Aquaglyceroporin-3’s Expression and Cellular Localization Is Differentially Modulated by Hypoxia in Prostate Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Flow Cytometry (AQP3 Expression and Cell Cycle)
2.3. mRNA Isolation and Quantitative RT–PCR
2.4. Immunocytofluorescence and Fluorescence Microscopy
2.5. Image Analysis and Classification
2.6. Statistical Analysis
3. Results
3.1. AQP3 Expression Levels under Normoxia and Hypoxia
3.2. AQP3 Expression Throughout the Cell Cycle
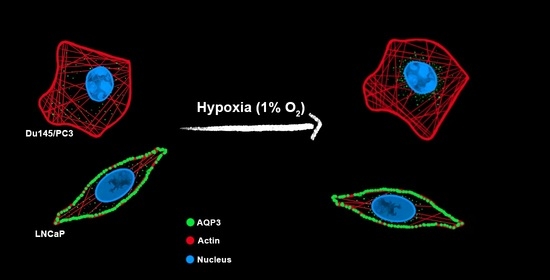
3.3. Cellular Distribution of AQP3
3.4. Classification of Cell Lines and Experimental Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verkman, A.S.; Anderson, M.O.; Papadopoulos, M.C. Aquaporins: Important but elusive drug targets. Nat. Rev. Drug Discov. 2014, 13, 259–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calamita, G.; Perret, J.; Delporte, C. Aquaglyceroporins: Drug Targets for Metabolic Diseases? Front. Physiol. 2018, 9, 851. [Google Scholar] [CrossRef]
- Aikman, B.; de Almeida, A.; Meier-Menches, S.M.; Casini, A. Aquaporins in cancer development: Opportunities for bioinorganic chemistry to contribute novel chemical probes and therapeutic agents. Metallomics 2018, 10, 696–712. [Google Scholar] [CrossRef] [Green Version]
- Hara-Chikuma, M.; Verkman, A.S. Roles of aquaporin-3 in the epidermis. J. Investig. Dermatol. 2008, 128, 2145–2151. [Google Scholar] [CrossRef] [Green Version]
- Mobasheri, A.; Wray, S.; Marples, D. Distribution of AQP2 and AQP3 water channels in human tissue microarrays. J. Mol. Histol. 2005, 36, 1–14. [Google Scholar] [CrossRef]
- Roudier, N.; Verbavatz, J.M.; Maurel, C.; Ripoche, P.; Tacnet, F. Evidence for the presence of aquaporin-3 in human red blood cells. J. Biol. Chem. 1998, 273, 8407–8412. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Satooka, H.; Watanabe, S.; Honda, T.; Miyachi, Y.; Watanabe, T.; Verkman, A.S. Aquaporin-3-mediated hydrogen peroxide transport is required for NF-kB signalling in keratinocytes and development of psoriasis. Nat. Commun. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribatti, D.; Ranieri, G.; Annese, T.; Nico, B. Aquaporins in cancer. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1550–1553. [Google Scholar] [CrossRef]
- Kang, B.W.; Kim, J.G.; Lee, S.J.; Chae, Y.S.; Jeong, J.Y.; Yoon, G.S.; Park, S.Y.; Kim, H.J.; Park, J.S.; Choi, G.S. Expression of aquaporin-1, aquaporin-3, and aquaporin-5 correlates with nodal metastasis in colon cancer. Oncology 2015, 88, 369–376. [Google Scholar] [CrossRef]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1596–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tanji, N.; Kikugawa, T.; Shudou, M.; Song, X.; Yokoyama, M. Expression of aquaporin 3 in the human prostate. Int. J. Urol. 2007, 14, 1088–1092. [Google Scholar] [CrossRef] [PubMed]
- Bründl, J.; Wallinger, S.; Breyer, J.; Weber, F.; Evert, M.; Georgopoulos, N.T.; Rosenhammer, B.; Burger, M.; Otto, W.; Rubenwolf, P. Expression, localisation and potential significance of aquaporins in benign and malignant human prostate tissue. BMC Urol. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Hwang, I.; Jung, S.-I.; Hwang, E.-C.; Song, S.H.; Lee, H.-S.; Kim, S.-O.; Kang, T.-W.; Kwon, D.; Park, K. Expression and Localization of Aquaporins in Benign Prostate Hyperplasia and Prostate Cancer. Chonnam Med. J. 2012, 48, 174. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, Y.-J.; Qu, F.; Sheng, J.-Z.; Huang, H.-F. Functions of water channels in male and female reproductive systems. Mol. Aspects Med. 2012, 33, 676–690. [Google Scholar] [CrossRef]
- Jablonski, E.M.; Adrian Mattocks, M.; Sokolov, E.; Koniaris, L.G.; Hughes, F.M.; Fausto, N.; Pierce, R.H.; McKillop, I.H. Decreased aquaporin expression leads to increased resistance to apoptosis in hepatocellular carcinoma. Cancer Lett. 2007, 250, 36–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonski, E.M.; Webb, A.N.; McConnell, N.A.; Riley, M.C.; Hughes, F.M. Plasma membrane aquaporin activity can affect the rate of apoptosis but is inhibited after apoptotic volume decrease. Am. J. Physiol. Cell Physiol. 2004, 286, C975–C985. [Google Scholar] [CrossRef] [PubMed]
- Conner, A.C.; Bill, R.M.; Conner, M.T. An emerging consensus on aquaporin translocation as a regulatory mechanism. Mol. Membr. Biol. 2013, 30, 101–112. [Google Scholar] [CrossRef] [Green Version]
- McKenna, D.J.; Errington, R.; Pors, K. Current challenges and opportunities in treating hypoxic prostate tumors. J. Cancer Metastasis Treat. 2018, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Yeldag, G.; Rice, A.; Hernández, A.D.R. Chemoresistance and the Self-Maintaining Tumor Microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butterworth, K.T.; McCarthy, H.O.; Devlin, A.; Ming, L.; Robson, T.; McKeown, S.R.; Worthington, J. Hypoxia selects for androgen independent LNCaP cells with a more malignant geno- And phenotype. Int. J. Cancer 2008, 123, 760–768. [Google Scholar] [CrossRef]
- Graeber, T.G.; Osmanian, C.; Jacks, T.; Housman, D.E.; Koch, C.J.; Lowe, S.W.; Giaccia, A.J. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 1996, 379, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Bergh, A. Hypoxia drives prostate tumour progression and impairs the effectiveness of therapy, but can also promote cell death and serve as a therapeutic target AU—Rudolfsson, Stina Häggström. Expert Opin. Ther. Targets 2009, 13, 219–225. [Google Scholar]
- Yang, M.; Gao, F.; Liu, H.; Yu, W.H.; Sun, S.Q. Temporal changes in expression of aquaporin3, -4, -5 and -8 in rat brains after permanent focal cerebral ischemia. Brain Res. 2009, 1290, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Hoogewijs, D.; Vogler, M.; Zwenger, E.; Krull, S. Oxygen-dependent regulation of aquaporin-3 expression. Hypoxia 2016, 4, 91–97. [Google Scholar]
- Hollborn, M.; Ulbricht, E.; Reichenbach, A.; Wiedemann, P.; Bringmann, A.; Kohen, L. Transcriptional regulation of aquaporin-3 in human retinal pigment epithelial cells. Mol. Biol. Rep. 2012, 39, 7949–7956. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, H.; Cheng, W.; Lin, S.; Shao, R.; Pan, H. Effects of hypoxia and ASIC3 on nucleus pulposus cells: From cell behavior to molecular mechanism. Biomed. Pharmacother. 2019, 117, 109061. [Google Scholar] [CrossRef]
- Echevarría, M.; Muñoz-Cabello, A.M.; Sánchez-Silva, R.; Toledo-Aral, J.J.; López-Barneo, J. Development of cytosolic hypoxia and hypoxia-inducible factor stabilization are facilitated by aquaporin-1 expression. J. Biol. Chem. 2007, 282, 30207–30215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galán-Cobo, A.; Sánchez-Silva, R.; Serna, A.; Abreu-Rodríguez, I.; Muñoz-Cabello, A.M.; Echevarría, M. Cellular overexpression of Aquaporins slows down the natural HIF-2α degradation during prolonged hypoxia. Gene 2013, 522, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, S.; Roy-Burman, P.; Lee, P.; Culig, Z. Current mouse and cell models in prostate cancer research. Endocr. Relat. Cancer 2013, 20, R155–R170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, A.E.; Khajah, M.A.; Khushaish, S.; Luqmani, Y.A. Aquaporin expression in breast cancer and their involvement in bleb formation, cell motility and invasion in endocrine resistant variant cells. Int. J. Oncol. 2020, 56, 1014–1024. [Google Scholar] [CrossRef]
- Chen, X.F.; Li, C.F.; Lü, L.; Mei, Z.C. Expression and clinical significance of aquaglyceroporins in human hepatocellular carcinoma. Mol. Med. Rep. 2016, 13, 5283–5289. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Kubota, M.; Iguchi, K.; Usui, S.; Kiho, T.; Hirano, K. Membrane trafficking of aquaporin 3 induced by epinephrine. Biochem. Biophys. Res. Commun. 2008, 373, 613–617. [Google Scholar] [CrossRef]
- Lee, S.E.; Kang, H.K.; Ryou, C.; Cheon, Y.P. Spatiotemporal expression of aquaporin 9 is critical for the antral growth of mouse ovarian follicles. Biol. Reprod. 2020, 103, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Zhou, J.; Shi, S.; Xu, H.-Y.; Qu, F.; Zhang, D.; Chen, Y.-D.; Yang, J.; Huang, H.-F.; Sheng, J.-Z. Identification of Estrogen Response Element in Aquaporin-3 Gene that Mediates Estrogen-induced Cell Migration and Invasion in Estrogen Receptor-positive Breast Cancer. Sci. Rep. 2015, 5, 12484. [Google Scholar] [CrossRef] [Green Version]
- Tanner, M.J.; Welliver, R.C., Jr.; Chen, M.; Shtutman, M.; Godoy, A.; Smith, G.; Mian, B.M.; Buttyan, R. Effects of androgen receptor and androgen on gene expression in prostate stromal fibroblasts and paracrine signaling to prostate cancer cells. PLoS ONE 2011, 6, e16027. [Google Scholar] [CrossRef]
- Harrison, H.; Rogerson, L.; Gregson, H.J.; Brennan, K.R.; Clarke, R.B.; Landberg, G. Contrasting hypoxic effects on breast cancer stem cell hierarchy is dependent on er-a status. Cancer Res. 2013, 73, 1420–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mylonis, I.; Simos, G.; Paraskeva, E. Hypoxia-Inducible Factors and the Regulation of Lipid Metabolism. Cells 2019, 8, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardelli, M.; Bruschi, F.V.; Claudel, T.; Moreno-Viedma, V.; Halilbasic, E.; Marra, F.; Herac, M.; Stulnig, T.M.; Trauner, M. AQP3 is regulated by PPARγ and JNK in hepatic stellate cells carrying PNPLA3 I148M. Sci. Rep. 2017, 7, 14661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.J.; Kim, P.; Lu, Y.F.; Feingold, K.R. PPARgamma Activators Stimulate Aquaporin 3 Expression in Keratinocytes/Epidermis. Exp. Dermathol. 2011, 20, 595–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, L.H.; Withers, H.G.; Garbens, A.; Love, H.D.; Magnoni, L.; Hayward, S.W.; Moyes, C.D. Hypoxia and the metabolic phenotype of prostate cancer cells. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 1433–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serocki, M.; Bartoszewska, S.; Janaszak-Jasiecka, A.; Ochocka, R.J.; Collawn, J.F.; Bartoszewski, R. miRNAs regulate the HIF switch during hypoxia: A novel therapeutic target. Angiogenesis 2018, 21, 183–202. [Google Scholar] [CrossRef] [Green Version]
- Belaiba, R.S.; Bonello, S.; Zähringer, C.; Schmidt, S.; Hess, J.; Kietzmann, T.; Görlach, A. Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol. Biol. Cell 2007, 18, 4691–4697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamboredon, S.; Ciais, D.; Desroches-Castan, A.; Savi, P.; Bono, F.; Feige, J.-J.; Cherradi, N. Hypoxia-inducible factor-1α mRNA: A new target for destabilization by tristetraprolin in endothelial cells. Mol. Biol. Cell 2011, 22, 3366–3378. [Google Scholar] [CrossRef]
- Saxena, K.; Jolly, M.K. Acute vs. Chronic vs. Cyclic Hypoxia: Their Differential Dynamics, Molecular Mechanisms, and Effects on Tumor Progression. Biomolecules 2019, 9, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouschop, K.M.A.; van den Beucken, T.; Dubois, L.; Niessen, H.; Bussink, J.; Savelkouls, K.; Keulers, T.; Mujcic, H.; Landuyt, W.; Voncken, J.W.; et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Investig. 2010, 120, 127–141. [Google Scholar] [CrossRef] [PubMed]
- White, E.; DiPaola, R.S. The double-edged sword of autophagy modulation in cancer. Clin. Cancer Res. 2009, 15, 5308–5316. [Google Scholar] [CrossRef] [Green Version]
- Bellot, G.; Garcia-Medina, R.; Gounon, P.; Chiche, J.; Roux, D.; Pouysségur, J.; Mazure, N.M. Hypoxia-Induced Autophagy Is Mediated through Hypoxia-Inducible Factor Induction of BNIP3 and BNIP3L via Their BH3 Domains. Mol. Cell. Biol. 2009, 29, 2570–2581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, Q.; Wang, M.; Yu, M.; Zhang, J.; Bristow, R.G.; Hill, R.P.; Tannock, I.F. Role of Autophagy as a Survival Mechanism for Hypoxic Cells in Tumors. Neoplasia 2016, 18, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Z.; Zhang, Q.; Wei, S.; Li, B.; Zhang, X.; Zhang, L.; Li, Q.; Xu, H.; Xu, Z. Silencing of AQP3 induces apoptosis of gastric cancer cells via downregulation of glycerol intake and downstream inhibition of lipogenesis and autophagy. OncoTargets Ther. 2017, 10, 2791–2804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zieseniss, A. Hypoxia and the modulation of the actin cytoskeleton—emerging interrelations. Hypoxia 2014, 2, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Bray, M.-A.; Singh, S.; Han, H.; Davis, C.T.; Borgeson, B.; Hartland, C.; Kost-Alimova, M.; Gustafsdottir, S.M.; Gibson, C.C.; Carpenter, A.E. Cell Painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat. Protoc. 2016, 11, 1757–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekaran, S.N.; Ceulemans, H.; Boyd, J.D.; Carpenter, A.E. Image-based profiling for drug discovery: Due for a machine-learning upgrade? Nat. Rev. Drug Discov. 2021, 20, 145–159. [Google Scholar] [CrossRef] [PubMed]





| Condition | Feature | Overall Classification Accuracy (%) | Classification Accuracy per Cell Line (%) | ||
|---|---|---|---|---|---|
| Du145 | PC3 | LNCaP | |||
| N | MD + cytoskeleton predictors | 75.5 | 65.0 | 88.0 | 69.0 |
| MD + nucleus predictors | 62.3 | 37.6 | 75.6 | 68.7 | |
| H 5 d | MD + cytoskeleton predictors | 82.8 | 75.1 | 85.7 | 87.8 |
| MD + nucleus predictors | 69.6 | 60.7 | 76.4 | 70.1 | |
| H 8 d | MD + cytoskeleton predictors | 75.1 | 63.2 | 74.9 | 90.5 |
| MD + nucleus predictors | 57.9 | 39.2 | 47.6 | 64.3 | |
| H 15 d | MD + cytoskeleton predictors | 88.3 | 81.4 | 93.5 | 86.6 |
| MD + nucleus predictors | 65.6 | 47.3 | 81.2 | 58.1 | |
| H 8 w | MD + cytoskeleton predictors | 72.7 | 52.6 | 75.8 | 88.5 |
| MD + nucleus predictors | 61.6 | 38.7 | 72.0 | 70.9 | |
| R 4 + 4 w | MD + cytoskeleton predictors | 69.4 | 77.4 | 52.0 | 81.1 |
| MD + nucleus predictors | 55.7 | 51.4 | 43.1 | 38.4 | |
| Cell Line | Feature | Overall Classification Accuracy (%) | Classification Accuracy per Time Course (%) | |||||
|---|---|---|---|---|---|---|---|---|
| N | H 5 d | H 8 d | H 15 d | H 8 w | R 4 + 4 w | |||
| Du145 | Texture | 70.2 | 75.2 | 63.4 | 57.6 | 73.0 | 69.2 | 77.8 |
| PC3 | 73.9 | 74 | 68.8 | 61.5 | 83.1 | 77.0 | 75.6 | |
| LNCaP | 51.0 | 61.4 | 43.3 | 34.8 | 59.2 | 58.6 | 45.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Almeida, A.; Parthimos, D.; Dew, H.; Smart, O.; Wiltshire, M.; Errington, R.J. Aquaglyceroporin-3’s Expression and Cellular Localization Is Differentially Modulated by Hypoxia in Prostate Cancer Cell Lines. Cells 2021, 10, 838. https://doi.org/10.3390/cells10040838
de Almeida A, Parthimos D, Dew H, Smart O, Wiltshire M, Errington RJ. Aquaglyceroporin-3’s Expression and Cellular Localization Is Differentially Modulated by Hypoxia in Prostate Cancer Cell Lines. Cells. 2021; 10(4):838. https://doi.org/10.3390/cells10040838
Chicago/Turabian Stylede Almeida, Andreia, Dimitris Parthimos, Holly Dew, Oliver Smart, Marie Wiltshire, and Rachel J. Errington. 2021. "Aquaglyceroporin-3’s Expression and Cellular Localization Is Differentially Modulated by Hypoxia in Prostate Cancer Cell Lines" Cells 10, no. 4: 838. https://doi.org/10.3390/cells10040838
APA Stylede Almeida, A., Parthimos, D., Dew, H., Smart, O., Wiltshire, M., & Errington, R. J. (2021). Aquaglyceroporin-3’s Expression and Cellular Localization Is Differentially Modulated by Hypoxia in Prostate Cancer Cell Lines. Cells, 10(4), 838. https://doi.org/10.3390/cells10040838