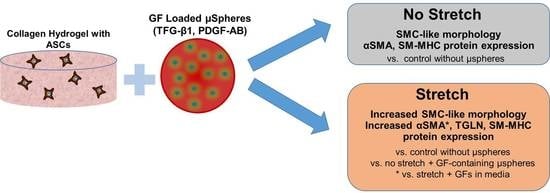
Controlled Growth Factor Delivery and Cyclic Stretch Induces a Smooth Muscle Cell-like Phenotype in Adipose-Derived Stem Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. ASCs Culture and Expansion
2.2. Production of μSpheres
2.3. Loading Growth Factor into μSpheres
2.4. Tissue Construct Production and Culture
2.5. Determining Growth Factor Release
2.6. Stretching Gels
2.7. Fluorescence Microscopy for Cell Shape
2.8. Compaction of Static Constructs
2.9. Immunocytochemistry and Analysis of Samples
2.10. Statistical Analysis
3. Results
3.1. Schematical Outline of Experimental Setup
3.2. Growth Factor Release from Genipin-Crosslinked Gelatin μSpheres
3.3. Compaction of Constructs Cultured with Specific Growth Factor Treatments
3.4. Morphology of ASCs Differentiated Using Growth Factor Treatments and Mechanical Stretch
3.5. The Shape of ASCs with Induced SMC Differentiation Using Growth Factor Treatments
3.6. Shape of ASCs Cultured with Growth Factor Treatments and Mechanical Stretch
3.7. Smooth Muscle-Associated Protein Expression by ASCs Cultured with Growth Factor Treatments and Mechanical Stretch
3.8. Correlations between Cell Morphology and SMC Marker Protein Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arias, E.; Heron, M.; Tejada-Vera, B. United States life tables eliminating certain causes of death, 1999–2001. Natl. Vital-Stat. Rep. Centers Dis. Control. Prev. Natl. Cent. Heal. Stat. Natl. Vital-Stat. Syst. 2014, 61, 1–128. [Google Scholar]
- Hart, M.L.; Neumayer, K.M.H.; Vaegler, M.; Daum, L.; Amend, B.; Sievert, K.D.; Di Giovanni, S.; Kraushaar, U.; Guenther, E.; Stenzl, A.; et al. Cell-Based Therapy for the Deficient Urinary Sphincter. Curr. Urol. Rep. 2013, 14, 476–487. [Google Scholar] [CrossRef]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The Potential of Adipose Stem Cells in Regenerative Medicine. Stem Cell Rev. Rep. 2011, 7, 269–291. [Google Scholar] [CrossRef]
- Fang, B.; Song, Y.; Liao, L.; Zhang, Y.; Zhao, R. Favorable Response to Human Adipose Tissue-Derived Mesenchymal Stem Cells in Steroid-Refractory Acute Graft-Versus-Host Disease. Transplant. Proc. 2007, 39, 3358–3362. [Google Scholar] [CrossRef]
- Cawthorn, W.P.; Scheller, E.L.; MacDougald, O.A. Adipose tissue stem cells: The great WAT hope. Trends Endocrinol. Metab. 2012, 23, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.-I.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hoang, A.C.; Yu, H.; Röszer, T. Transcriptional Landscaping Identifies a Beige Adipocyte Depot in the Newborn Mouse. Cells 2021, 10, 2368. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Z.; Svensson, K.J.; Tsai, L.; Zeng, X.; Roh, H.C.; Kong, X.; Rao, R.R.; Lou, J.; Lokurkar, I.; Baur, W.; et al. A Smooth Muscle-Like Origin for Beige Adipocytes. Cell Metab. 2014, 19, 810–820. [Google Scholar] [CrossRef] [Green Version]
- Shamsi, F.; Lynes, M.; Piper, M.; Ho, L.L.; Huang, T.; Tseng, Y.H. Vascular smooth muscle-derived TRPV1-positive progenitors are a source of cold-induced thermogenic adipocytes. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- An, Y.; Wang, G.; Diao, Y.; Long, Y.; Fu, X.; Weng, M.; Zhou, L.; Sun, K.; Cheung, T.H.; Ip, N.Y.; et al. A Molecular Switch Regulating Cell Fate Choice between Muscle Progenitor Cells and Brown Adipocytes. Dev. Cell 2017, 41, 382–391.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halvorsen, Y.-D.C.; Bond, A.; Sen, A.; Franklin, D.M.; Lea-Currie, Y.; Sujkowski, D.; Ellis, P.; Wilkison, W.O.; Gimble, J.M. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: Biochemical, cellular, and molecular analysis. Metabolism 2001, 50, 407–413. [Google Scholar] [CrossRef]
- Helder, M.N.; Knippenberg, M.; Klein-Nulend, J.; Wuisman, P.I. Stem Cells from Adipose Tissue Allow Challenging New Concepts for Regenerative Medicine. Tissue Eng. 2007, 13, 1799–1808. [Google Scholar] [CrossRef]
- Gao, L.; McBeath, R.; Chen, C. Stem Cell Shape Regulates a Chondrogenic Versus Myogenic Fate Through Rac1 and N-Cadherin. Stem Cells 2010, 28, 564–572. [Google Scholar] [CrossRef] [Green Version]
- Iyyanki, T.; Hubenak, J.; Liu, J.; Chang, E.I.; Beahm, E.K.; Zhang, Q. Harvesting Technique Affects Adipose-Derived Stem Cell Yield. Aesthetic Surg. J. 2015, 35, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Liang, L.; Dou, Y.; Huang, Z.; Mo, H.; Wang, Y.; Yu, B. Mechanical stretch inhibits mesenchymal stem cell adipogenic differentiation through TGFβ1/Smad2 signaling. J. Biomech. 2015, 48, 3656–3662. [Google Scholar] [CrossRef] [PubMed]
- Furuhata, Y.; Yoshitomi, T.; Kikuchi, Y.; Sakao, M.; Yoshimoto, K. Osteogenic Lineage Commitment of Adipose-Derived Stem Cells Is Predetermined by Three-Dimensional Cell Accumulation on Micropatterned Surface. ACS Appl. Mater. Interfaces 2017, 9, 9339–9347. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Cho, H.; Lee, E.; Won, Y.; Kim, C.; Ahn, W.; Lee, E.; Son, Y. Osteogenic stimulation of human adipose-derived stem cells by pre-treatment with fibroblast growth factor 2. Cell Tissue Res. 2016, 364, 137–147. [Google Scholar] [CrossRef]
- EEstes, B.T.; Wu, A.W.; Guilak, F. Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6. Arthritis Rheum. 2006, 54, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, A.; Esfandiari, E.; Hashemibeni, B.; Kazemi, M.; Mardani, M.; Esmaeili, A. Effects of FGF-2 on human adipose tissue derived adult stem cells morphology and chondrogenesis enhancement in Transwell culture. Biochem. Biophys. Res. Commun. 2012, 424, 234–238. [Google Scholar] [CrossRef]
- Lee, W.-C.C.; Maul, T.; Vorp, D.; Rubin, J.P.; Marra, K.G. Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech. Model. Mechanobiol. 2007, 6, 265–273. [Google Scholar] [CrossRef]
- Heydarkhan-Hagvall, S.; Schenke-Layland, K.; Yang, J.Q.; Heydarkhan, S.; Xu, Y.; Zuk, P.A.; MacLellan, W.R.; Beygui, R.E. Human Adipose Stem Cells: A Potential Cell Source for Cardiovascular Tissue Engineering. Cells Tissues Organs 2008, 187, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.J.; Abdollahi, H.; Zhang, P.; McIlhenny, S.; Tulenko, T.N.; DiMuzio, P.J. Differentiation of Adult Stem Cells into Smooth Muscle for Vascular Tissue Engineering. J. Surg. Res. 2011, 168, 306–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moses, H.L.; Roberts, A.B.; Derynck, R. The Discovery and Early Days of TGF-beta: A Historical Perspective. Cold Spring Harb. Perspect. Biol. 2016, 8, a021865. [Google Scholar] [CrossRef] [Green Version]
- Solorio, L.D.; Dhami, C.D.; Dang, P.N.; Vieregge, E.L.; Alsberg, E. Spatiotemporal Regulation of Chondrogenic Differentiation with Controlled Delivery of Transforming Growth Factor-β1 from Gelatin Microspheres in Mesenchymal Stem Cell Aggregates. Stem Cells Transl. Med. 2012, 1, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Solorio, L.D.; Phillips, L.M.; McMillan, A.; Cheng, C.W.; Dang, P.N.; Samorezov, J.E.; Yu, X.; Murphy, W.L.; Alsberg, E. Spatially organized differentiation of mesenchymal stem cells within biphasic microparticle-incorporated high cell density osteochondral tissues. Adv. Health Mater. 2015, 4, 2306–2313. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.; Xie, A.; Emani, S.; Yamato, M.; Okano, T.; Emani, S.M.; Wong, J.Y. A Comparison of Human Smooth Muscle and Mesenchymal Stem Cells as Potential Cell Sources for Tissue-Engineered Vascular Patches. Tissue Eng. Part A 2012, 18, 986–998. [Google Scholar] [CrossRef]
- Park, W.S.; Heo, S.C.; Jeon, E.S.; Hong, D.H.; Son, Y.K.; Ko, J.-H.; Kim, H.K.; Lee, S.Y.; Kim, J.H.; Han, J. Functional expression of smooth muscle-specific ion channels in TGF-β(1)-treated human adipose-derived mesenchymal stem cells. Am. J. Physiol. 2013, 305, C377–C391. [Google Scholar] [CrossRef] [Green Version]
- Brun, J.; Lutz, K.A.; Neumayer, K.M.H.; Klein, G.; Seeger, T.; Uynuk-Ool, T.; Wörgötter, K.; Schmid, S.; Kraushaar, U.; Guenther, E.; et al. Smooth Muscle-Like Cells Generated from Human Mesenchymal Stromal Cells Display Marker Gene Expression and Electrophysiological Competence Comparable to Bladder Smooth Muscle Cells. PLoS ONE 2015, 10, e0145153. [Google Scholar] [CrossRef] [Green Version]
- Brun, J.; Abruzzese, T.; Rolauffs, B.; Aicher, W.K.; Hart, M.L. Choice of xenogenic-free expansion media significantly influences the myogenic differentiation potential of human bone marrow–derived mesenchymal stromal cells. Cytotherapy 2016, 18, 344–359. [Google Scholar] [CrossRef]
- Parvizi, M.; Bolhuis-Versteeg, L.A.; Poot, A.A.; Harmsen, M.C. Efficient generation of smooth muscle cells from adipose-derived stromal cells by 3D mechanical stimulation can substitute the use of growth factors in vascular tissue engineering. Biotechnol. J. 2016, 11, 932–944. [Google Scholar] [CrossRef]
- Yang, L.; Geng, Z.; Nickel, T.; Johnson, C.; Gao, L.; Dutton, J.; Hou, C.; Zhang, J. Differentiation of Human Induced-Pluripotent Stem Cells into Smooth-Muscle Cells: Two Novel Protocols. PLoS ONE 2016, 11, e0147155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, E.S.; Moon, H.J.; Lee, M.J.; Song, H.Y.; Kim, Y.M.; Bae, Y.C.; Jung, J.S.; Kim, J.H. Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-β-dependent mechanism. J. Cell Sci. 2006, 119, 4994–5005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Yin, S.; Cen, L.; Liu, Q.; Liu, W.; Cao, Y.; Cui, L. Differentiation of Adipose-Derived Stem Cells into Contractile Smooth Muscle Cells Induced by Transforming Growth Factor-β1 and Bone Morphogenetic Protein-4. Tissue Eng. Part A 2010, 16, 1201–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aji, K.; Maimaijiang, M.; Aimaiti, A.; Rexiati, M.; Azhati, B.; Tusong, H.; Cui, L. Differentiation of Human Adipose Derived Stem Cells into Smooth Muscle Cells Is Modulated by CaMKIIγ. Stem Cells Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Elçin, A.E.; Parmaksiz, M.; Dogan, A.; Seker, S.; Durkut, S.; Dalva, K.; Elçin, Y.M. Differential gene expression profiling of human adipose stem cells differentiating into smooth muscle-like cells by TGFβ1/BMP4. Exp. Cell Res. 2017, 352, 207–217. [Google Scholar] [CrossRef]
- Boroujeni, S.M.; Mashayekhan, S.; Vakilian, S.; Ardeshirylajimi, A.; Soleimani, M. The synergistic effect of surface topography and sustained release of TGF-β1 on myogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2016, 104, 1610–1621. [Google Scholar] [CrossRef]
- Solorio, L.; Zwolinski, C.; Lund, A.; Farrell, M.J.; Stegemann, J.P. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J. Tissue Eng. Regen. Med. 2010, 4, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Turner, P.A.; Thiele, J.S.; Stegemann, J.P. Growth factor sequestration and enzyme-mediated release from genipin-crosslinked gelatin microspheres. J. Biomater. Sci. Polym. Ed. 2017, 28, 1826–1846. [Google Scholar] [CrossRef]
- Kawai, K.; Suzuki, S.; Tabata, Y.; Ikada, Y.; Nishimura, Y. Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials 2000, 21, 489–499. [Google Scholar] [CrossRef]
- Strobel, H.A.; Dikina, A.D.; Levi, K.; Solorio, L.D.; Alsberg, E.; Rolle, M.W. Cellular Self-Assembly with Microsphere Incorporation for Growth Factor Delivery Within Engineered Vascular Tissue Rings. Tissue Eng. Part A 2017, 23, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Galvin, D.J.; Watson, R.W.G.; Gillespie, J.I.; Brady, H.; Fitzpatrick, J.M. Mechanical stretch regulates cell survival in human bladder smooth muscle cells in vitro. Am. J. Physiol. Physiol. 2002, 283, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kahn, C.J.; Chen, H.-Q.; Tran, N.; Wang, X. Effect of uniaxial stretching on rat bone mesenchymal stem cell: Orientation and expressions of collagen types I and III and tenascin-C. Cell Biol. Int. 2008, 32, 344–352. [Google Scholar] [CrossRef]
- Ghazanfari, S.; Tafazzoli-Shadpour, M.; Shokrgozar, M.A. Effects of cyclic stretch on proliferation of mesenchymal stem cells and their differentiation to smooth muscle cells. Biochem. Biophys. Res. Commun. 2009, 388, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Maul, T.; Chew, D.W.; Nieponice, A.; Vorp, D.A. Mechanical stimuli differentially control stem cell behavior: Morphology, proliferation, and differentiation. Biomech. Model. Mechanobiol. 2011, 10, 939–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.-J.; Sun, J.; Lu, S.; Qi, Y.-X.; Wang, Y. Prolonged Mechanical Stretch Initiates Intracellular Calcium Oscillations in Human Mesenchymal Stem Cells. PLoS ONE 2014, 9, e109378. [Google Scholar] [CrossRef] [Green Version]
- Rothdiener, M.; Hegemann, M.; Uynuk-Ool, T.; Walters, B.; Papugy, P.; Nguyen, P.; Claus, V.; Seeger, T.; Stoeckle, U.; Boehme, K.A.; et al. Stretching human mesenchymal stromal cells on stiffness-customized collagen type I generates a smooth muscle marker profile without growth factor addition. Sci. Rep. 2016, 6, 35840. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Tafazzoli-Shadpour, M.; Shokrgozar, M.A.; Janmaleki, M.; Teymoori, M. Cyclic Stretch Effects on Adipose-Derived Stem Cell Stiffness, Morphology and Smooth Muscle Cell Gene Expression. Tissue Eng. Regen. Med. 2017, 14, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Kanda, K.; Matsuda, T.; Oka, T. Two-dimensional Orientational Response of Smooth Muscle Cells to Cyclic Stretching. ASAIO J. 1992, 14, 382–385. [Google Scholar] [CrossRef]
- Liu, B.; Qu, M.-J.; Qin, K.-R.; Li, H.; Li, Z.-K.; Shen, B.-R.; Jiang, Z.-L. Role of Cyclic Strain Frequency in Regulating the Alignment of Vascular Smooth Muscle Cells In Vitro. Biophys. J. 2008, 94, 1497–1507. [Google Scholar] [CrossRef] [Green Version]
- Wanjare, M.; Agarwal, N.; Gerecht, S. Biomechanical strain induces elastin and collagen production in human pluripotent stem cell-derived vascular smooth muscle cells. Am. J. Physiol. Physiol. 2015, 309, C271–C281. [Google Scholar] [CrossRef] [Green Version]
- Kurpinski, K.; Park, J.; Thakar, R.G.; Li, S. Regulation of vascular smooth muscle cells and mesenchymal stem cells by mechanical strain. Mol. Cell Biomech. 2006, 3, 21–34. [Google Scholar]
- Walters, B.; Uynuk-Ool, T.; Rothdiener, M.; Palm, J.; Hart, M.L.; Stegemann, J.P.; Rolauffs, B. Engineering the geometrical shape of mesenchymal stromal cells through defined cyclic stretch regimens. Sci. Rep. 2017, 7, 6640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wang, P.; Wei, B.; Mo, X.; Cui, F. Electrospun collagen–chitosan nanofiber: A biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 2010, 6, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Sun, M.B.; Lee, J.; Abdeen, A.A.; Kilian, K.A. C ell shape and the presentation of adhesion ligands guide smooth muscle myogenesis. J. Biomed. Mater. Res. Part A 2016, 104, 1212–1220. [Google Scholar] [CrossRef]
- Lee, J.; Abdeen, A.; Zhang, D.; Kilian, K.A. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 2013, 34, 8140–8148. [Google Scholar] [CrossRef]
- Yang, Y.; Relan, N.; Przywara, D.; Schuger, L. Embryonic mesenchymal cells share the potential for smooth muscle differentiation: Myogenesis is controlled by the cell’s shape. Development 1999, 126, 3027–3033. [Google Scholar] [CrossRef]
- Tay, C.Y.; Yu, H.; Pal, M.; Leong, W.S.; Tan, N.S.; Ng, K.W.; Leong, D.T.; Tan, L.P. Micropatterned matrix directs differentiation of human mesenchymal stem cells towards myocardial lineage. Exp. Cell Res. 2010, 316, 1159–1168. [Google Scholar] [CrossRef]
- Matsuoka, F.; Takeuchi, I.; Agata, H.; Kagami, H.; Shiono, H.; Kiyota, Y.; Honda, H.; Kato, R. Morphology-Based Prediction of Osteogenic Differentiation Potential of Human Mesenchymal Stem Cells. PLoS ONE 2013, 8, e55082. [Google Scholar] [CrossRef]
- Matsuoka, F.; Takeuchi, I.; Agata, H.; Kagami, H.; Shiono, H.; Kiyota, Y.; Honda, H.; Kato, R. Characterization of time-course morphological features for efficient prediction of osteogenic potential in human mesenchymal stem cells. Biotechnol. Bioeng. 2014, 111, 1430–1439. [Google Scholar] [CrossRef] [Green Version]
- Uynuk-Ool, T.; Rothdiener, M.; Walters, B.; Hegemann, M.; Palm, J.; Nguyen, P.; Seeger, T.; Stöckle, U.; Stegemann, J.P.; Aicher, W.K.; et al. The geometrical shape of mesenchymal stromal cells measured by quantitative shape descriptors is determined by the stiffness of the biomaterial and by cyclic tensile forces. J. Tissue Eng. Regen. Med. 2017, 11, 3508–3522. [Google Scholar] [CrossRef] [PubMed]
- Stegemann, J.P.; Nerem, R.M. Phenotype Modulation in Vascular Tissue Engineering Using Biochemical and Mechanical Stimulation. Ann. Biomed. Eng. 2003, 31, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Peterson, A.W.; Ceccarelli, J.; Putnam, A.; Stegemann, J.P. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 2012, 15, 253–264. [Google Scholar] [CrossRef] [PubMed]
- El-Taji, O.M.; Khattak, A.Q.; Hussain, S. Bladder reconstruction: The past, present and future. Oncol. Lett. 2015, 10, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaj, S.; Liu, G.; Shi, Y.; Markert, C.; Andersson, K.-E.; Atala, A.; Zhang, Y. Characterization of Urine-Derived Stem Cells Obtained from Upper Urinary Tract for Use in Cell-Based Urological Tissue Engineering. Tissue Eng. Part A 2011, 17, 2123–2132. [Google Scholar] [CrossRef]
- Andersson, K.-E.; Arner, A. Urinary Bladder Contraction and Relaxation: Physiology and Pathophysiology. Physiol. Rev. 2004, 84, 935–986. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Hart, M.L.; Rolauffs, B.; Stegemann, J.P.; Annamalai, R.T. Bioresponsive microspheres for on-demand delivery of anti-inflammatory cytokines for articular cartilage repair. J. Biomed. Mater. Res. Part A 2020, 108, 722–733. [Google Scholar] [CrossRef]
- Gomes, L.R.; Terra, L.F.; Wailemann, R.A.; Labriola, L.; Sogayar, M.C. TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer 2012, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Feng, C.; Hu, J.; Liu, C.; Liu, S.; Liao, G.; Song, L.; Zeng, X. Association of 17-β Estradiol with Adipose-Derived Stem Cells: New Strategy to Produce Functional Myogenic Differentiated Cells with a Nano-Scaffold for Tissue Engineering. PLoS ONE 2016, 11, e0164918. [Google Scholar] [CrossRef] [Green Version]
- Floren, M.; Bonani, W.; Dharmarajan, A.; Motta, A.; Migliaresi, C.; Tan, W. Human mesenchymal stem cells cultured on silk hydrogels with variable stiffness and growth factor differentiate into mature smooth muscle cell phenotype. Acta Biomater. 2016, 31, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Jiang, W.; Alraies, A.; Liu, Q.; Gudla, V.; Oni, J.; Wei, X.; Sloan, A.; Ni, L.; Agarwal, M. Bladder Smooth Muscle Cells Differentiation from Dental Pulp Stem Cells: Future Potential for Bladder Tissue Engineering. Stem Cells Int. 2016, 2016, 6979368. [Google Scholar] [CrossRef] [Green Version]
- Solorio, L.D.; Fu, A.S.; Hernández-Irizarry, R.; Alsberg, E. Chondrogenic differentiation of human mesenchymal stem cell aggregates via controlled release of TGF-β1 from incorporated polymer microspheres. J. Biomed. Mater. Res. Part A 2010, 92, 1139–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Yu, H.; Fan, C.; Kong, Q.; Liu, D.; Meng, L. Differentiate into urothelium and smooth muscle cells from adipose tissue-derived stem cells for ureter reconstruction in a rabbit model. Am. J. Transl. Res. 2016, 8, 3757–3768. [Google Scholar] [PubMed]
- ZZhang, X.; Bendeck, M.P.; Simmons, C.A.; Santerre, J.P. Deriving vascular smooth muscle cells from mesenchymal stromal cells: Evolving differentiation strategies and current understanding of their mechanisms. Biomaterials 2017, 145, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zohar, R.; McCulloch, C.A. Multiple roles of α-smooth muscle actin in mechanotransduction. Exp. Cell Res. 2006, 312, 205–214. [Google Scholar] [CrossRef]
- Lauer, J.; Selig, M.; Hart, M.; Kurz, B.; Rolauffs, B. Articular Chondrocyte Phenotype Regulation through the Cytoskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Understanding of the Intersection between Actin Dynamics and Chondrogenic Function. Int. J. Mol. Sci. 2021, 22, 3279. [Google Scholar] [CrossRef]
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; Van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef] [Green Version]
- Talele, N.; Fradette, J.; Davies, J.E.; Kapus, A.; Hinz, B. Expression of α-Smooth Muscle Actin Determines the Fate of Mesenchymal Stromal Cells. Stem Cell Rep. 2015, 4, 1016–1030. [Google Scholar] [CrossRef] [Green Version]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Bochaton-Piallat, M.-L.; Gabbiani, G.; Hinz, B. The myofibroblast in wound healing and fibrosis: Answered and unanswered questions. F1000Research 2016, 5, 752. [Google Scholar] [CrossRef] [Green Version]
- Desai, V.D.; Hsia, H.C.; Schwarzbauer, J.E. Reversible Modulation of Myofibroblast Differentiation in Adipose-Derived Mesenchymal Stem Cells. PLoS ONE 2014, 9, e86865. [Google Scholar] [CrossRef] [Green Version]
- Scharenberg, M.A.; Pippenger, B.; Sack, R.; Zingg, D.; Ferralli, J.; Schenk, S.; Martin, I.; Chiquet-Ehrismann, R. TGF-β-induced differentiation into myofibroblasts involves specific regulation of two MKL1 isoforms. J. Cell Sci. 2014, 127, 1079–1091. [Google Scholar] [CrossRef] [Green Version]
- Froese, A.R.; Shimbori, C.; Bellaye, P.S.; Inman, M.; Obex, S.; Fatima, S.; Jenkins, G.; Gauldie, J.; Kolb, M. Stretch Induced Activation of TGF-β1 in Pulmonary Fibrosis. Am. J. Respitory Crit. Care. 2016, 194, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, F.; Chow, M.L.; Koehler, A.; Boo, S.; Buscemi, L.; Quinn, T.M.; Costell, M.; Alman, B.A.; Genot, E.; Hinz, B. Prestress in the extracellular matrix sensitizes latent TGF-β1 for activation. J. Cell Biol. 2014, 207, 283–297. [Google Scholar] [CrossRef]
- Van De Water, L.; Varney, S.; Tomasek, J.J. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv. Wound Care 2013, 2, 122–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girão-Silva, T.; Bassaneze, V.; Campos, L.C.G.; Barauna, V.G.; Dallan, L.A.O.; Krieger, J.E.; Miyakawa, A.A. Short-term mechanical stretch fails to differentiate human adipose-derived stem cells into cardiovascular cell phenotypes. Biomed. Eng. Online 2014, 13, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walters, B.; Turner, P.A.; Rolauffs, B.; Hart, M.L.; Stegemann, J.P. Controlled Growth Factor Delivery and Cyclic Stretch Induces a Smooth Muscle Cell-like Phenotype in Adipose-Derived Stem Cells. Cells 2021, 10, 3123. https://doi.org/10.3390/cells10113123
Walters B, Turner PA, Rolauffs B, Hart ML, Stegemann JP. Controlled Growth Factor Delivery and Cyclic Stretch Induces a Smooth Muscle Cell-like Phenotype in Adipose-Derived Stem Cells. Cells. 2021; 10(11):3123. https://doi.org/10.3390/cells10113123
Chicago/Turabian StyleWalters, Brandan, Paul A. Turner, Bernd Rolauffs, Melanie L. Hart, and Jan P. Stegemann. 2021. "Controlled Growth Factor Delivery and Cyclic Stretch Induces a Smooth Muscle Cell-like Phenotype in Adipose-Derived Stem Cells" Cells 10, no. 11: 3123. https://doi.org/10.3390/cells10113123
APA StyleWalters, B., Turner, P. A., Rolauffs, B., Hart, M. L., & Stegemann, J. P. (2021). Controlled Growth Factor Delivery and Cyclic Stretch Induces a Smooth Muscle Cell-like Phenotype in Adipose-Derived Stem Cells. Cells, 10(11), 3123. https://doi.org/10.3390/cells10113123