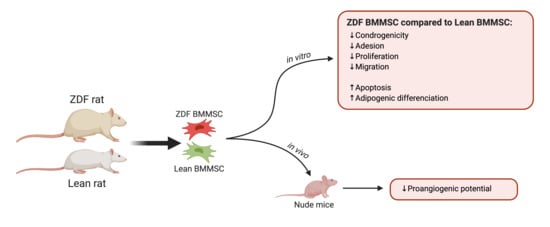
Experimental Type 2 Diabetes Differently Impacts on the Select Functions of Bone Marrow-Derived Multipotent Stromal Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Isolation of Rat Bone Marrow-Derived Multipotent Stromal Cells
2.3. Fibroblastic Colony-Forming Unit (CFU-F) Assay
2.4. Cell Adhesion
2.5. Cell Proliferation
2.6. Apoptosis
2.7. Cell Migration
2.8. Reactive Oxygen Species (ROS) Release
2.9. Differentiation Assays
2.9.1. Adipogenic Differentiation
2.9.2. Osteogenic Differentiation
2.9.3. Angiogenic Differentiation and Potential
2.10. Matrigel Plug Assay
2.11. Quantitative Polymerase Chain Reaction (qRT-PCR)
2.12. Statistical Analyses
3. Results
3.1. Animals
3.2. T2DM Affects the Number of Bone Marrow Mononuclear Cells and Select Functions of the Expanded BMMSCs
3.3. T2DM Differently Affects the Differentiation Potential of the In Vitro BMMSCs
3.4. T2DM Reduces the BMMSC Angiogenic Potential
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, A.V. Marrow Fat and Bone: Review of Clinical Findings. Front. Endocrinol. 2015, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2013, 13, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Marin, C.; Luyten, F.P.; Van Der Schueren, B.; Kerckhofs, G.; Vandamme, K. The Impact of Type 2 Diabetes on Bone Fracture Healing. Front. Endocrinol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Caliaperoumal, G.; Souyet, M.; Bensidhoum, M.; Petite, H.; Anagnostou, F. Type 2 diabetes impairs angiogenesis and osteogenesis in calvarial defects: MicroCT study in ZDF rats. Bone 2018, 112, 161–172. [Google Scholar] [CrossRef]
- Spinetti, G.; Cordella, D.; Fortunato, O.; Sangalli, E.; Losa, S.; Gotti, A.; Carnelli, F.; Rosa, F.; Riboldi, S.; Sessa, F.; et al. Global remodeling of the vascular stem cell niche in bone marrow of diabetic patients: Implication of the microRNA-155/FOXO3a signaling pathway. Circ. Res. 2013, 112, 510–522. [Google Scholar] [CrossRef] [Green Version]
- Piccinin, M.A.; Khan, Z.A. Pathophysiological role of enhanced bone marrow adipogenesis in diabetic complications. Adipocyte 2014, 3, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.Y.; Schafer, A.L. Diabetes and Bone Marrow Adiposity. Curr. Osteoporos. Rep. 2016, 14, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxson, S.; Lopez, E.A.; Yoo, D.; Danilkovitch-Miagkova, A.; Leroux, M.A. Concise Review: Role of Mesenchymal Stem Cells in Wound Repair. Stem Cells Transl. Med. 2012, 1, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Watt, S.M.; Gullo, F.; Van Der Garde, M.; Markeson, D.; Camicia, R.; Khoo, C.P.; Zwaginga, J.J. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br. Med. Bull. 2013, 108, 25–53. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.R.; Kim, J.W.; Jun, H.S.; Roh, J.Y.; Lee, H.E.; Hong, I.S. Stem Cell Secretome and Its Effect on Cellular Mechanisms Relevant to Wound Healing. Mol. Ther. 2017, 26, 606–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fijany, A.; Sayadi, L.R.; Khoshab, N.; Banyard, D.A.; Shaterian, A.; Alexander, M.; Lakey, J.R.T.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Mesenchymal stem cell dysfunction in diabetes. Mol. Biol. Rep. 2019, 46, 1459–1475. [Google Scholar] [CrossRef] [PubMed]
- Van De Vyver, M. Intrinsic Mesenchymal Stem Cell Dysfunction in Diabetes Mellitus: Implications for Autologous Cell Therapy. Stem Cells Dev. 2017, 26, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.; Abu-Shahba, N.; Azmy, O.; El-Badri, N. Impact of Diabetes Mellitus on Human Mesenchymal Stromal Cell Biology and Functionality: Implications for Autologous Transplantation. Stem Cell Rev. Rep. 2019, 15, 194–217. [Google Scholar] [CrossRef] [PubMed]
- Davey, G.C.; Patil, S.B.; O’Loughlin, A.; O’Brien, T. Mesenchymal Stem Cell-Based Treatment for Microvascular and Secondary Complications of Diabetes Mellitus. Front. Endocrinol. 2013, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Gang, X.; Sun, C.; Wang, G. Mesenchymal Stem Cells Improve Healing of Diabetic Foot Ulcer. J. Diabetes Res. 2017, 2017, 9328347. [Google Scholar] [CrossRef]
- Soria-Juan, B.; Escacena, N.; Capilla-González, V.; Aguilera, Y.; Llanos, L.; Tejedo, J.R.; Bedoya, F.J.; Juan, V.; De La Cuesta, A.; Ruiz-Salmerón, R.; et al. Cost-Effective, Safe, and Personalized Cell Therapy for Critical Limb Ischemia in Type 2 Diabetes Mellitus. Front. Immunol. 2019, 10, 1151. [Google Scholar] [CrossRef]
- Etgen, G.; Oldham, B. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism 2000, 49, 684–688. [Google Scholar] [CrossRef]
- Reinwald, S.; Peterson, R.G.; Allen, M.R.; Burr, D.B. Skeletal changes associated with the onset of type 2 diabetes in the ZDF and ZDSD rodent models. Am. J. Physiol. Metab. 2009, 296, E765–E774. [Google Scholar] [CrossRef] [Green Version]
- Prisby, R.D.; Swift, J.M.; Bloomfield, S.A.; Hogan, H.A.; Delp, M.D. Altered bone mass, geometry and mechanical properties during the development and progression of type 2 diabetes in the Zucker diabetic fatty rat. J. Endocrinol. 2008, 199, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Stabley, J.N.; Prisby, R.D.; Behnke, B.J.; Delp, M.D. Type 2 diabetes alters bone and marrow blood flow and vascular control mechanisms in the ZDF rat. J. Endocrinol. 2015, 225, 47–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeitoun, D.; Caliaperoumal, G.; Bensidhoum, M.; Constans, J.M.; Anagnostou, F.; Bousson, V. Microcomputed tomography of the femur o diabetic rats: Alterations of trabecular and cortical bone microarchitecture and vasculature: A feasibility study. Eur. Radiol. Exp. 2019, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Ribot, J.; Caliaperoumal, G.; Paquet, J.; Boisson-Vidal, C.; Petite, H.; Anagnostou, F. Type 2 diabetes alters mesenchymal stem cell secretome composition and angiogenic properties. J. Cell. Mol. Med. 2017, 21, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Shin, L.; Peterson, D.A. Impaired therapeutic capacity of autologous stem cells in a model of type 2 diabetes. Stem Cells Transl. Med. 2012, 1, 125–135. [Google Scholar] [CrossRef]
- Weinberg, E.; Maymon, T.; Weinreb, M. AGEs induce caspase-mediated apoptosis of rat BMSCs via TNFα production and oxidative stress. J. Mol. Endocrinol. 2013, 52, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Liu, N.; Shi, H.; Wu, H.; Gao, Y.; He, H.; Gu, B.; Liu, H. High glucose microenvironments inhibit the proliferation and migration of bone mesenchymal stem cells by activating GSK3β. J. Bone Miner. Metab. 2016, 34, 140–150. [Google Scholar] [CrossRef]
- Raghavan, S.; Malayaperumal, S.; Mohan, V.; Balasubramanyam, M. A comparative study on the cellular stressors in mesenchymal stem cells (MSCs) and pancreatic β-cells under hyperglycemic milieu. Mol. Cell. Biochem. 2020, 1–13. [Google Scholar] [CrossRef]
- Stolzing, A.; Sellers, D.J.; Llewelyn, O.; Scutt, A. Diabetes Induced Changes in Rat Mesenchymal Stem Cells. Cells Tissues Organs 2010, 191, 453–465. [Google Scholar] [CrossRef]
- Zhao, Y.-F.; Zeng, D.-L.; Xia, L.; Zhang, S.-M.; Xu, L.-Y.; Jiang, X.; Zhang, F. Osteogenic potential of bone marrow stromal cells derived from streptozotocin-induced diabetic rats. Int. J. Mol. Med. 2013, 31, 614–620. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kang, H.J.; Hong, M.H.; Kang, W.S.; Choe, N.; Kook, H.; Jeong, M.H.; Ahn, Y. Angiopoietin-Like 4 Is Involved in the Poor Angiogenic Potential of High Glucose-Insulted Bone Marrow Stem Cells. Korean Circ. J. 2014, 44, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.C.; Sampaio, P.; Fernandes, M.H.; Gomes, P.S. The Osteogenic Priming of Mesenchymal Stem Cells is Impaired in Experimental Diabetes. J. Cell. Biochem. 2015, 116, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.I.; Coimbra, L.S.; Tian, C.; Alblowi, J.; Kayal, R.A.; Einhorn, T.A.; Gerstenfeld, L.C.; Pignolo, R.L.; Graves, D.V. Diabetes reduces mesenchymal stem cells in fracture healing through a TNFalpha-mediated mechanism. Diabetologia 2015, 58, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, M.A.; Zhang, E.; Natarajan, R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia 2015, 58, 443–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, C.; Tonks, K.T.; Center, J.R.; Samocha-Bonet, D.; Greenfield, J.R. Complex interplay among adiposity, insulin resistance and bone health. Clin. Obes. 2018, 8, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, S.V.; Renovato-Martins, M.; Ribeiro-Pereira, C.; Citelli, M.; Barja-Fidalgo, C. Obesity modifies bone marrow microenvironment and directs bone marrow mesenchymal cells to adipogenesis. Obesity 2016, 24, 2522–2532. [Google Scholar] [CrossRef]
- Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Gödtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Smieszek, A.; Kornicka-Garbowska, K.; Szłapka-Kosarzewska, J.; Androvic, P.; Valihrach, L.; Langerova, L.; Rohlova, E.; Kubista, M.; Marycz, K. Metformin Increases Proliferative Activity and Viability of Multipotent Stromal Stem Cells Isolated from Adipose Tissue Derived from Horses with Equine Metabolic Syndrome. Cells 2019, 8, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berezin, A.E. Metabolic memory phenomenon in diabetes mellitus: Achieving and perspectives. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S176–S183. [Google Scholar] [CrossRef]
- Delić, D.; Eisele, C.; Schmid, R.; Luippold, G.; Mayoux, E.; Grempler, R. Characterization of Micro-RNA Changes during the Progression of Type 2 Diabetes in Zucker Diabetic Fatty Rats. Int. J. Mol. Sci. 2016, 17, 665. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Zhang, X. The Profiling and Role of miRNAs in Diabetes Mellitus. J. Diabetes Clin. Res. 2019, 1, 5–23. [Google Scholar] [CrossRef]
- Alicka, M.; Major, P.; Wysocki, M.; Marycz, K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. J. Clin. Med. 2019, 8, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Tie, G.; Wang, S.; Messina, K.E.; Didato, S.; Guo, S.; Messina, L.M. Type 2 Diabetes Restricts Multipotency of Mesenchymal Stem Cells and Impairs Their Capacity to Augment Postischemic Neovascularization in db/db Mice. J. Am. Heart Assoc. 2012, 1, e002238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhira, S.L.; Challa, S.S.; Chalasani, M.; Nappanveethl, G.; Bhonde, R.R.; Ajumeera, R.; Venkatesan, V. Promise(s) of Mesenchymal Stem Cells as an In Vitro Model System to Depict Pre-Diabetic/Diabetic Milieu in WNIN/GR-Ob Mutant Rats. PLoS ONE 2012, 7, e48061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferland-McCollough, D.; Maselli, D.; Spinetti, G.; Sambataro, M.; Sullivan, N.; Blom, A.; Beltrami, A.P. MCP-1 Feedback Loop Between Adipocytes and Mesenchymal Stromal Cells Causes Fat Accumulation and Contributes to Hematopoietic Stem Cell Rarefaction in the Bone Marrow of Patients With Diabetes. Diabetes 2018, 67, 1380–1394. [Google Scholar] [CrossRef] [Green Version]
- Moseley, K.F.; Doyle, M.E.; De Beur, S.M.J. Diabetic serum from older women increases adipogenic differentiation in mesenchymal stem cells. Endocr. Res. 2018, 43, 155–165. [Google Scholar] [CrossRef]
- Brewster, L.P.; Robinson, S.; Wang, R.; Griffiths, S.; Li, H.; Peister, A.; Copland, I.; McDevitt, T.; Wang, R. Expansion and angiogenic potential of mesenchymal stem cells from patients with critical limb ischemia. J. Vasc. Surg. 2017, 65, 826–838.e1. [Google Scholar] [CrossRef] [Green Version]
- Rezaie, J.; Mehranjani, M.S.; Rahbarghazi, R.; Shariatzadeh, M.A. Angiogenic and Restorative Abilities of Human Mesenchymal Stem Cells Were Reduced Following Treatment with Serum From Diabetes Mellitus Type 2 Patients. J. Cell. Biochem. 2018, 119, 524–535. [Google Scholar] [CrossRef]
- Rezabakhsh, A.; Cheraghi, O.; Nourazarian, A.; Hassanpour, M.; Kazemi, M.; Ghaderi, S.; Faraji, E.; Rahbarghazi, R.; Avci, C.B.; Bagca, B.G.; et al. Type 2 Diabetes Inhibited Human Mesenchymal Stem Cells Angiogenic Response by Over-Activity of the Autophagic Pathway. J. Cell. Biochem. 2017, 118, 1518–1530. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Lin, C.-H.; Huang, T.H.; Chuang, S.-Y. In Vivo Rodent Models of Type 2 Diabetes and Their Usefulness for Evaluating Flavonoid Bioactivity. Nutrients 2019, 11, 530. [Google Scholar] [CrossRef] [Green Version]
- Engel, H.; Xiong, L.; Reichenberger, M.; Germann, G.; Roth, C.; Hirche, C. Rodent models of diet-induced type 2 diabetes mellitus: A literature review and selection guide. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 195–200. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribot, J.; Denoeud, C.; Frescaline, G.; Landon, R.; Petite, H.; Pavon-Djavid, G.; Bensidhoum, M.; Anagnostou, F. Experimental Type 2 Diabetes Differently Impacts on the Select Functions of Bone Marrow-Derived Multipotent Stromal Cells. Cells 2021, 10, 268. https://doi.org/10.3390/cells10020268
Ribot J, Denoeud C, Frescaline G, Landon R, Petite H, Pavon-Djavid G, Bensidhoum M, Anagnostou F. Experimental Type 2 Diabetes Differently Impacts on the Select Functions of Bone Marrow-Derived Multipotent Stromal Cells. Cells. 2021; 10(2):268. https://doi.org/10.3390/cells10020268
Chicago/Turabian StyleRibot, Jonathan, Cyprien Denoeud, Guilhem Frescaline, Rebecca Landon, Hervé Petite, Graciela Pavon-Djavid, Morad Bensidhoum, and Fani Anagnostou. 2021. "Experimental Type 2 Diabetes Differently Impacts on the Select Functions of Bone Marrow-Derived Multipotent Stromal Cells" Cells 10, no. 2: 268. https://doi.org/10.3390/cells10020268
APA StyleRibot, J., Denoeud, C., Frescaline, G., Landon, R., Petite, H., Pavon-Djavid, G., Bensidhoum, M., & Anagnostou, F. (2021). Experimental Type 2 Diabetes Differently Impacts on the Select Functions of Bone Marrow-Derived Multipotent Stromal Cells. Cells, 10(2), 268. https://doi.org/10.3390/cells10020268