A.; Suzuki, K.; Tsuboi, M.; Ishii, G. Organoid culture containing cancer cells and stromal cells reveals that podoplanin-positive cancer-associated fibroblasts enhance proliferation of lung cancer cells. Lung Cancer 2019, 134, 100–107. [Google Scholar] [CrossRef]
- Endo, H.; Okami, J.; Okuyama, H.; Kumagai, T.; Uchida, J.; Kondo, J.; Takehara, T.; Nishizawa, Y.; Imamura, F.; Higashiyama, M.; et al. Spheroid culture of primary lung cancer cells with neuregulin 1/HER3 pathway activation. J. Thorac. Oncol. 2013, 8, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Kim, S.M.; Lim, S.; Lee, J.Y.; Choi, S.J.; Yang, S.D.; Yun, M.R.; Kim, C.G.; Gu, S.R.; Park, C.; et al. Modeling Clinical Responses to Targeted Therapies by Patient-Derived Organoids of Advanced Lung Adenocarcinoma. Clin. Cancer Res. 2021, 27, 4397–4409. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Chen, D.; Meng, Z.; Chen, W.; Huang, W. Protocol for generation of lung adenocarcinoma organoids from clinical samples. STAR Protocols 2021, 2, 100239. [Google Scholar] [CrossRef] [PubMed]
- Vilgelm, A.E.; Bergdorf, K.; Wolf, M.; Bharti, V.; Shattuck-Brandt, R.; Blevins, A.; Jones, C.; Phifer, C.; Lee, M.; Lowe, C.; et al. Fine-Needle Aspiration-Based Patient-Derived Cancer Organoids. Iscience 2020, 23, 101408. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, Y.H.; Park, S.; Cho, S.W. Organoid engineering with microfluidics and biomaterials for liver, lung disease, and cancer modeling. Acta Biomater. 2021, 132, 37–51. [Google Scholar] [CrossRef]
- Abou-El-Enein, M.; Elsallab, M.; Feldman, S.A.; Fesnak, A.D.; Heslop, H.E.; Marks, P.; Till, B.G.; Bauer, G.; Savoldo, B. Scalable Manufacturing of CAR T cells for Cancer Immunotherapy. Blood Cancer Discov. 2021, 2, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Nabhan, A.N.; Brownfield, D.G.; Harbury, P.B.; Krasnow, M.A.; Desai, T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 2018, 359, 1118–1123. [Google Scholar] [CrossRef] [Green Version]
- Hegab, A.E.; Arai, D.; Gao, J.; Kuroda, A.; Yasuda, H.; Ishii, M.; Naoki, K.; Soejima, K.; Betsuyaku, T. Mimicking the niche of lung epithelial stem cells and characterization of several effectors of their in vitro behavior. Stem Cell Res. 2015, 15, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Armendariz, A.I.; Heiner, M.; El Agha, E.; Salwig, I.; Hoek, A.; Hessler, M.C.; Shalashova, I.; Shrestha, A.; Carraro, G.; Mengel, J.P.; et al. Multilineage murine stem cells generate complex organoids to model distal lung development and disease. EMBO J. 2020, 39, e103476. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Onaitis, M.W.; Rawlins, E.L.; Lu, Y.; Clark, C.P.; Xue, Y.; Randell, S.H.; Hogan, B.L. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. USA 2009, 106, 12771–12775. [Google Scholar] [CrossRef] [Green Version]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahin, U. Studying Tumor-ReacTive T Cells, A Personalized Organoid Model. Cell Stem Cell 2018, 23, 318–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kersten, K.; de Visser, K.E.; van Miltenburg, M.H.; Jonkers, J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol. Med. 2017, 9, 137–153. [Google Scholar] [CrossRef]
- Chung, W.J.; Daemen, A.; Cheng, J.H.; Long, J.E.; Cooper, J.E.; Wang, B.E.; Tran, C.; Singh, M.; Gnad, F.; Modrusan, Z.; et al. Kras mutant genetically engineered mouse models of human cancers are genomically heterogeneous. Proc. Natl. Acad. Sci. USA 2017, 114, E10947–E10955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Totoki, Y.; Takahashi, H.; Nakamura, H.; Hama, N.; Kohno, T.; Tsuta, K.; Yoshida, A.; Asamura, H.; Mutoh, M.; et al. Mouse model for ROS1-rearranged lung cancer. PLoS ONE 2013, 8, e56010. [Google Scholar] [CrossRef] [PubMed]
- Dost, A.F.M.; Moye, A.L.; Vedaie, M.; Tran, L.M.; Fung, E.; Heinze, D.; Villacorta-Martin, C.; Huang, J.; Hekman, R.; Kwan, J.H.; et al. Organoids Model Transcriptional Hallmarks of Oncogenic KRAS Activation in Lung Epithelial Progenitor Cells. Cell Stem Cell 2020, 27, 663–678. [Google Scholar] [CrossRef] [PubMed]
| Organoid (Reference) | Isolated Cell | WNT Signaling | BMP Inhibitor | FGF | EGF | TGFβ Inhibitor | ALK Inhibitor | p38 MAPKi | ROCK Inhibitor | Supplement | Etc. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WNT 3A | CHIR 99021 | R-Spondin | Noggin | FGF 7 | FGF 10 | A83-01 | SB 431542 | SB 202190 | Y-27632 | NA, B27, NAC | ||||
| Airway organoid [16] | Lung epithelial cells | |||||||||||||
| Alveolar organoid [22] | Embryonic lung epithelial tip cells | |||||||||||||
| Alveolosphere [14] | AT 2 cells (HTII-280+ cells) | BIRB-786 | Heparin, IL-1β | |||||||||||
| Distal lung organoid [13] | Lung epithelial cells (EPCAM+ cells) | |||||||||||||
| Alveolosphere [15] | AT 2 cells (HTII-280+ cells) | Afamin-Wnt-3A | FGF-2, IGF-1 | |||||||||||
| 3D alveolar stem cell culture [23] | AT 2 cells (HTII-280+ cells) | |||||||||||||
| P.I. (Year) | Cancer Type and Origin | Success Rate [Validation Methods] | Culture Media or Remarks | Reference |
|---|---|---|---|---|
| Inoue (2013) | Lung cancer cells | 80% | Embryonic stem cell culture media, Plus FGF2 | [38] |
| Voest (2018) | AC (n = 3), SQC (n = 2), NOS (n = 1) Biopsy (n = 2), resection (n = 4) | NSCLC organoid from 6 patients One sample contained normal airway organoid | AO media containing R-spondin1, FGF7, FGF10, Noggin, A83-01, SB202109, B27, NAC, and NA | [33] |
| Clevers (2019) | AD, SQC, LCNEC (n = 34) Biopsy of metastatic lesion, resection | Resection: 88% (n = 16): normal tissue contained. Biopsy: 28% (n = 18) Orthotopic transplantation: 30% (n = 12) (morphology, histology, whole-genome sequencing) | AO media: 5uM Nutlin-3a | [16] |
| Jang (2019) | AD, SQC, ASC, LCC, SCLC (n = 23) biopsy, resection | Long term expansion (>6 months) : 87% (n = 20) (SNP genotype, VAF distribution) | MBM containing basic FGF, N2, B27, ROCK inhibitor (deletion of Wnt3a and noggin) | [10] |
| Voest (2020) | AC, SQC (n = 58), biopsy (n = 30), resection (n = 28), | Overall: 17% (n = 9), resection: 18% (n = 5) Biopsy: 13% (n = 4) (Copy number profile, IHC) | Media containing Noggin, FGF-7, FGF-10, A83-01, SB202190, 5 μM Nutlin-3a | [36] |
| Tsao (2020) | AD (n = 19), SQC (n = 15), AD-PDX (n = 16), SQC-PDX (n = 26) | Short-term culture (1–3 months, 1–9 passages): 72% (n = 47) Long-term culture (>3 months, >10 passages): 15% (n = 10) (Whole-exome and RNA sequencing) | M26 containing CHIR 99021, A83-01, EGF, FGF-4, FGF-10, SAG | [12] |
| Yamatsuji (2021) | AD (n = 29), SQC (n = 7), ACIS (n = 1), SCLC(n = 2), PC (n = 2) | Long term culture (>13 months, >36 passages) Overall: 7% (n = 3), Primary tumor 3.6% (n = 1) LN: 100% (n = 1), ME: 50% (n = 1) (Karyotyping of chromosomes) | AO media was superior to 3 different media (media of Jang, Tsao’s, and Inoue’s groups) | [35] |
| Cho (2021) | Advanced AD (n = 100) | 83.0% (n = 83), ME (n = 77), Brain metastasis (n = 3), Bone metastasis (n = 1), lung primary tumor (n = 2) (Whole-exome, RNA sequencing) | AO media | [39] |
| Jeong (2021) | SCLC (n = 10) | Long-term expansion (>9 months) 80% (n = 8) (Morphology, molecular characteristics, genomic profile) | EGF, FGF-based media ± WNT3A or R-spondin-1 | [34] |
| Lung Epithelial Cell | Co-Culture | Effect | Reference |
|---|---|---|---|
| Mouse airway basal stem cell (ABSC) | Lung fibroblast, endothelial cell | Mesenchymal cells Influence ABSC’s proliferation and differentiation in vitro | [42] |
| Mouse bronchioalveolar stem cell (BASC) | Endothelial cell | BASC differentiates to alveolar lineage | [21] |
| Mouse AEC2 (HT-280+ cell) | PDGFRα+ stromal cells | AEC2s self-renewal and differentiate to AEC1s, forming alveolosphere | [40] |
| Mouse BASC | Lung-resident mesenchymal cells | BASC forms bronchioalveolar lung organoid (BALO) that express markers of airway and alveoli | [46] |
| Mouse AEC2 | Fibroblast | Wnt-secreting fibroblasts maintain AT2 stemness and prevents differentiation to AT1 | [44] |
| Human basal cell | Fibroblast | Basal cell forms tracheospheres containing basal, ciliated, and mucosecretory cells | [47] |
| Human lung cancer PC9 cell | Podoplanin+ cancer-associated fibroblast (CAF) | With CAFs, PC9 cell form cancer organoid CAF promotes cancer cell growth | [37] |
| Human lung cancer organoid | Lung cancer organoid | Test the effect of immunotherapy | [33] |
| Topic of Study | Study Type Phase | Outcome Measures | Status/Location | Reference |
|---|---|---|---|---|
| Prospective primary human lung cancer organoids to predict treatment response | Observational, prospective | Biobanking of lung cancer organoid PDX models of lung cancer Tumor response | Recruiting/Zuyderland Medical Center, The Netherlands | NCT04859166 |
| Patient-derived organoid model and circulating tumor cells for predicting treatment response of lung cancer | Observational, prospective | Biobanking of patient-derived organoid Correlation of PDO and circulating tumor cell | Recruiting/M.D. Anderson Cancer Center, the United States | NCT03655015 |
| Drug sensitivity correlation between patient-derived organoid model and clinical response in NSCLC patients | Observational, cross-sectional | Correlation of ex vivo sensitivity test on patient derived organoid models | Unknown/People’s Hospital of Hebei, Province, China | NCT03453307 |
| TCR-T cell for immunotherapy of lung cancer | Phase 1 | Coculture of organoid and TIL will be utilized to screen tumor responsive T cell | Recruiting/Hospital of Guangzhou Medical University, China | NCT03778814 |
| High-dose vitamin C intravenous infusion in patients with solid tumor | Phase 2 | 3 month DCR, in vitro activity of vitamin C in tumor organoids | Recruiting/New York-Presbyterian Hospital, the United States | NCT03146962 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Kim, Y.; Chung, C. Scientific Validation and Clinical Application of Lung Cancer Organoids. Cells 2021, 10, 3012. https://doi.org/10.3390/cells10113012
Lee D, Kim Y, Chung C. Scientific Validation and Clinical Application of Lung Cancer Organoids. Cells. 2021; 10(11):3012. https://doi.org/10.3390/cells10113012
Chicago/Turabian StyleLee, Dahye, Yoonjoo Kim, and Chaeuk Chung. 2021. "Scientific Validation and Clinical Application of Lung Cancer Organoids" Cells 10, no. 11: 3012. https://doi.org/10.3390/cells10113012
APA StyleLee, D., Kim, Y., & Chung, C. (2021). Scientific Validation and Clinical Application of Lung Cancer Organoids. Cells, 10(11), 3012. https://doi.org/10.3390/cells10113012