Intra-Tumoral Nerve-Tracing in a Novel Syngeneic Model of High-Grade Serous Ovarian Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Immunohistochemistry (IHC)
2.2. Scoring of IHC Staining
2.3. Double Immunofluorescent Staining
2.4. Ovarian Cancer Tumor Model
2.5. Preparation and Injection of Trp53 -/- Pten -/- Cell Lines for Innervation Studies
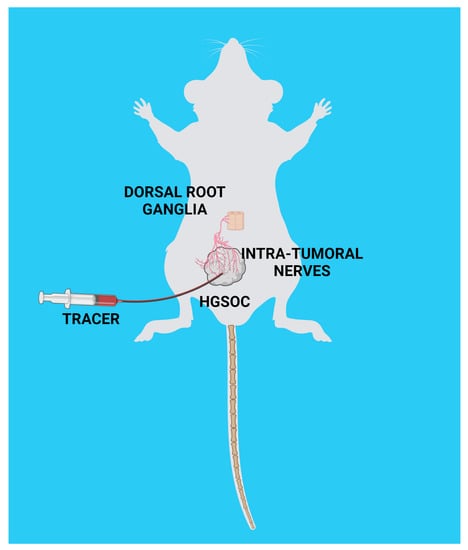
2.6. Ultrasound-Guided Injection of Axonal Tracer and Collection of Peripheral Ganglia
2.7. Human Studies
3. Results
3.1. Sensory Nerves Innervate HGSOCs
3.2. Syngeneic Model of HGSOC
3.3. Nerve Tracing in HGSOC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, L.; Cui, C.; Shi, O.; Lu, X.; Li, Y.-K.; Wang, W.; Li, Y.; Wang, Q. Incidence and mortality of ovarian cancer at the global, regional, and national levels, 1990–2017. Gynecol. Oncol. 2020, 159, 239–247. [Google Scholar] [CrossRef]
- Wild, C.; Weiderpass, E.; Stewart, B. (Eds.) World Cancer Report: Cancer Research for Cancer Prevention; IARC: Lyon, France, 2020. [Google Scholar]
- Jacobs, I.; Menon, U. Progress and Challenges in Screening for Early Detection of Ovarian Cancer. Mol. Cell. Proteom. 2004, 3, 355–366. [Google Scholar] [CrossRef] [Green Version]
- Badgwell, D.; Bast, R.C., Jr. Early detection of ovarian cancer. Dis. Markers 2007, 23, 397–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, N.E.; Woodman, M.; DiSilvestro, P.A.; Ribeiro, J.R. The Perfect Combination: Enhancing Patient Response to PD-1-Based Therapies in Epithelial Ovarian Cancer. Cancers 2020, 12, 2150. [Google Scholar] [CrossRef]
- Iyer, S.; Zhang, S.; Yucel, S.; Horn, H.; Smith, S.G.; Reinhardt, F.; Hoefsmit, E.; Assatova, B.; Casado, J.; Meinsohn, M.-C.; et al. Genetically Defined Syngeneic Mouse Models of Ovarian Cancer as Tools for the Discovery of Combination Immunotherapy. Cancer Discov. 2021, 11, 384–407. [Google Scholar] [CrossRef]
- Zhang, S.; Iyer, S.; Ran, H.; Dolgalev, I.; Gu, S.; Wei, W.; Foster, C.J.; Loomis, C.A.; Olvera, N.; Dao, F.; et al. Genetically Defined, Syngeneic Organoid Platform for Developing Combination Therapies for Ovarian Cancer. Cancer Discov. 2021, 11, 362–383. [Google Scholar] [CrossRef] [PubMed]
- Maniati, E.; Berlato, C.; Gopinathan, G.; Heath, O.; Kotantaki, P.; Lakhani, A.; McDermott, J.; Pegrum, C.; Delaine-Smith, R.M.; Pearce, O.; et al. Mouse Ovarian Cancer Models Recapitulate the Human Tumor Microenvironment and Patient Response to Treatment. Cell Rep. 2020, 30, 525–540.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017, 8, 1093. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Park, E.Y.; Klinkebiel, D.L.; Pack, S.D.; Shin, Y.-H.; Abdullaev, Z.; Emerson, R.E.; Coffey, D.M.; Kwon, S.Y.; Creighton, C.J.; et al. In vivo modeling of metastatic human high-grade serous ovarian cancer in mice. PLoS Genet. 2020, 16, e1008808. [Google Scholar] [CrossRef]
- Perets, R.; Drapkin, R. It’s Totally Tubular…Riding The New Wave of Ovarian Cancer Research. Cancer Res. 2016, 76, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Kroeger, P.T., Jr.; Drapkin, R. Pathogenesis and heterogeneity of ovarian cancer. Curr. Opin. Obstet. Gynecol. 2017, 29, 26–34. [Google Scholar] [CrossRef]
- Gysler, S.M.; Drapkin, R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef]
- Saloman, J.L.; Albers, K.M.; Rhim, A.D.; Davis, B. Can Stopping Nerves, Stop Cancer? Trends Neurosci. 2016, 39, 880–889. [Google Scholar] [CrossRef] [Green Version]
- Zahalka, A.H.; Frenette, P.S. Nerves in cancer. Nat. Rev. Cancer 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Li, Z.J.; Cho, C.H. Neurotransmitters, more than meets the eye—Neurotransmitters and their perspectives in cancer development and therapy. Eur. J. Pharmacol. 2011, 667, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Reavis, H.D.; Chen, H.I.; Drapkin, R. Tumor Innervation: Cancer Has Some Nerve. Trends Cancer 2020, 6, 1059–1067. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic Nerve Development Contributes to Prostate Cancer Progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, A.; Hayama, Y.; Kato, S.; Shimomura, A.; Shimomura, T.; Irie, K.; Kaneko, R.; Yanagawa, Y.; Kobayashi, K.; Ochiya, T. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat. Neurosci. 2019, 22, 1289–1305. [Google Scholar] [CrossRef] [PubMed]
- Keskinov, A.A.; Tapias, V.; Watkins, S.C.; Ma, Y.; Shurin, M.R.; Shurin, G.V. Impact of the Sensory Neurons on Melanoma Growth In Vivo. PLoS ONE 2016, 11, e0156095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.-M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [Green Version]
- Renz, B.W.; Tanaka, T.; Sunagawa, M.; Takahashi, R.; Jiang, Z.; Macchini, M.; Dantes, Z.; Valenti, G.; White, R.A.; Middelhoff, M.A.; et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018, 8, 1458–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papka, R.; Cotton, J.; Traurig, H. Comparative distribution of neuropeptide tyrosine-,vasoactive intestinal polypeptide-, substance P-immunoreactive, acetylcholinesterase-positive and noradrenergic nerves in the reproductive tract of the female rat. Z. Für Zellforsch. Und Mikrosk. Anat. 1985, 242, 475–490. [Google Scholar] [CrossRef]
- Burden, H.W.; Zary, J.T. Localization of calretinin in the rat ovary and in relation to nerve cell bodies in dorsal root and paravertebral ganglia projecting to the ovary. Microsc. Res. Tech. 2002, 59, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Pastelín, C.F.; Rosas, N.H.; Morales-Ledesma, L.; Linares, R.; Domínguez, R.; Morán, C. Anatomical organization and neural pathways of the ovarian plexus nerve in rats. J. Ovarian Res. 2017, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Brauer, M.M.; Smith, P.G. Estrogen and female reproductive tract innervation: Cellular and molecular mechanisms of autonomic neuroplasticity. Auton. Neurosci. 2015, 187, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Borges, L.F.; Sidman, R.L. Axonal transport of lectins in the peripheral nervous system. J. Neurosci. 1982, 2, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Dumas, M.; Schwab, M.E.; Thoenen, H. Retrograde axonal transport of specific macromolecules as a tool for characterizing nerve terminal membranes. J. Neurobiol. 1979, 10, 179–197. [Google Scholar] [CrossRef]
- Fabian, R.H.; Coulter, J.D. Transneuronal transport of lectins. Brain Res. 1985, 344, 41–48. [Google Scholar] [CrossRef]
- Rousselot, P.; Poulain, D.A.; Theodosis, D.T. Ultrastructural visualization and neurochemical characterization of spinal projections of primary sensory afferents from the nipple: Combined use of transganglionic transport of HRP-WGA and glutamate immunocytochemistry. J. Histochem. Cytochem. 1994, 42, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Ciriello, J.; Caverson, M.M. Effect of estrogen on vagal afferent projections to the brainstem in the female. Brain Res. 2016, 1636, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Itaya, S.K.; van Hoesen, G.W. WGA-HRP as a transneuronal marker in the visual pathways of monkey and rat. Brain Res. 1982, 236, 199–204. [Google Scholar] [CrossRef]
- Levy, S.; White, J.J.; Lackey, E.P.; Schwartz, L.; Sillitoe, R.V. WGA—Alexa Conjugates for Axonal Tracing. Curr. Protoc. Neurosci. 2017, 79, 1.28.1–1.28.24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karst, A.M.; Levanon, K.; Drapkin, R. Modeling high-grade serous ovarian carcinogenesis from the fallopian tube. Proc. Natl. Acad. Sci. USA 2011, 108, 7547–7552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karst, A.M.; Drapkin, R. Primary culture and immortalization of human fallopian tube secretory epithelial cells. Nat. Protoc. 2012, 7, 1755–1764. [Google Scholar] [CrossRef]
- Walton, J.; Blagih, J.; Ennis, D.; Leung, E.; Dowson, S.; Farquharson, M.; Tookman, L.A.; Orange, C.; Athineos, D.; Mason, S.; et al. CRISPR/Cas9-Mediated Trp53 and Brca2 Knockout to Generate Improved Murine Models of Ovarian High-Grade Serous Carcinoma. Cancer Res. 2016, 76, 6118–6129. [Google Scholar] [CrossRef] [Green Version]
- Malin, S.A.; Davis, B.; Molliver, D. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat. Protoc. 2007, 2, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; de Araujo, I.E. Dissection and surgical approaches to the mouse jugular-nodose ganglia. STAR Protoc. 2021, 2, 100474. [Google Scholar] [CrossRef] [PubMed]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef] [Green Version]
- Lucido, C.; Wynja, E.; Madeo, M.; Williamson, C.S.; Schwartz, L.E.; Imblum, B.A.; Drapkin, R.; Vermeer, P.D. Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecol. Oncol. 2019, 154, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Ferrandina, G.; Zannoni, G.F.; Martinelli, E.; Paglia, A.; Gallotta, V.; Mozzetti, S.; Scambia, G.; Ferlini, C. Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin. Cancer Res. 2006, 12, 2774–2779. [Google Scholar] [CrossRef] [Green Version]
- Perets, R.; Wyant, G.A.; Muto, K.W.; Bijron, J.G.; Poole, B.B.; Chin, K.T.; Chen, J.Y.H.; Ohman, A.; Stepule, C.D.; Kwak, S.; et al. Transformation of the Fallopian Tube Secretory Epithelium Leads to High-Grade Serous Ovarian Cancer in Brca;Tp53;Pten Models. Cancer Cell 2013, 24, 751–765. [Google Scholar] [CrossRef] [Green Version]
- McCool, K.W.; Freeman, Z.; Zhai, Y.; Wu, R.; Hu, K.; Liu, C.-J.; Tomlins, S.A.; Fearon, E.R.; Magnuson, B.; Kuick, R.; et al. Murine Oviductal High-Grade Serous Carcinomas Mirror the Genomic Alterations, Gene Expression Profiles, and Immune Microenvironment of Their Human Counterparts. Cancer Res. 2020, 80, 877–889. [Google Scholar] [CrossRef]
- Zhai, Y.; Wu, R.; Kuick, R.; Sessine, M.S.; Schulman, S.; Green, M.; Fearon, E.R.; Cho, K.R. High-grade serous carcinomas arise in the mouse oviduct via defects linked to the human disease. J. Pathol. 2017, 243, 16–25. [Google Scholar] [CrossRef]
- Wu, R.-C.; Wang, P.; Lin, S.-F.; Zhang, M.; Song, Q.; Chu, T.; Wang, B.G.; Kurman, R.J.; Vang, R.; Kinzler, K.; et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J. Pathol. 2019, 248, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Ducie, J.; Dao, F.; Considine, M.; Olvera, N.; Shaw, P.A.; Kurman, R.J.; Shih, I.-M.; Soslow, R.A.; Cope, L.; Levine, D.A. Molecular analysis of high-grade serous ovarian carcinoma with and without associated serous tubal intra-epithelial carcinoma. Nat. Commun. 2017, 8, 990. [Google Scholar] [CrossRef]
- Eckert, M.; Pan, S.; Hernandez, K.M.; Loth, R.M.; Andrade, J.; Volchenboum, S.L.; Faber, P.; Montag, A.; Lastra, R.; Peter, M.E.; et al. Genomics of Ovarian Cancer Progression Reveals Diverse Metastatic Trajectories Including Intraepithelial Metastasis to the Fallopian Tube. Cancer Discov. 2016, 6, 1342–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindelberger, D.W.; Lee, Y.; Miron, A.; Hirsch, M.S.; Feltmate, C.; Medeiros, F.; Callahan, M.J.; Garner, E.O.; Gordon, R.W.; Birch, C.; et al. Intraepithelial Carcinoma of the Fimbria and Pelvic Serous Carcinoma: Evidence for a Causal Relationship. Am. J. Surg. Pathol. 2007, 31, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Miron, A.; Drapkin, R.; Nucci, M.R.; Medeiros, F.; Saleemuddin, A.; Garber, J.; Birch, C.; Mou, H.; Gordon, R.W.; et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol. 2007, 211, 26–35. [Google Scholar] [CrossRef]
- Shih, I.-M.; Wang, Y.; Wang, T.-L. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am. J. Pathol. 2021, 191, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Skírnisdóttir, I.; Seidal, T. Prognostic Impact of Concomitant p53 and PTEN on Outcome in Early Stage (FIGO I-II) Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2011, 21, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar]
- Roh, M.H.; Yassin, Y.; Miron, A.; Mehra, K.K.; Mehrad, M.; Monte, N.M.; Mutter, G.L.; Nucci, M.R.; Ning, G.; Mckeon, F.D.; et al. High-grade fimbrial-ovarian carcinomas are unified by altered p53, PTEN and PAX2 expression. Mod. Pathol. 2010, 23, 1316–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCallum, G.A.; Shiralkar, J.; Suciu, D.; Covarrubias, G.; Yu, J.S.; Karathanasis, E.; Durand, D.M. Chronic neural activity recorded within breast tumors. Sci. Rep. 2020, 10, 14824. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.; Dietrich, A.; Kasparek, M.S.; Benhaqi, P.; Schneider, M.R.; Schemann, M.; Seeliger, H.; Kreis, M.E. Extrinsic intestinal denervation modulates tumor development in the small intestine of ApcMin/+ mice. J. Exp. Clin. Cancer Res. 2015, 34, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Erin, N.; Barkan, G.A.; Harms, J.F.; Clawson, G.A. Vagotomy enhances experimental metastases of 4THMpc breast cancer cells and alters Substance P level. Regul. Pept. 2008, 151, 35–42. [Google Scholar] [CrossRef]
- Erin, N.; Duymuş, Ö.; Öztürk, S.; Demir, N. Activation of vagus nerve by semapimod alters substance P levels and decreases breast cancer metastasis. Regul. Pept. 2012, 179, 101–108. [Google Scholar] [CrossRef]
- Shiers, S.; Klein, R.M.; Price, T.J. Quantitative differences in neuronal subpopulations between mouse and human dorsal root ganglia demonstrated with RNAscope in situ hybridization. Pain 2020, 161, 2410–2424. [Google Scholar] [CrossRef]
- Devor, M.; Janig, W.; Michaelis, M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J. Neurophysiol. 1994, 71, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, D.; Hurtado, M.L.; De Gregorio, C.; Oñate, M.; Martínez, G.; Catenaccio, A.; Wishart, T.M.; Court, F.A. Collateral Sprouting of Peripheral Sensory Neurons Exhibits a Unique Transcriptomic Profile. Mol. Neurobiol. 2020, 57, 4232–4249. [Google Scholar] [CrossRef] [PubMed]
- Sverrisdottir, Y.B.; Martin, S.C.; Hadjipavlou, G.; Kent, A.R.; Paterson, D.J.; FitzGerald, J.J.; Green, A.L. Human Dorsal Root Ganglion Stimulation Reduces Sympathetic Outflow and Long-Term Blood Pressure. JACC Basic Transl. Sci. 2020, 5, 973–985. [Google Scholar] [CrossRef]
- Utzschneider, D.; Kocsis, J.; Devor, M. Mutual excitation among dorsal root ganglion neurons in the rat. Neurosci. Lett. 1992, 146, 53–56. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barr, J.L.; Kruse, A.; Restaino, A.C.; Tulina, N.; Stuckelberger, S.; Vermeer, S.J.; Williamson, C.S.; Vermeer, D.W.; Madeo, M.; Stamp, J.; et al. Intra-Tumoral Nerve-Tracing in a Novel Syngeneic Model of High-Grade Serous Ovarian Carcinoma. Cells 2021, 10, 3491. https://doi.org/10.3390/cells10123491
Barr JL, Kruse A, Restaino AC, Tulina N, Stuckelberger S, Vermeer SJ, Williamson CS, Vermeer DW, Madeo M, Stamp J, et al. Intra-Tumoral Nerve-Tracing in a Novel Syngeneic Model of High-Grade Serous Ovarian Carcinoma. Cells. 2021; 10(12):3491. https://doi.org/10.3390/cells10123491
Chicago/Turabian StyleBarr, Jeffrey L., Allison Kruse, Anthony C. Restaino, Natalia Tulina, Sarah Stuckelberger, Samuel J. Vermeer, Caitlin S. Williamson, Daniel W. Vermeer, Marianna Madeo, Jillian Stamp, and et al. 2021. "Intra-Tumoral Nerve-Tracing in a Novel Syngeneic Model of High-Grade Serous Ovarian Carcinoma" Cells 10, no. 12: 3491. https://doi.org/10.3390/cells10123491
APA StyleBarr, J. L., Kruse, A., Restaino, A. C., Tulina, N., Stuckelberger, S., Vermeer, S. J., Williamson, C. S., Vermeer, D. W., Madeo, M., Stamp, J., Bell, M., Morgan, M., Yoon, J. -Y., Mitchell, M. A., Budina, A., Omran, D. K., Schwartz, L. E., Drapkin, R., & Vermeer, P. D. (2021). Intra-Tumoral Nerve-Tracing in a Novel Syngeneic Model of High-Grade Serous Ovarian Carcinoma. Cells, 10(12), 3491. https://doi.org/10.3390/cells10123491