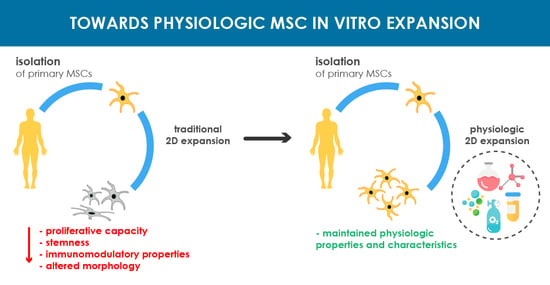
Towards Physiologic Culture Approaches to Improve Standard Cultivation of Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Basal Media for Isolation and Expansion
3. Glucose
4. Amino Acids
5. Lipids
6. Growth Factors
6.1. Fibroblast Growth Factor-2 and -4
6.2. Platelet Derived Growth Factor-BB
6.3. Epidermal Growth Factor
7. Trace Elements
8. Ascorbic Acid
9. Human Platelet Lysate
10. Serum-, Xeno-Free-, and Chemically Defined Media
11. Hypoxia
12. Isolation and Passage Cell Seeding Densities
13. Cell Detaching Methods
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| ADCF | animal-derived component free |
| BM-MSCs | bone marrow-mesenchymal stem cells |
| EAAs | essential amino acids |
| EGF | epidermal growth factor |
| FBS | fetal bovine serum |
| FGF-2 and -4 | fibroblast growth factor-2 and -4 |
| GMP | good manufacturing practice |
| HB-EGF | heparin-Binding Epidermal Growth Factor |
| hPL | human platelet lysate |
| HIF1 | hypoxia inducible factor I |
| IGF | insulin-like growth factor |
| ISCT | International Society for Cellular Therapy |
| MSC(s) | mesenchymal stem cell(s) |
| NEAAs | non-essential amino-acids |
| PDGF-BB | platelet derived growth factor-BB |
| PCs | platelet concentrates |
| ROS | reactive oxygen species |
| PRP | platelet rich plasma |
| SF | serum-free |
| VEGF | endothelial growth factor |
References
- Hanna, E.; Rmuzat, C.; Auquier, P.; Toumi, M. Advanced therapy medicinal products: Current and future perspectives. J. Mark. Access Health Policy 2016, 4, 31036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mount, N.M.; Ward, S.J.; Kefalas, P.; Hyllner, J. Cell-based therapy technology classifications and translational challenges. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20150017. [Google Scholar] [CrossRef]
- Buzhor, E.; Leshansky, L.; Blumenthal, J.; Barash, H.; Warshawsky, D.; Mazor, Y.; Shtrichman, R. Cell-based therapy approaches: The hope for incurable diseases. Regen. Med. 2014, 9, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Chagastelles, P.C.; Nardi, N.B. Biology of stem cells: An overview. Kidney Int. Suppl. 2011, 1, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Gardner, O.F.; Alini, M.; Stoddart, M.J. Mesenchymal Stem Cells Derived from Human Bone Marrow. Methods Mol. Biol. 2015, 1340, 41–52. [Google Scholar] [CrossRef]
- Mahmoudifar, N.; Doran, P.M. Mesenchymal Stem Cells Derived from Human Adipose Tissue. Methods Mol. Biol. 2015, 1340, 53–64. [Google Scholar] [CrossRef]
- Van Pham, P.; Truong, N.C.; Le, P.T.; Tran, T.D.; Vu, N.B.; Bui, K.H.; Phan, N.K. Isolation and proliferation of umbilical cord tissue derived mesenchymal stem cells for clinical applications. Cell Tissue Bank. 2016, 17, 289–302. [Google Scholar] [CrossRef]
- Ouryazdanpanah, N.; Dabiri, S.; Derakhshani, A.; Vahidi, R.; Farsinejad, A. Peripheral Blood-Derived Mesenchymal Stem Cells: Growth Factor-Free Isolation, Molecular Characterization and Differentiation. Iran. J. Pathol. 2018, 13, 461–466. [Google Scholar]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal Stem Cells Derived from Dental Pulp: A Review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef] [PubMed]
- Kropp, C.; Massai, D.; Zweigerdt, R. Progress and challenges in large-scale expansion of human pluripotent stem cells. Process. Biochem. 2017, 59, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Rafiq, Q.A.; Coopman, K.; Hewitt, C.J. Scale-up of human mesenchymal stem cell culture: Current technologies and future challenges. Curr. Opin. Chem. Eng. 2013, 2, 8–16. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Mead, B.E.; Safaee, H.; Langer, R.; Karp, J.M.; Levy, O. Engineering Stem Cell Organoids. Cell Stem Cell 2016, 18, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shu, Z.; Qian, K.; Wang, J.; Zhu, H. Harnessing the Properties of Biomaterial to Enhance the Immunomodulation of Mesenchymal Stem Cells. Tissue Eng. Part. B Rev. 2019, 25, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Hanson, S.; D’Souza, R.N.; Hematti, P. Biomaterial-mesenchymal stem cell constructs for immunomodulation in composite tissue engineering. Tissue Eng. Part A 2014, 20, 2162–2168. [Google Scholar] [CrossRef]
- Schneider, R.K.; Knüchel, R.; Neuss, S. Mesenchymal stem cells and their interaction with biomaterials: Potential applications in tissue engineering. Pathologe 2011, 32 (Suppl. S2), 296–303. [Google Scholar] [CrossRef]
- Azandeh, S.; Mohammad Gharravi, A.; Orazizadeh, M.; Khodadi, A.; Hashemi Tabar, M. Improvement of mesenchymal stem cell differentiation into the endoderm lineage by four step sequential method in biocompatible biomaterial. Bioimpacts 2016, 6, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Roosloot, R.; van Pel, M.; Schepers, K.; Driessen, M.; Fibbe, W.E.; de Bruijn, J.D.; Roelofs, H. Preparing for cell culture scale-out: Establishing parity of bioreactor- and flask-expanded mesenchymal stromal cell cultures. J. Transl. Med. 2019, 17, 241. [Google Scholar] [CrossRef] [Green Version]
- Jossen, V.; van den Bos, C.; Eibl, R.; Eibl, D. Manufacturing human mesenchymal stem cells at clinical scale: Process and regulatory challenges. Appl. Microbiol. Biotechnol. 2018, 102, 3981–3994. [Google Scholar] [CrossRef] [Green Version]
- Lawson, T.; Kehoe, D.E.; Schnitzler, A.C.; Rapiejko, P.J.; Der, K.A.; Philbrick, K.; Punreddy, S.; Rigby, S.; Smith, R.; Feng, Q.; et al. Process development for expansion of human mesenchymal stromal cells in a 50L single-use stirred tank bioreactor. Biochem. Eng. J. 2017, 120, 49–62. [Google Scholar] [CrossRef]
- Hoch, A.I.; Duhr, R.; Di Maggio, N.; Mehrkens, A.; Jakob, M.; Wendt, D. Expansion of Bone Marrow Mesenchymal Stromal Cells in Perfused 3D Ceramic Scaffolds Enhances In Vivo Bone Formation. Biotechnol. J. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Sung, J.H. Organ-on-a-chip technology for reproducing multiorgan physiology. Adv. Healthc. Mater. 2018, 7, 1700419. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef] [Green Version]
- Frith, J.E.; Thomson, B.; Genever, P.G. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng. Part C Methods 2010, 16, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Stiehler, M.; Bünger, C.; Baatrup, A.; Lind, M.; Kassem, M.; Mygind, T. Effect of dynamic 3-D culture on proliferation, distribution, and osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. A 2009, 89, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Itaka, K.; Ohba, S.; Nishiyama, N.; Chung, U.I.; Yamasaki, Y.; Kataoka, K. 3D spheroid culture system on micropatterned substrates for improved differentiation efficiency of multipotent mesenchymal stem cells. Biomaterials 2009, 30, 2705–2715. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Latzinik, N.W.; Grosheva, A.G.; Gorskaya, U.F. Marrow microenvironment transfer by heterotopic transplantation of freshly isolated and cultured cells in porous sponges. Exp. Hematol. 1982, 10, 217–227. [Google Scholar]
- Wexler, S.A.; Donaldson, C.; Denning-Kendall, P.; Rice, C.; Bradley, B.; Hows, J.M. Adult bone marrow is a rich source of human mesenchymal ‘stem’cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol. 2003, 121, 368–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Sotome, S.; Wang, J.; Orii, H.; Uemura, T.; Shinomiya, K. Correlation of in vivo bone formation capability and in vitro differentiation of human bone marrow stromal cells. J. Med. Dent. Sci. 2005, 52, 27–34. [Google Scholar]
- Yang, Y.-H.K.; Ogando, C.R.; Wang See, C.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Han, Z.B.; Liu, J.F.; Wang, Y.W.; Zhang, J.Z.; Li, C.T.; Xin, P.L.; Han, Z.C.; Zhu, X.P. Serum-free media and the immunoregulatory properties of mesenchymal stem cells in vivo and in vitro. Cell. Physiol. Biochem. 2014, 33, 569–580. [Google Scholar] [CrossRef]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.G.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT (R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [CrossRef]
- Sotiropoulou, P.A.; Perez, S.A.; Salagianni, M.; Baxevanis, C.N.; Papamichail, M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells 2006, 24, 462–471. [Google Scholar] [CrossRef] [Green Version]
- Dhanasekaran, M.; Indumathi, S.; Rashmi, M.; Rajkumar, J.S.; Sudarsanam, D. Unravelling the retention of proliferation and differentiation potency in extensive culture of human subcutaneous fat-derived mesenchymal stem cells in different media. Cell Prolif. 2012, 45, 516–526. [Google Scholar] [CrossRef]
- Hagmann, S.; Moradi, B.; Frank, S.; Dreher, T.; Kämmerer, P.W.; Richter, W.; Gotterbarm, T. Different culture media affect growth characteristics, surface marker distribution and chondrogenic differentiation of human bone marrow-derived mesenchymal stromal cells. BMC Musculoskelet. Disord. 2013, 14, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haack-Sorensen, M.; Friis, T.; Bindslev, L.; Mortensen, S.; Johnsen, H.E.; Kastrup, J. Comparison of different culture conditions for human mesenchymal stromal cells for clinical stem cell therapy. Scand. J. Clin. Lab. Investig. 2008, 68, 192–203. [Google Scholar] [CrossRef]
- Woerle, H.J.; Gerich, J.E. Glucose Physiology, Normal. In Encyclopedia of Endocrine Diseases; Martini, L., Ed.; Elsevier: New York, NY, USA, 2004; pp. 263–270. [Google Scholar]
- Nuschke, A.; Rodrigues, M.; Wells, A.W.; Sylakowski, K.; Wells, A. Mesenchymal stem cells/multipotent stromal cells (MSCs) are glycolytic and thus glucose is a limiting factor of in vitro models of MSC starvation. Stem Cell Res. Ther. 2016, 7, 179. [Google Scholar] [CrossRef] [Green Version]
- Keats, E.; Khan, Z.A. Unique responses of stem cell-derived vascular endothelial and mesenchymal cells to high levels of glucose. PLoS ONE 2012, 7, e38752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, T.; Ho, J.H.; Yang, M.-H.; Lee, O.K. Glucose Reduction Prevents Replicative Senescence and Increases Mitochondrial Respiration in Human Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Weil, B.R.; Abarbanell, A.M.; Herrmann, J.L.; Wang, Y.; Meldrum, D.R. High glucose concentration in cell culture medium does not acutely affect human mesenchymal stem cell growth factor production or proliferation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1735–R1743. [Google Scholar] [CrossRef] [Green Version]
- D’Esposito, V.; Lecce, M.; Marenzi, G.; Cabaro, S.; Ambrosio, M.R.; Sammartino, G.; Misso, S.; Migliaccio, T.; Liguoro, P.; Oriente, F.; et al. Platelet-rich plasma counteracts detrimental effect of high-glucose concentrations on mesenchymal stem cells from Bichat fat pad. J. Tissue Eng. Regen. Med. 2020. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Nan, L.-P.; Wang, F.; Zhou, S.-F.; Wang, J.-C.; Feng, X.-M.; Zhang, L. The effect of high glucose on the biological characteristics of nucleus pulposus-derived mesenchymal stem cells. Cell Biochem. Funct. 2020. [Google Scholar] [CrossRef]
- Chang, T.-C.; Hsu, M.-F.; Wu, K.K. High Glucose Induces Bone Marrow-Derived Mesenchymal Stem Cell Senescence by Upregulating Autophagy. PLoS ONE 2015, 10, e0126537. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Li, H.; Tao, Y.; Zhou, X.; Li, F.; Chen, G.; Chen, Q. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J. Transl. Med. 2012, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-M.; Schilling, T.; Benisch, P.; Zeck, S.; Meissner-Weigl, J.; Schneider, D.; Limbert, C.; Seufert, J.; Kassem, M.; Schütze, N.; et al. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem. Biophys. Res. Commun. 2007, 363, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ejtehadifar, M.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P.; Dehdilani, N.; Abbasi, P.; Molaeipour, Z.; Saleh, M. The effect of hypoxia on mesenchymal stem cell biology. Adv. Pharm. Bull. 2015, 5, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Deschepper, M.; Oudina, K.; David, B.; Myrtil, V.; Collet, C.; Bensidhoum, M.; Logeart-Avramoglou, D.; Petite, H. Survival and function of mesenchymal stem cells (MSCs) depend on glucose to overcome exposure to long-term, severe and continuous hypoxia. J. Cell. Mol. Med. 2011, 15, 1505–1514. [Google Scholar] [CrossRef]
- Jedrzejczak-Silicka, M. History of Cell Culture. In New Insights into Cell Culture Technology; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Salazar, A.; Keusgen, M.; von Hagen, J. Amino acids in the cultivation of mammalian cells. Amino Acids 2016, 48, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doverskog, M.; Ljunggren, J.; Öhman, L.; Häggström, L. Physiology of cultured animal cells. J. Biotechnol. 1997, 59, 103–115. [Google Scholar] [CrossRef]
- Kontoravdi, C.; Wong, D.; Lam, C.; Lee, Y.Y.; Yap, M.G.S.; Pistikopoulos, E.N.; Mantalaris, A. Modeling Amino Acid Metabolism in Mammalian Cells-Toward the Development of a Model Library. Biotechnol. Prog. 2007, 23, 1261–1269. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of The Cell; Norton & Company: New York, NY, USA, 2007; Volume 230. [Google Scholar]
- Choi, W.; Kwon, S.-J.; Jin, H.J.; Jeong, S.Y.; Choi, S.J.; Oh, W.; Yang, Y.S.; Jeon, H.B.; Jeon, E.S. Optimization of culture conditions for rapid clinical-scale expansion of human umbilical cord blood-derived mesenchymal stem cells. Clin. Transl. Med. 2017, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Higuera, G.A.; Schop, D.; Spitters, T.W.G.M.; van Dijkhuizen-Radersma, R.; Bracke, M.; de Bruijn, J.D.; Martens, D.; Karperien, M.; van Boxtel, A.; van Blitterswijk, C.A. Patterns of amino acid metabolism by proliferating human mesenchymal stem cells. Tissue Eng. Part A 2012, 18, 654–664. [Google Scholar] [CrossRef]
- Eagle, H.; Oyama, V.I.; Levy, M.A. The growth response of mammalian cells in tissue culture to L-glutamine and L-glutamic acid. J. Biol. Chem. 1956, 218, 607–616. [Google Scholar] [CrossRef]
- Dos Santos, G.G.; Hastreiter, A.A.; Sartori, T.; Borelli, P.; Fock, R.A. L-Glutamine in vitro Modulates some Immunomodulatory Properties of Bone Marrow Mesenchymal Stem Cells. Stem Cell Rev. Rep. 2017, 13, 482–490. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, Y.; Chen, Q.; Xie, L.; Li, J. Glutamine Metabolism Is Essential for Stemness of Bone Marrow Mesenchymal Stem Cells and Bone Homeostasis. Stem Cells Int. 2019, 2019, 8928934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartori, T.; Santos, A.C.A.A. Branched chain amino acids improve mesenchymal stem cell proliferation, reducing nuclear factor kappa B expression and modulating some inflammatory properties. Nutrition 2020, 78, 110935. [Google Scholar] [CrossRef] [PubMed]
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef]
- Van Meer, G.; de Kroon, A.I.P.M. Lipid map of the mammalian cell. J. Cell Sci. 2011, 124, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clémot, M.; Sênos Demarco, R.; Jones, D.L. Lipid Mediated Regulation of Adult Stem Cell Behavior. Front. Cell Dev. Biol. 2020, 8, 115. [Google Scholar] [CrossRef] [Green Version]
- Grammatikos, S.I.; Subbaiah, P.V.; Victor, T.A.; Miller, W.M. Diverse effects of essential (n-6 and n-3) fatty acids on cultured cells. In Cell Culture Engineering I.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 31–50. [Google Scholar]
- Whitford, W.; Manwaring, J. Lipids in Cell Culture Media. Fish. Appl. Notes 2004, 152–154. [Google Scholar]
- Rampler, E.; Egger, D.; Schoeny, H.; Rusz, M.; Pacheco, M.P.; Marino, G.; Kasper, C.; Naegele, T.; Koellensperger, G. The power of LC-MS based multiomics: Exploring adipogenic differentiation of human mesenchymal stem/stromal cells. Molecules 2019, 24, 3615. [Google Scholar] [CrossRef] [Green Version]
- Chatgilialoglu, A.; Rossi, M.; Alviano, F.; Poggi, P.; Zannini, C.; Marchionni, C.; Ricci, F.; Tazzari, P.L.; Taglioli, V.; Calder, P.C.; et al. Restored in vivo-like membrane lipidomics positively influence in vitro features of cultured mesenchymal stromal/stem cells derived from human placenta. Stem Cell Res. Ther. 2017, 8, 31. [Google Scholar] [CrossRef] [Green Version]
- Kilpinen, L.; Tigistu-Sahle, F.; Oja, S.; Greco, D.; Parmar, A.; Saavalainen, P.; Nikkilä, J.; Korhonen, M.; Lehenkari, P.; Käkelä, R.; et al. Aging bone marrow mesenchymal stromal cells have altered membrane glycerophospholipid composition and functionality. J. Lipid Res. 2013, 54, 622–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Chen, Y.; Wang, H.; Bai, Y.; Zhao, J.; Zhang, X.; Liang, L.; Chen, Y.; Ye, C.; Li, Y.; et al. Integrated Lipidomics and Transcriptomics Characterization upon Aging-Related Changes of Lipid Species and Pathways in Human Bone Marrow Mesenchymal Stem Cells. J. Proteome Res. 2019, 18, 2065–2077. [Google Scholar] [CrossRef]
- Fillmore, N.; Huqi, A.; Jaswal, J.S.; Mori, J.; Paulin, R.; Haromy, A.; Onay-Besikci, A.; Ionescu, L.; Thbaud, B.; Michelakis, E.; et al. Effect of fatty acids on human bone marrow mesenchymal stem cell energy metabolism and survival. PLoS ONE 2015, 10, e0120257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Wang, C.; He, L.; Han, X. Novel strategies for enhancing shotgun lipidomics for comprehensive analysis of cellular lipidomes. TrAC Trends Anal. Chem. 2019, 120, 115330. [Google Scholar] [CrossRef] [PubMed]
- Goracci, L.; Tortorella, S.; Tiberi, P.; Pellegrino, R.M.A. Lipostar, a Comprehensive Platform-Neutral Cheminformatics Tool for Lipidomics. Anal. Chem. 2017, 89, 6257–6264. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Molendijk, J.; Hill, M.M. Lipidr: A Software Tool for Data Mining and Analysis of Lipidomics Datasets. J. Proteome Res. 2020, 19, 2890–2897. [Google Scholar] [CrossRef] [PubMed]
- MERCK. Lipids and Lipid Carriers—Solubilizing Agents|Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/life-science/cell-culture/cell-culture-products.html?TablePage=22608488 (accessed on 12 January 2021).
- Rodrigues, M.; Griffith, L.G.; Wells, A. Growth factor regulation of proliferation and survival of multipotential stromal cells. Stem Cell Res. Ther. 2010, 1, 32. [Google Scholar] [CrossRef] [Green Version]
- Franz, K.C.; Suschek, C.V.; Grotheer, V.; Akbas, M.; Pallua, N. Impact of growth factor content on proliferation of mesenchymal stromal cells derived from adipose tissue. PLoS ONE 2020, 15, e0230265. [Google Scholar] [CrossRef]
- Gharibi, B.; Hughes, F.J. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl. Med. 2012, 1, 771–782. [Google Scholar] [CrossRef]
- Kabiri, A.; Esfandiari, E.; Hashemibeni, B.; Kazemi, M.; Mardani, M.; Esmaeili, A. Effects of FGF-2 on human adipose tissue derived adult stem cells morphology and chondrogenesis enhancement in Transwell culture. Biochem. Biophys. Res. Commun. 2012, 424, 234–238. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Penick, K.; Porter, J.D.; Goldberg, V.M.; Caplan, A.I.; Welter, J.F. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J. Cell. Physiol. 2005, 203, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Shimazu, A.; Miyazaki, K.; Pan, H.; Koike, C.; Yoshida, E.; Takagishi, K.; Kato, Y. Retention of Multilineage Differentiation Potential of Mesenchymal Cells during Proliferation in Response to FGF. Biochem. Biophys. Res. Commun. 2001, 288, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Eom, Y.W.; Oh, J.-E.; Lee, J.I.; Baik, S.K.; Rhee, K.-J.; Shin, H.C.; Kim, Y.M.; Ahn, C.M.; Kong, J.H.; Kim, H.S.; et al. The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2014, 445, 16–22. [Google Scholar] [CrossRef]
- Jung, S.; Sen, A.; Rosenberg, L.; Behie, L.A. Identification of growth and attachment factors for the serum-free isolation and expansion of human mesenchymal stromal cells. Cytotherapy 2010, 12, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sawada, R.; Fujiwara, Y.; Seyama, Y.; Tsuchiya, T. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-β2. Biochem. Biophys. Res. Commun. 2007, 359, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, F.; Boucher, S.; Koh, S.; Sastry, K.S.R.; Chase, L.; Lakshmipathy, U.; Choong, C.; Yang, Z.; Vemuri, M.C.; Rao, M.S.; et al. PDGF, TGF-β, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): Transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood 2008, 112, 295–307. [Google Scholar] [CrossRef]
- Gruber, R.; Karreth, F.; Kandler, B.; Fuerst, G.; Rot, A.; Fischer, M.B.; Watzek, G. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets 2004, 15, 29–35. [Google Scholar] [CrossRef]
- Fierro, F.; Illmer, T.; Jing, D.; Schleyer, E.; Ehninger, G.; Boxberger, S.; Bornhäuser, M. Inhibition of platelet-derived growth factor receptorbeta by imatinib mesylate suppresses proliferation and alters differentiation of human mesenchymal stem cells in vitro. Cell Prolif. 2007, 40, 355–366. [Google Scholar] [CrossRef]
- Tamama, K.; Fan, V.H.; Griffith, L.G.; Blair, H.C.; Wells, A. Epidermal Growth Factor as a Candidate for Ex Vivo Expansion of Bone Marrow–Derived Mesenchymal Stem Cells. Stem Cells 2006, 24, 686–695. [Google Scholar] [CrossRef]
- Krampera, M.; Pasini, A.; Rigo, A.; Scupoli, M.T.; Tecchio, C.; Malpeli, G.; Scarpa, A.; Dazzi, F.; Pizzolo, G.; Vinante, F. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: Inducing cell expansion and reversibly preventing multilineage differentiation. Blood 2005, 106, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.-Y.; Kim, H.J.; Choi, B.Y.; Sohn, M.; Chung, T.N.; Suh, S.W. Zinc Promotes Adipose-Derived Mesenchymal Stem Cell Proliferation and Differentiation towards a Neuronal Fate. Stem Cells Int. 2018, 2018, 5736535. [Google Scholar] [CrossRef] [Green Version]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siti Noor Fazliah, M.N.; Yusuf, M.M.; Abdullah, T.K.; Zuhailawati, H. Human Mesenchymal Stem Cells Response to Magnesium-based Biomaterials. Proc. Chem. 2016, 19, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Barradas, A.M.; Fernandes, H.A.; Groen, N.; Chai, Y.C.; Schrooten, J.; van de Peppel, J.; van Leeuwen, J.P.; van Blitterswijk, C.A.; de Boer, J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Chen, J.; Huang, W.; Wu, D.; Lin, H.; Lai, Y. Proteomic analysis of the effect of extracellular calcium ions on human mesenchymal stem cells: Implications for bone tissue engineering. Chem Biol. Interact. 2015, 233, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Caldarelli, I.; Speranza, M.C.; Scianguetta, S.; Tramontano, A.; Bencivenga, D.; Stampone, E.; Negri, A.; Nobili, B.; Locatelli, F.; et al. Iron overload enhances human mesenchymal stromal cell growth and hampers matrix calcification. Biochim. Biophys. Acta 2016, 1860, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Ebert, R.; Ulmer, M.; Zeck, S.; Meissner-Weigl, J.; Schneider, D.; Stopper, H.; Schupp, N.; Kassem, M.; Jakob, F. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells 2006, 24, 1226–1235. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.H.; Yoon, B.S.; Jun, E.K.; Lee, G.; Kim, I.Y.; You, S. Additive effect of bFGF and selenium on expansion and paracrine action of human amniotic fluid-derived mesenchymal stem cells. Stem Cell Res. 2018, 9, 293. [Google Scholar] [CrossRef]
- Rodríguez, J.; Ríos, S.; González, M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef]
- Choi, K.-M.; Seo, Y.-K.; Yoon, H.-H.; Song, K.-Y.; Kwon, S.-Y.; Lee, H.-S.; Park, J.-K. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J. Biosci. Bioeng. 2008, 105, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Potdar, P.D.; D’souza, S.B. Ascorbic acid induces in vitro proliferation of human subcutaneous adipose tissue derived mesenchymal stem cells with upregulation of embryonic stem cell pluripotency markers Oct4 and SOX 2. Hum. Cell 2010, 23, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Hara, K.; Takami, T.; Okada, S.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. Evaluation of the effects of ascorbic acid on metabolism of human mesenchymal stem cells. Stem Cell Res. 2018, 9, 93. [Google Scholar] [CrossRef]
- Brunner, D.; Frank, J.; Appl, H.; Schoffl, H.; Pfaller, W.; Gstraunthaler, G. Serum-free cell culture: The serum-free media interactive online database. ALTEX 2010, 27, 53–62. [Google Scholar] [CrossRef]
- Van der Valk, J.; Brunner, D.; De Smet, K.; Fex Svenningsen, A.; Honegger, P.; Knudsen, L.E.; Lindl, T.; Noraberg, J.; Price, A.; Scarino, M.L.; et al. Optimization of chemically defined cell culture media–replacing fetal bovine serum in mammalian in vitro methods. Toxicol. Vitr. 2010, 24, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Van der Valk, J.; Mellor, D.; Brands, R.; Fischer, R.; Gruber, F.; Gstraunthaler, G.; Hellebrekers, L.; Hyllner, J.; Jonker, F.H.; Prieto, P.; et al. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol. Vitr. 2004, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gstraunthaler, G. Alternatives to the use of fetal bovine serum: Serum-free cell culture. Altex Altern Tierexp 2003, 20, 275–281. [Google Scholar]
- Van der Valk, J.; Bieback, K.; Buta, C.; Cochrane, B.; Dirks, W.G.; Fu, J.; Hickman, J.J.; Hohensee, C.; Kolar, R.; Liebsch, M.; et al. Fetal Bovine Serum (FBS): Past—Present—Future. Altex 2018, 35, 99–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieback, K.; Hecker, A.; Kocaömer, A.; Lannert, H.; Schallmoser, K.; Strunk, D.; Klüter, H. Human Alternatives to Fetal Bovine Serum for the Expansion of Mesenchymal Stromal Cells from Bone Marrow. Stem Cells 2009, 27, 2331–2341. [Google Scholar] [CrossRef]
- Carrancio, S.; Lopez-Holgado, N.; Sanchez-Guijo, F.M.; Villarn, E.; Barbado, V.; Tabera, S.; Dez-Campelo, M.; Blanco, J.A. Optimization of mesenchymal stem cell expansion procedures by cell separation and culture conditions modification. Exp. Hematol. 2008, 36, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Copland, I.B.; Garcia, M.A.; Waller, E.K.; Roback, J.D.; Galipeau, J. The effect of platelet lysate fibrinogen on the functionality of MSCs in immunotherapy. Biomaterials 2013, 34, 7840–7850. [Google Scholar] [CrossRef] [PubMed]
- Cowper, M.; Frazier, T.; Wu, X.; Curley, J.; Ma, M.; Mohiuddin, O.; Dietrich, M.; McCarthy, M.; Bukowska, J.; Gimble, J. Human Platelet Lysate as a Functional Substitute for Fetal Bovine Serum in the Culture of Human Adipose Derived Stromal/Stem Cells. Cells 2019, 8, 724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessels, C.; Durandt, C.; Pepper, M.S. Comparison of human platelet lysate alternatives using expired and freshly isolated platelet concentrates for adipose-derived stromal cell expansion. Platelets 2019, 30, 356–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsdottir-Buch, S.M.; Lieder, R.; Sigurjonsson, O.E. Platelet Lysates Produced from Expired Platelet Concentrates Support Growth and Osteogenic Differentiation of Mesenchymal Stem Cells. PLoS ONE 2013, 8, e68984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Ilzarbe, M.; Dez-Campelo, M.; Aranda, P.; Tabera, S.; Lopez, T.; del Caizo, C.; Merino, J.; Moreno, C.; Andreu, E.J.; Prsper, F.; et al. Comparison of ex vivo expansion culture conditions of mesenchymal stem cells for human cell therapy. Transfusion 2009, 49, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Albiero, E.; Alghisi, A.; Chieregato, K.; Lievore, C.; Madeo, D.; Rodeghiero, F.; Astori, G. Production of human platelet lysate by use of ultrasound for ex vivo expansion of human bone marrow–derived mesenchymal stromal cells. Cytotherapy 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Doucet, C.; Ernou, I.; Zhang, Y.; Llense, J.R.; Begot, L.; Holy, X.; Lataillade, J.J. Platelet lysates promote mesenchymal stem cell expansion: A safety substitute for animal serum in cell-based therapy applications. J. Cell. Physiol. 2005, 205, 228–236. [Google Scholar] [CrossRef]
- Kinzebach, S.; Dietz, L.; Klter, H.; Thierse, H.J.; Bieback, K. Functional and differential proteomic analyses to identify platelet derived factors affecting ex vivo expansion of mesenchymal stromal cells. BMC Cell Biol. 2013, 14. [Google Scholar] [CrossRef] [Green Version]
- Muraglia, A.; Ottonello, C. Span. Biological activity of a standardized freeze-dried platelet derivative to be used as cell culture medium supplement. Platelets 2014, 25, 211–220. [Google Scholar] [CrossRef]
- Naaijkens, B.A.; Niessen, H.W.M.; Prins, H.J.; Krijnen, P.A.J.; Kokhuis, T.J.A. Human platelet lysate as a fetal bovine serum substitute improves human adipose-derived stromal cell culture for future cardiac repair applications. Cell Tissue Res. 2012, 348, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Reinisch, A.; Bartmann, C.; Rohde, E.; Schallmoser, K.; Bjelic-Radisic, V.; Lanzer, G.; Linkesch, W.; Strunk, D. Humanized system to propagate cord blood-derived multipotent mesenchymal stromal cells for clinical application. Regen. Med. 2007, 2, 371–382. [Google Scholar] [CrossRef]
- Witzeneder, K.; Lindenmair, A.; Gabriel, C.; Höller, K.; Theiß, D.; Redl, H.; Hennerbichler, S. Human-Derived Alternatives to Fetal Bovine Serum in Cell Culture. Transfus. Med. Hemother. 2013, 40, 417–423. [Google Scholar] [CrossRef] [Green Version]
- Burnouf, T.; Strunk, D.; Koh, M.B.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Iudicone, P.; Fioravanti, D.; Bonanno, G.; Miceli, M.; Lavorino, C.; Totta, P.; Frati, L.; Nuti, M.; Pierelli, L. Pathogen-free, plasma-poor platelet lysate and expansion of human mesenchymal stem cells. J. Transl. Med. 2014, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenhani, F.; Durand, V.; Ben Azouna, N.; Thallet, S.; Ben Othmen, T.; Bejaoui, M.; Domenech, J. Human cytokine expression profile in various conditioned media for in vitro expansion bone marrow and umbilical cord blood immunophenotyped mesenchymal stem cells. Transpl. Proc. 2011, 43, 639–643. [Google Scholar] [CrossRef]
- Xia, W.; Li, H.; Wang, Z.; Xu, R.; Fu, Y.; Zhang, X.; Ye, X.; Huang, Y.; Xiang, A.P.; Yu, W. Human platelet lysate supports ex vivo expansion and enhances osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol. Int. 2011, 35, 639–643. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.; Benedetti, E.; Preslar, A.; Jacobson, P.; Jin, P.; Stroncek, D.F.; Reems, J.-A. Comparative analyses of industrial-scale human platelet lysate preparations. Transfusion 2017, 57, 2858–2869. [Google Scholar] [CrossRef] [PubMed]
- Bandeiras, C.; Koc, J.R.; Ma, Y.; Samberg, M.; Finkelstein, S.; Ferreira, F. Cost effectiveness analysis of allogeneic, just-in-time expansion of mesenchymal stem cells with PLUS™ human platelet lysate for a clinical trial. Cytotherapy 2018, 20, S60. [Google Scholar] [CrossRef]
- Kakudo, N.; Morimoto, N.; Ma, Y.; Kusumoto, K. Differences between the Proliferative Effects of Human Platelet Lysate and Fetal Bovine Serum on Human Adipose-Derived Stem Cells. Cells 2019, 8, 1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadjian, M.; Senger, A.-S.; Essers, C.; Wilkesmann, S.; Heller, R.; Fellenberg, J.; Simon, R.; Westhauser, F. Human Platelet Lysate Can Replace Fetal Calf Serum as a Protein Source to Promote Expansion and Osteogenic Differentiation of Human Bone-Marrow-Derived Mesenchymal Stromal Cells. Cells 2020, 9, 918. [Google Scholar] [CrossRef] [Green Version]
- Lensch, M.; Muise, A.; White, L.; Badowski, M.; Harris, D. Comparison of Synthetic Media Designed for Expansion of Adipose-Derived Mesenchymal Stromal Cells. Biomedicines 2018, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Shih, D.T.-B.; Chen, J.-C.; Chen, W.-Y.; Kuo, Y.-P.; Su, C.-Y.; Burnouf, T. Expansion of adipose tissue mesenchymal stromal progenitors in serum-free medium supplemented with virally inactivated allogeneic human platelet lysate. Transfusion 2011, 51, 770–778. [Google Scholar] [CrossRef]
- Sargent, B. What Does Xeno-Free Really Mean, and Why Does It Matter to Cell Culture Scientists Today? Available online: https://cellculturedish.com/what-does-xeno-free-really-mean-and-why-does-it-matter-to-cell-culture-scientists-today/ (accessed on 27 February 2021).
- Bahsoun, S.; Coopman, K.; Forsyth, N.R.; Akam, E.C. The Role of Dissolved Oxygen Levels on Human Mesenchymal Stem Cell Culture Success, Regulatory Compliance, and Therapeutic Potential. Stem Cells Dev. 2018, 27, 1303–1321. [Google Scholar] [CrossRef] [Green Version]
- Gottipamula, S.; Muttigi, M.S.; Kolkundkar, U.; Seetharam, R.N. Serum-free media for the production of human mesenchymal stromal cells: A review. Cell Prolif. 2013, 46, 608–627. [Google Scholar] [CrossRef]
- Gottipamula, S.; Ashwin, K.M.; Muttigi, M.S.; Kannan, S.; Kolkundkar, U.; Seetharam, R.N. Isolation, expansion and characterization of bone marrow-derived mesenchymal stromal cells in serum-free conditions. Cell Tissue Res. 2014, 356, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, L.; Iscove, N. Partial replacement of serum by selenite, transferrin, albumin and lecithin in haemopoitec cell cultures. Nature 1976, 263, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Masui, H.; Sato, G.H.; Sueoka, N.; Chow, T.P.; Kano-Sueoka, T. Growth of hybridoma cells in serum-free medium: Ethanolamine is an essential component. Proc. Natl. Acad. Sci. USA 1982, 79, 1158–1162. [Google Scholar] [CrossRef] [Green Version]
- Sargent, B. Albumin in Cell Culture Media—An Examination of Quality and Function. Available online: https://cellculturedish.com/albumin-in-cell-culture-media-an-examination-of-quality-and-function/ (accessed on 30 November 2020).
- Jung, S.; Panchalingam, K.M.; Rosenberg, L.; Behie, L.A. Ex vivo expansion of human mesenchymal stem cells in defined serum-free media. Stem Cells Int. 2012, 2012, 123030. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-H.; Wu, M.-L.; Hwang, S.-M. Optimization of serum free medium for cord blood mesenchymal stem cells. Biochem. Eng. J. 2007, 33, 1–9. [Google Scholar] [CrossRef]
- Marshak, D.R. US5908782A—Chemically Defined Medium for Human Mesenchymal Stem Cells—Google Patents; Patent and Trademark Office: Washington, DC, USA, 1999. [Google Scholar]
- ThermoFisher Scientific. L-Glutamine & GlutaMAX Cell Culture Supplements. Available online: https://www.thermofisher.com/at/en/home/life-science/cell-culture/mammalian-cell-culture/media-supplements/glutamax-media.html?gclid=EAIaIQobChMIuYj0ya7F6wIV05TVCh0CCQFFEAAYASAAEgJXG_D_BwE&s_kwcid=AL!3652!3!345664482627!p!!g!!glutamax&ef_id=EAIaIQobChMIuYj0ya7F6wIV05TVCh0CCQFFEAAYASAAEgJXG_D_BwE:G:s&s_kwcid=AL!3652!3!345664482627!p!!g!!glutamax&cid=bid_clb_cce_r01_co_cp0000_pjt0000_bid00000_0se_gaw_bt_pur_con (accessed on 27 February 2021).
- Sigma Aldrich. Folic Acid and Tetrahydrofolates in Cell Culture. Available online: https://www.sigmaaldrich.com/life-science/cell-culture/learning-center/media-expert/folic-acid.html (accessed on 27 February 2021).
- Sigma Aldrich. Biotin in Cell Culture. Available online: https://www.sigmaaldrich.com/life-science/cell-culture/learning-center/media-expert/biotin.html (accessed on 25 February 2021).
- Boucek, R.J.; Alvarez, T.R. 5-Hydroxytryptamine: A cytospecific growth stimulator of cultured fibroblasts. Science 1970, 167, 898–899. [Google Scholar] [CrossRef]
- Wu, X.; Kang, H.; Liu, X.; Gao, J.; Zhao, K.; Ma, Z. Serum and xeno-free, chemically defined, no-plate-coating-based culture system for mesenchymal stromal cells from the umbilical cord. Cell Prolif. 2016, 49, 579–588. [Google Scholar] [CrossRef] [PubMed]
- ThermoFisher Scientific. Gibco™ 2-Mercaptoethanol. Available online: https://www.thermofisher.com/order/catalog/product/21985023#/21985023 (accessed on 27 February 2021).
- Zhao, X.; Liu, L.; Liu, D.; Fan, H.; Wang, Y.; Hu, Y.; Hou, Y. Progesterone Enhances Immunoregulatory Activity of Human Mesenchymal Stem Cells Via PGE 2 and IL-6. Am. J. Reprod. Immunol. 2012, 68, 290–300. [Google Scholar] [CrossRef]
- Devireddy, L.R.; Myers, M.; Screven, R.; Liu, Z.; Boxer, L. A serum-free medium formulation efficiently supports isolation and propagation of canine adipose-derived mesenchymal stem/stromal cells. PLoS ONE 2019, 14, e0210250. [Google Scholar] [CrossRef]
- Cell Culture Technologies LLC. Cell Culture Technologies. Available online: https://www.cellculture.com/ (accessed on 2 March 2021).
- Li, E.; Zhang, Z.; Jiang, B.; Yan, L.; Park, J.W.; Xu, R.H. Generation of mesenchymal stem cells from human embryonic stem cells in a complete serum-free condition. Int. J. Biol. Sci. 2018, 14, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Al-Saqi, S.H.; Saliem, M.; Quezada, H.C.; Ekblad, A.; Jonasson, A.F.; Hovatta, O.; Gotherstrom, C. Defined serum- and xeno-free cryopreservation of mesenchymal stem cells. Cell Tissue Bank. 2015, 16, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Oikonomopoulos, A.; Van Deen, W.K.; Manansala, A.R.; Lacey, P.N.; Tomakili, T.A.; Ziman, A.; Hommes, D.W. Optimization of human mesenchymal stem cell manufacturing: The effects of animal/xeno-free media. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chase, L.G.; Yang, S.; Zachar, V.; Yang, Z.; Lakshmipathy, U.; Bradford, J.; Boucher, S.E.; Vemuri, M.C. Development and Characterization of a Clinically Compliant Xeno-Free Culture Medium in Good Manufacturing Practice for Human Multipotent Mesenchymal Stem Cells. Stem Cells Transl. Med. 2012, 1, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Chase, L.G.; Lakshmipathy, U.; Solchaga, L.A.; Rao, M.S.; Vemuri, M.C. A novel serum-free medium for the expansion of human mesenchymal stem cells. Stem Cell Res. Ther. 2010, 1, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.Y.; Teo, K.L.; Lim, J.F.Y.; Chen, A.K.L.; Reuveny, S.; Oh, S.K.W. Serum-free media formulations are cell line-specific and require optimization for microcarrier culture. Cytotherapy 2015, 17, 1152–1165. [Google Scholar] [CrossRef]
- Bhat, S.; Viswanathan, P.; Chandanala, S.; Prasanna, S.J.; Seetharam, R.N. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci. Rep. 2021, 11, 3403. [Google Scholar] [CrossRef]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Park, S.-H.; Park, S.G.; Choi, J.-S.; Xia, Y.; Sung, J.-H. The Pivotal Role of Reactive Oxygen Species Generation in the Hypoxia-Induced Stimulation of Adipose-Derived Stem Cells. Stem Cells Dev. 2011, 20, 1753–1761. [Google Scholar] [CrossRef] [Green Version]
- Wenger, R.H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002, 16, 1151–1162. [Google Scholar] [CrossRef] [Green Version]
- BioSpherix. C-Chamber Incubator Subchamber. Available online: https://www.biospherix.com/products/c-chamber (accessed on 20 February 2021).
- Stemcell Technologies. Hypoxia Incubator Chamber. Available online: https://www.stemcell.com/hypoxia-incubator-chamber.html (accessed on 20 February 2021).
- Kay, A.G.; Dale, T.P.; Akram, K.M.; Mohan, P.; Hampson, K.; Maffulli, N.; Spiteri, M.A.; El Haj, A.J.; Forsyth, N.R. BMP2 repression and optimized culture conditions promote human bone marrow-derived mesenchymal stem cell isolation. Regen. Med. 2015, 10, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Grayson, W.L.; Zhao, F.; Bunnell, B.; Ma, T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2007, 358, 948–953. [Google Scholar] [CrossRef]
- Dos Santos, F.; Andrade, P.Z.; Boura, J.S.; Abecasis, M.M.; da Silva, C.L.; Cabral, J.M. Ex vivo expansion of human mesenchymal stem cells: A more effective cell proliferation kinetics and metabolism under hypoxia. J. Cell. Physiol. 2010, 223, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lavrentieva, A.; Majore, I.; Kasper, C.; Hass, R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun. Signal. 2010, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.C.; Chen, Y.J.; Yew, T.L.; Chen, L.L.; Wang, J.Y.; Chiu, C.H.; Hung, S.C. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood 2011, 117, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Basciano, L.; Nemos, C.; Foliguet, B.; de Isla, N.; de Carvalho, M.; Tran, N.; Dalloul, A. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 2011, 12, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valorani, M.G.; Montelatici, E.; Germani, A.; Biddle, A.; D’Alessandro, D.; Strollo, R.; Patrizi, M.P.; Lazzari, L.; Nye, E.; Otto, W.R.; et al. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012, 45, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, S.M.; Tsai, T.L.; Li, W.J. Macrophage migration inhibitory factor regulates AKT signaling in hypoxic culture to modulate senescence of human mesenchymal stem cells. Stem Cells Dev. 2014, 23, 852–865. [Google Scholar] [CrossRef]
- Choi, J.R.; Pingguan-Murphy, B.A. In Situ Normoxia Enhances Survival and Proliferation Rate of Human Adipose Tissue-Derived Stromal Cells without Increasing the Risk of Tumourigenesis. PLoS ONE 2015, 10, e0115034. [Google Scholar] [CrossRef]
- Ali, N.M.; Boo, L.; Yeap, S.K.; Ky, H.; Satharasinghe, D.A.; Liew, W.C.; Ong, H.K.; Cheong, S.K.; Kamarul, T. Probable impact of age and hypoxia on proliferation and microRNA expression profile of bone marrow-derived human mesenchymal stem cells. PeerJ 2016, 2016, e1536. [Google Scholar] [CrossRef]
- Pachón-Peña, G.; Serena, C.; Ejarque, M.; Petriz, J.; Duran, X.; Oliva-Olivera, W.; Simó, R.; Tinahones, F.J. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells from Adipose Tissue. Stem Cells Transl. Med. 2016, 5, 464–475. [Google Scholar] [CrossRef]
- Peng, L.; Shu, X.; Lang, C.; Yu, X. Effects of hypoxia on proliferation of human cord blood-derived mesenchymal stem cells. Cytotechnology 2016, 68, 1615–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratushnyy, A.Y.; Rudimova, Y.V.; Buravkova, L.B. Alteration of Hypoxia-Associated Gene Expression in Replicatively Senescent Mesenchymal Stromal Cells under Physiological Oxygen Level. Biochemistry 2019, 84, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Hwang, O.K.; Noh, Y.W.; Hong, J.T.; Lee, J.W. Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-Derived Stem Cells via Vascular Endothelial Growth Factor. Tissue Eng. Regen. Med. 2020, 17, 335–350. [Google Scholar] [CrossRef]
- Holzwarth, C.; Vaegler, M.; Gieseke, F.; Pfister, S.M.; Handgretinger, R.; Kerst, G.; Mller, I. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biol. 2010, 11, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pezzi, A.; Amorin, B.; Laureano, A.; Valim, V.; Dahmer, A.; Zambonato, B.; Sehn, F.; Wilke, I.; Bruschi, L.; Silva, M.; et al. Effects Of Hypoxia in Long-Term In Vitro Expansion of Human Bone Marrow Derived Mesenchymal Stem Cells. J Cell Biochem 2017, 118, 3072–3079. [Google Scholar] [CrossRef]
- Rosová, I.; Dao, M.; Capoccia, B.; Link, D.; Nolta, J.A. Hypoxic Preconditioning Results in Increased Motility and Improved Therapeutic Potential of Human Mesenchymal Stem Cells. Stem Cells 2008, 26, 2173–2182. [Google Scholar] [CrossRef] [Green Version]
- Dionigi, B.; Ahmed, A.; Pennington, E.C.; Zurakowski, D.; Fauza, D.O. A comparative analysis of human mesenchymal stem cell response to hypoxia in vitro: Implications to translational strategies. J. Pediatr. Surg. 2014, 49, 915–918. [Google Scholar] [CrossRef]
- Egger, D.; Oliveira, A.C.; Mallinger, B.; Hemeda, H.; Charwat, V.; Kasper, C. From 3D to 3D: Isolation of mesenchymal stem/stromal cells into a three-dimensional human platelet lysate matrix. Stem Cell Res. Ther. 2019, 10, 248. [Google Scholar] [CrossRef] [Green Version]
- Bartmann, C.; Rohde, E.; Schallmoser, K.; Pürstner, P.; Lanzer, G.; Linkesch, W.; Strunk, D. Two steps to functional mesenchymal stromal cells for clinical application. Transfusion 2007, 47, 1426–1435. [Google Scholar] [CrossRef]
- Both, S.K.; Muijsenberg, A.J.C.V.D.; Blitterswijk, C.A.V.; Boer, J.D.; Bruijn, J.D.D. A Rapid and Efficient Method for Expansion of Human Mesenchymal Stem Cells. Tissue Eng. 2007, 13, 3–9. [Google Scholar] [CrossRef]
- Sekiya, I.; Larson, B.L.; Smith, J.R.; Pochampally, R.; Cui, J.G.; Prockop, D.J. Expansion of human adult stem cells from bone marrow stroma: Conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells 2002, 20, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Colter, D.C.; Class, R.; DiGirolamo, C.M.; Prockop, D.J. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc. Natl. Acad. Sci. USA 2000, 97, 3213. [Google Scholar] [CrossRef]
- Prockop, D.J.; Sekiya, I.; Colter, D.C. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy 2001, 3, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, M.W.; Lee, T.H.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Cell culture density affects the stemness gene expression of adipose tissue-derived mesenchymal stem cells. Biomed. Rep. 2017, 6, 300–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.W.; Kim, D.S.; Yoo, K.H.; Kim, H.R.; Jang, I.K.; Lee, J.H.; Kim, S.Y.; Son, M.H.; Lee, S.H.; Jung, H.L.; et al. Human bone marrow-derived mesenchymal stem cell gene expression patterns vary with culture conditions. Blood Res. 2013, 48, 107–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binato, R.; de Souza Fernandez, T.; Lazzarotto-Silva, C.; Du Rocher, B.; Mencalha, A.; Pizzatti, L.; Bouzas, L.F.; Abdelhay, E. Stability of human mesenchymal stem cells during in vitro culture: Considerations for cell therapy. Cell Prolif. 2013, 46, 10–22. [Google Scholar] [CrossRef]
- Tsuji, K.; Ojima, M.; Otabe, K.; Horie, M.; Koga, H.; Sekiya, I.; Muneta, T. Effects of Different Cell-Detaching Methods on the Viability and Cell Surface Antigen Expression of Synovial Mesenchymal Stem Cells. Cell Transplant. 2017, 26, 1089–1102. [Google Scholar] [CrossRef] [Green Version]
- Heng, B.C.; Cowan, C.M.; Basu, S. Comparison of Enzymatic and Non-Enzymatic Means of Dissociating Adherent Monolayers of Mesenchymal Stem Cells. Biol. Proc. Online 2009, 11, 161. [Google Scholar] [CrossRef] [Green Version]
- Salzig, D.; Schmiermund, A.; Grace, P.P.; Elseberg, C.; Weber, C.; Czermak, P. Enzymatic detachment of therapeutic mesenchymal stromal cells grown on glass carriers in a bioreactor. Open Biomed. Eng. J. 2013, 7, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Salzig, D.; Leber, J.; Merkewitz, K.; Lange, M.C.; Köster, N.; Czermak, P. Attachment, Growth, and Detachment of Human Mesenchymal Stem Cells in a Chemically Defined Medium. Stem Cells Int. 2016, 2016, 5246584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della Bella, E.; Stoddart, M.J. Cell detachment rapidly induces changes in noncoding RNA expression in human mesenchymal stromal cells. BioTechniques 2019, 67, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ojima, M.; Tsuji, K.; Otabe, K.; Horie, M.; Koga, H.; Sekiya, I.; Muneta, T. Different methods of detaching adherent cells significantly affect the detection of stem cell antigens in synovial mesenchymal stem cells. Osteoarthr. Cartil. 2016, 24, S509–S510. [Google Scholar] [CrossRef]
- Kozanoglu, I.; Boga, C.; Ozdogu, H.; Maytalman, E.; Ovali, E.; Sozer, O. A detachment technique based on the thermophysiologic responses of cultured mesenchymal cells exposed to cold. Cytotherapy 2008, 10, 686–689. [Google Scholar] [CrossRef]
- Liao, T.; Moussallem, M.D.; Kim, J.; Schlenoff, J.B.; Ma, T. N-isopropylacrylamide-based thermoresponsive polyelectrolyte multilayer films for human mesenchymal stem cell expansion. Biotechnol. Prog. 2010, 26, 1705–1713. [Google Scholar] [CrossRef]
- Patel, N.G.; Cavicchia, J.P.; Zhang, G.; Zhang Newby, B.-M. Rapid cell sheet detachment using spin-coated pNIPAAm films retained on surfaces by an aminopropyltriethoxysilane network. Acta Biomater. 2012, 8, 2559–2567. [Google Scholar] [CrossRef]
- Nagase, K.; Hatakeyama, Y.; Shimizu, T.; Matsuura, K.; Yamato, M.; Takeda, N.; Okano, T. Thermoresponsive Cationic Copolymer Brushes for Mesenchymal Stem Cell Separation. Biomacromolecules 2015, 16, 532–540. [Google Scholar] [CrossRef]
- Nash, M.E.; Fan, X.; Carroll, W.M.; Gorelov, A.V.; Barry, F.P.; Shaw, G.; Rochev, Y.A. Thermoresponsive substrates used for the expansion of human mesenchymal stem cells and the preservation of immunophenotype. Stem Cell Rev. Rep. 2013, 9, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Cheng, F.; Liu, T.; Lu, J.R.; Song, K.; Jiang, L.; Wu, S.; Guo, W. Comparison of mesenchymal stem cells released from poly(N -isopropylacrylamide) copolymer film and by trypsinization. Biomed. Mater. 2012, 7, 035003. [Google Scholar] [CrossRef] [PubMed]
- Kurashina, Y.; Imashiro, C.; Hirano, M.; Kuribara, T.; Totani, K.; Ohnuma, K.; Friend, J.; Takemura, K. Enzyme-free release of adhered cells from standard culture dishes using intermittent ultrasonic traveling waves. Commun. Biol. 2019, 2, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; García, I.; Liz-Marzán, L.M. Plasmonic Surfaces for Cell Growth and Retrieval Triggered by Near-Infrared Light. Angew. Chem. Int. Ed. 2016, 55, 974–978. [Google Scholar] [CrossRef]
- Ikeda, T.; Ichikawa, K.; Shigeto, H.; Ishida, T.; Hirota, R.; Funabashi, H.; Kuroda, A. Arginine-mediated dissociation of single cells and cell sheets from a polystyrene culture dish. Biosci. Biotechnol. Biochem. 2019, 83, 2272–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhry, M.A.; Bowen, B.D.; Piret, J.M. Culture pH and osmolality influence proliferation and embryoid body yields of murine embryonic stem cells. Biochem. Eng. J. 2009, 45, 126–135. [Google Scholar] [CrossRef]
- Fekrazad, R.; Asefi, S.; Allahdadi, M.; Kalhori, K.A.M. Effect of Photobiomodulation on Mesenchymal Stem Cells. Photomed. Laser Surg. 2016, 34, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Stolzing, A.; Scutt, A. Effect of reduced culture temperature on antioxidant defences of mesenchymal stem cells. Free Radic. Biol. Med. 2006, 41, 326–338. [Google Scholar] [CrossRef]
- Ahmadyan, S.; Kabiri, M.; Hanaee-Ahvaz, H.; Farazmand, A. Osmolyte Type and the Osmolarity Level Affect Chondrogenesis of Mesenchymal Stem Cells. Appl. Biochem. Biotechnol. 2018, 185, 507–523. [Google Scholar] [CrossRef]
- Buyl, K.; Merimi, M.; Rodrigues, R.M.; Moussa Agha, D.; Melki, R.; Vanhaecke, T.; Bron, D.; Lewalle, P.; Meuleman, N.; Fahmi, H.; et al. The Impact of Cell-Expansion and Inflammation on The Immune-Biology of Human Adipose Tissue-Derived Mesenchymal Stromal Cells. J. Clin. Med. 2020, 9, 696. [Google Scholar] [CrossRef] [Green Version]

| Media Name | Description |
|---|---|
| DMEM | Dulbecco’s modified Eagle’s medium (MEM) |
| DMEM/LG/L-G | Dulbecco’s MEM (DMEM) with 1000 mg/mL glucose and L-glutamine |
| DMEM/HG/L-G | DMEM with 4500 mg/mL glucose and L-glutamine |
| DMEM/HG/GL | DMEM with 4500 mg/mL glucose and Glutamax |
| IMDM | Iscove’s modified Dulbecco’s medium with L-glutamine |
| aMEM | MEM alpha |
| aMEM/L-G | MEM alpha with L-glutamine |
| aMEM/GL | MEM alpha with Glutamax |
| Starting Material | Solution | Pooled (PC) | Platelet Counts (×109/mL) | Platelet Lysis | Expanded Cells | Supplementation | Outperformed FBS? | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| (Freeze/Thaw) | (Others) | ||||||||
| Aph-PC | Plasma | 10 | 1 | −80 °C | - | BM-MSC | 5% | yes | [120] |
| BC-PC | Plasma | 10–13 | 0.95 | −30 °C | - | UCB-MSC | 10% | yes | [124] |
| Exp Aph-PC | Plasma | yes | - | −80 °C | - | BM-MSC | 10% | yes | [113] |
| Exp Aph-PC | Plasma | - | - | −80 °C | - | BM-MSC | 5% | no | [118] |
| Aph-PC | Plasma | - | - | −80 °C | - | BM-MSC | 8% | no | [129] |
| Aph-PC | Plasma | - | 1.0–1.3 | - | S/D | AD-MSC | 10% | no | [135] |
| Aph-PC | Plasma | 10 | - | −80 °C | - | BM-MSC, UCB-MSC | 5–10% | yes | [128] |
| BC-PC, leuko depleted | Plasma | - | - | −80 °C | - | AD-SC | 5% | yes | [123] |
| Aph-PC | Plasma | 4–6 don | - | −80 °C | Sonication | BM-MSC | 10% | yes | [119] |
| BC-PC | Plasma | 2 | - | −30 °C | - | BM-MSC, AD-MSC | 2.5–10% | yes | [121] |
| BC-PC | Plasma | 2 | - | −30 °C | Thrombin | BM-MSC, AD-MSC | 2.5–10% | yes | [121] |
| BC-PC | Saline | 6 | 3.34 | −80 °C | - | AD-MSC | 5% | yes | [125] |
| BC-PC | Plasma | 6 | 3.58 | −80 °C | - | AD-MSC | 5% | yes | [125] |
| Exp Aph-PC | Plasma | 5 don | 1 | −80 °C | CaCl2 | BM-MSC | 10% | yes | [114] |
| Exp BC-PC | Plasma | yes | - | −80 °C | - | BM-MSC | 10% | yes | [117] |
| BC-PC, path. Inactivated | Add Sol | 12 don | 1 | −80 °C | - | BM-MSC | 10% | yes | [127] |
| BC-PRP | Plasma | 10–20 | 10 | −196 °C | Lyophilization, Irradiation | BM-MSC | 5% | yes | [122] |
| PL-Serum | - | 49–109 | - | - | CaCl2 20% w/v | commercial BM-MSC | 10% | yes | [130] |
| BC-PC | Plasma | 2 | 2.03 | −25 °C | - | AD-MSC | 1–10% | yes | [116] |
| BC-PC | TSOL | 2 | 0.91 | −25 °C | - | AD-MSC | 1–10% | yes | [116] |
| Exp BC-PC | Plasma | 2 | 0.41 | −25 °C | - | AD-MSC | 1–10% | yes | [116] |
| Exp BC-PC | TSOL | 2 | 0.19 | −25 °C | - | AD-MSC | 1–10% | yes | [116] |
| Exp Aph-PC | - | 3–4 don | - | −80 °C | - | AD-MSC | 0.1–1% | yes | [115] |
| Brand Name | Supplier | Pooled (PC) | Platelet Counts (×109/mL) | Platelet Lysis | Expanded Cells | Supplementation | Outperformed FBS? | REF | |
| (freeze/thaw) | (others) | ||||||||
| PLTMAX | Sigma Aldrich | yes | - | - | - | AD-MSC | 5% | yes | [134] |
| MesenCult hPL media | Stemcell Technologies | yes | - | - | - | AD-MSC | 10% | yes | [134] |
| PLUS™ hPL | Compass Biomedical | yes | - | - | - | BM-MSC | 5% | yes | [131] |
| PLTmax | MERCK | yes | - | - | - | AD-MSC | 1–10% | yes | [132] |
| phPL | PL BioScience | yes | - | - | BM-MSC | 10% | yes | [133] | |
| Basal Medium | Supplier | Add. Supplements | Cells Expanded | Cultivation Time | Outperformed CTL Medium? | Diff Capacity | Cell Surface Markers | Ref. |
|---|---|---|---|---|---|---|---|---|
| MSCGM-CD | Lonza | - | UC-MSC | 5–7 passages | n.t. | unaltered | unaltered | [38] |
| MesenCult | Stemcell Technologies | - | hESC-derived MSCs | - | yes | n.t. | unaltered | [155] |
| Mesencult-XF™ | Stemcell Technologies | - | AD-MSC, BM-MSC | - | yes | improved (AD-MSC), decreased (BM-MSC) | altered (BM-MSC) | [156] |
| StemPro MSC SFM XenoFree™ | Life Technologies | - | BM-MSC | up to p4 | no | n.t. | n.t. | [139] |
| Mesencult-XF™ | Stemcell Technologies | - | BM-MSC | up to p4 | no | unaltered | unaltered | [139] |
| BD Mosaic™ Mesenchymal Stem Cell Serum-Free media | BD Biosciences | - | BM-MSC | up to p4 | no | unaltered | unaltered | [139] |
| StemPro® MSC SFM XenoFree, Invitrogen | Life Technologies | - | AD-MSC, BM-MSC | - | yes | altered | unaltered | [157] |
| StemPro MSC SFM XenoFree™ | Life Technologies | PDGF-BB, bFGF, TGF-β1 | ASC line, BM-MSC | up to p9 | yes | unaltered | unaltered | [158] |
| StemPro® MSC SFM | Life Technologies | PDGF-BB, bFGF, TGF-β1 | BM-MSC | 8 passages | no | unaltered | unaltered | [159] |
| StemPro MSC SFM Xenofree | Life Technologies | - | BM-MSC, UC-MSC, AD-MSC | 7 days | yes | n.t. | n.t. | [160] |
| MSC Nutristem XF | Biological Industries | - | BM-MSC, UC-MSC, AD-MSC | 7 days | yes | n.t. | n.t. | [160] |
| MesenCult-XF | Stemcell Technologies | - | BM-MSC, UC-MSC, AD-MSC | 7 days | yes | n.t. | n.t. | [160] |
| StemXVivo SFM Human MSC Expansion Medium | R&D Systems | - | BM-MSC, UC-MSC, AD-MSC | 7 days | yes | n.t. | n.t. | [160] |
| RoosterNourish-MSC XF | RoosterBio, Inc. | BM-MSC | up to p5 | no | unaltered | unaltered | [161] | |
| StemMACS-MSC Expansion Media Kit XF | Miltenyi Biotec | BM-MSC | up to p5 | no | unaltered | unaltered | [161] | |
| MSC NutriStem XF | Biological Industries | BM-MSC | up to p5 | no | unaltered | unaltered | [161] | |
| StemXVivo SFM Human MSC Expansion Medium | R&D Systems | BM-MSC | up to p5 | no | unaltered | unaltered | [161] |
| Ref. | [144] | [145] | [150] |
| Basal Media | IMDM | IMDM | 17.7 g/L IDMD |
| Supplemented With | 17.91 ng bovine FGF/mL | 5 mg/mL human serum albumin | 5 mM l-glutamine |
| 2.80 mg/mL human albumin | 100 μg/mL human Ex-Cyte lipoprotein | 3.024 g/L sodium bicarbonate | |
| 27. 65 µM hydrocortisone | 2 μg/mL saturated human transferrin | 10 mg/L rh insulin | |
| 1.18% SITE (S4920; containing 0.5 µg/mL sodium selenite, 1.0 mg/mL bovine insulin, 0.55 mg/mL human transferrin, 0.2 mg/mL ethanolamine; 100-fold concentrate) | 10 μg/mL rh insulin | 10 mg/L rh transferrin | |
| 1.0% 100 × MEM vitamins | 4 g/L rh serum albumin | ||
| 0.89% MEM essential amino acids | 55 μM β-mercaptoethanol | ||
| 0.4% MEM nonessential amino acids | 0.1% chemically defined lipid concentrate | ||
| 1 mM sodium pyruvate | 2% MEM essential amino acids solution | ||
| 1 mM GlutaMAX-I supplement | 1% MEM non-essential amino acid solution | ||
| 10 μg/mL folic acid | 1% Vitamins solution | ||
| 10 μM ascorbic acid 2-phosphate | 0.1% trace elements solution | ||
| 1.0 μg/mL Biotin | 50 μg/L hydrocortisone | ||
| 1.36 μg/mL vitamin B12 mix | 50 mg/L l-ascorbic acid-2-phosphate | ||
| 500fold diluted trace element mix | 5 mg/L rh fibronectin | ||
| 4 × 10−8 M FeSO4 | 5 μg/L progesterone | ||
| 10 μg/mL nucleoside mix (ribonucleosides, 2′-deoxyribo-nucleosides, uridine, and thymidine) | 10 mg/L putrescine | ||
| 2 mg/L serotonin | |||
| 10 ng/mL rh EGF | |||
| 1.0% antibiotic/antimycotic | 10 ng/mL rh basic FGF | ||
| 10–20 ng/mL rh PDGF ββ homodimer or 10−5 to 10−6 M 5-hydroxytryptamine | 10 ng/mL rh PDGF | ||
| 10 ng/mL rh IGF |
| Cells | O2 (%) | Exposure Time | Outperformed 21% O2? | Diff Capacity | Cell Surface Markers | Other Cultivation Parameters Analyzed | Ref. |
|---|---|---|---|---|---|---|---|
| BM-MSC | 2 | 6 weeks | yes | unaltered | n.t. | [169] | |
| BM-MSC | 1–3 | 16 h | no | n.t. | n.t. | HGF stimulation | [184] |
| BM-MSC | 5 | up to p4 | yes | unaltered | unaltered | hPL | [113] |
| BM-MSC | 2 | 14 d | yes | n.t. | unaltered | [113] | |
| UC-MSC | 1.5–5 | 3 d | yes | n.t. | n.t. | [171] | |
| BM-MSC | 1–5 | 14 d | no | decreased | unaltered | [182] | |
| BM-MSC | 1 | 84 days | yes | improved | unaltered | [172] | |
| BM-MSC | 5 | up to p3 | yes | improved | unaltered | [173] | |
| AD-MSC | 2 | 7 d | yes | improved | unaltered | [174] | |
| BM-MSC | 1 | up to 90 d | yes | improved | upregulated | [175] | |
| BM-MSC, AD-MSC, AF-MSC, UCB-MSC | 1 | 7 d | depending on cell source (prenatal yes, postnatal no) | n.t. | n.t. | prenatal + postnatal material | [185] |
| AD-MSC | 2 | up to 21 d | yes | unaltered | unaltered | [176] | |
| UCB-MSC | 5 | 5 d | yes | n.t. | unaltered | [179] | |
| AD-MSC | 5 | up to 14 d | yes | n.t. | slightly altered | lean + obese donors | [178] |
| BM-MSC | 5 | up to p15 | yes | unaltered | n.t. | donor age | [177] |
| BM-MSC | 1–4 | up to p2 | no | unaltered | unaltered | [183] | |
| UCB-MSC | 3 | 5 d | yes | unaltered | unaltered | Ca2+ | [61] |
| AD-MSC | 5 | up to p28 | yes (until passage 23) | n.t. | n.t. | [180] | |
| AD-MSC | 1 | 48 h | yes | decreased (osteogenic) increased (chondrogenic) | unaltered | [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolits, I.; Nebel, S.; Egger, D.; Kreß, S.; Kasper, C. Towards Physiologic Culture Approaches to Improve Standard Cultivation of Mesenchymal Stem Cells. Cells 2021, 10, 886. https://doi.org/10.3390/cells10040886
Nikolits I, Nebel S, Egger D, Kreß S, Kasper C. Towards Physiologic Culture Approaches to Improve Standard Cultivation of Mesenchymal Stem Cells. Cells. 2021; 10(4):886. https://doi.org/10.3390/cells10040886
Chicago/Turabian StyleNikolits, Ilias, Sabrina Nebel, Dominik Egger, Sebastian Kreß, and Cornelia Kasper. 2021. "Towards Physiologic Culture Approaches to Improve Standard Cultivation of Mesenchymal Stem Cells" Cells 10, no. 4: 886. https://doi.org/10.3390/cells10040886
APA StyleNikolits, I., Nebel, S., Egger, D., Kreß, S., & Kasper, C. (2021). Towards Physiologic Culture Approaches to Improve Standard Cultivation of Mesenchymal Stem Cells. Cells, 10(4), 886. https://doi.org/10.3390/cells10040886