Incorporation of 5-Bromo-2′-deoxyuridine into DNA and Proliferative Behavior of Cerebellar Neuroblasts: All That Glitters Is Not Gold
Abstract
:1. Introduction
2. The Thymidine Analogue 5-Bromo-2′-Deoxyuridine: An Overview
3. The Cerebellum: A Model to Assess the Effects of Bromodeoxyuridine Administration
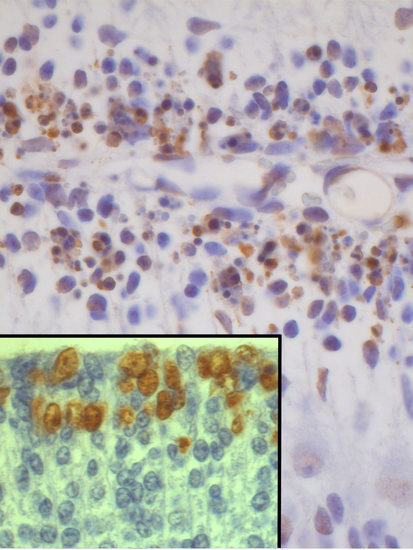
4. Effect of Bromodeoxyuridine Exposure on the Cerebellar Neuroepithelium
5. Effects of Bromodeoxyuridine Exposure on the Cerebellar External Granular Layer Neuroblasts
6. Inferring Purkinje Cells and Deep Cerebellar Neurons Developmental Timetables with Bromodeoxyuridine or Tritiated Thymidine. What Is the Most Suitable Marker?
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rakic, P. Pre and post-development neurogenesis in primates. Clinic. Neurosci. Res. 2002, 2, 29–39. [Google Scholar] [CrossRef]
- Breunig, J.J.; Arellano, J.I.; Macklis, J.D.; Rakic, P. Everything that glitters isn’t gold: A critical review of postnatal neural precursor analyses. Cell Stem Cell 2007, 1, 612–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duque, A.; Rakic, P. Different effects of BrdU and 3H-Thymidine incorporation into DNA on cell proliferation, position and fate. J. Neurosci. 2011, 31, 15205–15217. [Google Scholar] [CrossRef]
- Martí, J.; Santa-Cruz, M.C.; Serra, R.; Hervás, J.P. Systematic differences in time of cerebellar-neuron origin derived from bromodeoxyuridine immunoperoxidase staining protocols and tritiated thymidine autoradiographic: A comparative study. Int. J. Dev. Neurosci. 2015, 47, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Martí, J.; Molina, V.; Santa-Cruz, M.C.; Hervás, J.P. Developmental injury to the cerebellar cortex following hydroxyurea treatment in early postnatal life: An immunohistochemical and electron microscopic study. Neurotox. Res. 2017, 31, 187–203. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, L.; Vons, O.; Valero, O.; Martí, J. Hydroxyurea exposure and developmental of the cerebellar external granular layer: Effects on granule cell precursors, Bergmann glial and microglial cells. Neurotox. Res. 2019, 35, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Duque, A.; Rakic, P. Identification of proliferating and migration cell by BrdU and other thymidine analogs: Benefits and limitations. In Immunocytochemistry and Related Techniques; Merighi, A., Lossi, L., Eds.; Humana Press: New York, NY, USA, 2015; Volume 101, Chapter 7; pp. 123–139. [Google Scholar]
- Lehner, B.; Sandner, B.; Marschallinger, J.; Lehner, C.; Furtner, T.; Couillard-Despres, S.; Rivera, F.J.; Brockhoff, G.; Bauer, H.C.; Weidner, N.; et al. The dark side of BrdU in neural stem cell biology: Detrimental effects on cell cycle, differentiation and survival. Cell Tissue Res. 2011, 345, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Sekerkova, G.; Ilijic, E.; Mugnaini, E. Bromodeoxyuridine administered during neurogenesis of the projection neurons causes cerebellar defects in rat. J. Comp. Neurol. 2004, 470, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, L.; Martí, J. Administration of 5-bromo-2′deoxyuridine interferes with neuroblast proliferation and promotes apoptotic cell death in the rat cerebellar neuroepithelium. J. Comp. Neurol. 2021, 529, 1081–1096. [Google Scholar] [CrossRef]
- Duque, A.; Spector, R. A balanced evaluation of the evidence for adult neurogenesis in humans: Implications for the Neuropsychiatry disorders. Brain Struct. Funct. 2019, 224, 2281–2295. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, C.; Parolisi, R.; Bonfanti, L. Brain structural plasticity: From adult neurogenesis to immature neurons. Front. Neurosci. 2020, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, R.S.; Lewin, S.B.; Miller, M.W. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J. Neurocytol. 1989, 18, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Gratzner, H.G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine. A new reagent for detection of DNA replication. Science 1982, 218, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Molina, V.; Rodríguez-Vázquez, L.; Owen, D.; Valero, O.; Martí, J. Cell cycle analysis in the rat external granular layer evaluated by several bromodeoxyuridine immunoperoxidase staining protocols. Histochem. Cell Biol. 2017, 148, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hidalgo, A. Adult neurogenesis in the Drosophila brain: The evidence and the void. Int. J. Mol. Sci. 2020, 21, 6653. [Google Scholar] [CrossRef]
- Larson, T.A.; Thatra, N.M.; Hou, D.; Hu, R.A.; Brenowitz, E.A. Seasonal change in neuronal turnover in a forebrain nucleus in adult songbirds. J. Comp. Neurol. 2019, 527, 767–779. [Google Scholar] [CrossRef]
- Lanctot, A.A.; Guo, Y.; Le, Y.; Edens, B.M.; Nowakowski, R.S.; Feng, Y. Loss of brap results in premature G1/S phase transition and impeded neural progenitor differentiation. Cell Rep. 2017, 20, 1148–1160. [Google Scholar] [CrossRef] [Green Version]
- Rash, B.G.; Duque, A.; Morozov, Y.M.; Arellano, J.I.; Micali, N.; Rakic, P. Gliogenesis in the outer subventricular zone promotes enlargement and gyrification of the primate cerebrum. Proc. Natl. Acad. Sci. USA 2019, 116, 7089–7094. [Google Scholar] [CrossRef] [Green Version]
- Martí, J.; Rodríguez-Vázquez, L. An immunocytochemical approach to the analysis of the cell division cycle in the cerebellar neuroepithelium. Cell Cycle 2020, 19, 2451–2459. [Google Scholar] [CrossRef]
- Costandi, M. Warning on neural technique. Nature 2011. [Google Scholar] [CrossRef]
- Levkoff, L.H.; Marshall, G.P., 2nd; Ross, H.H.; Caldeira, M.; Reynolds, B.A.; Cakiroglu, M.; Mariani, C.L.; Streit, W.J.; Laywell, E.D. Bromodeoxyuridine inhibits cancer cell proliferation in vitro and in vivo. Neoplasia 2008, 10, 804–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michishita, E.; Nakabayashi, K.; Suzuki, T.; Kaul, S.C.; Ogino, H.; Fujii, M.; Mitsui, Y.; Ayusawa, D. 5-Bromodeoxyuridine induces senescence-like phenomena in mammalian cells regardless of cell type or species. J. Biochem. 1999, 126, 1052–1059. [Google Scholar] [PubMed]
- Ross, H.H.; Levkoff, L.H.; Marshall, G.P.; Caldeira, M.; Steindler, D.A.; Reynolds, B.A.; Laywell, E.D. Bromodeoxyuridine induces senescence in neural stem and progenitor cells. Stem Cells 2008, 26, 3218–3227. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, M.A.; He, X.; Svendsen, C.N. 5-Bromo-2’-deoxyuridine is selectively toxic to neural precursors in vitro. Eur. J. Neurosci. 2005, 22, 2965–2970. [Google Scholar] [CrossRef]
- Taupin, P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007, 53, 198–214. [Google Scholar] [CrossRef]
- Schneider, L.; di Fagagna, F.A. Neural stem cells exposed to BrdU lose their global DNA methylation and undergo astrocytic differentiation. Nucleic Acids Res. 2012, 40, 5332–5342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowell, J.J.; Ragsdale, C.W. BrdU birth dating can produce errors in cell fate specification in chick brain development. J. Histochem. Cytochem. 2012, 60, 801–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggers, W.J.; Barnea, E.R.; Sanyal, M.K. Anomalous neural differentiation induced by 5-bromo-2’-deoxyuridine during organogenesis in the rat. Teratology 1987, 35, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Kolb, B.; Pedersen, B.; Ballermann, M.; Gibb, R.; Whishaw, I.Q. Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J. Neurosci. 1999, 19, 2337–2346. [Google Scholar] [CrossRef] [Green Version]
- Kuwagata, M.; Ogawa, T.; Nagata, T.; Shioda, S. The evaluation of early embryonic neurogenesis after exposure to the genotoxic agent 5-bromo-2’-deoxyuridine in mice. Neurotoxicology 2007, 28, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, S.; Nakabayashi, K.; Fujii, M.; Scherer, S.W.; Ayusawa, D. Early BrdU-responsive genes constitute a novel class of senescence-associated genes in human cells. Exp. Cell Res. 2005, 304, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Minagawa, S.; Michishita, E.; Ogino, H.; Fujii, M.; Mitsui, Y.; Ayusawa, D. Induction of senescence-associated genes by 5-bromodeoxyuridine in HeLa cells. Exp. Gerontol. 2001, 36, 465–474. [Google Scholar] [CrossRef]
- Jacobs, B.; Johnson, N.L.; Wahl, D.; Schall, M.; Maseko, B.C.; Lewandowski, A.; Raghanti, M.A.; Wicinski, B.; Butti, C.; Hopkins, W.D.; et al. Comparative neuronal morphology of the cerebellar cortex in afrotherians, carnivores, cetartiodactyls, and primates. Front. Neuroanat. 2014, 8, 24. [Google Scholar]
- Sugahara, F.; Murakami, Y.; Pascual-Anaya, J.; Kuratani, S. Reconstructing the ancestral vertebrate braim. Dev. Growth Differ. 2017, 59, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Macrì, S.; Di-Poï, N. Heterochronic developmental shifts underlying squamate cerebellar diversity unveil the key features of ammiote cerebellogenesis. Front. Cell. Dev. Biol. 2020, 8, 593377. [Google Scholar] [CrossRef]
- Sillitoe, R.V.; Joyner, A.L. Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol. 2007, 23, 549–577. [Google Scholar] [CrossRef] [PubMed]
- Sudarov, A.; Joyner, A.L. Cerebellum morphogenesis: The foliation pattern is orchestrated by multi-cellular anchoring centers. Neural Dev. 2007, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Altman, J.; Bayer, S.A. Development of the Cerebellar System: In Relation to Its Evolution, Structure and Functions; CRC Press, Inc.: Boca Raton, FL, USA, 1997. [Google Scholar]
- Green, M.J.; Wingate, R.J.T. Developmental origins of diversity in cerebellar output nuclei. Neural Dev. 2014, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Houck, B.D.; Person, A.L. Cerebellar loops: A review of the nucleocortical pathway. Cerebellum 2014, 13, 378–385. [Google Scholar] [CrossRef] [Green Version]
- Cerminara, N.L.; Lang, E.J.; Sillitoe, R.V.; Apps, R. Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat. Rev. Neurosci. 2015, 16, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Schilling, K.; Oberdick, J.; Rossi, F.; Baader, S.L. Besides Purkinje cells and granule neurons: An appraisal of the cell biology of the interneurons of the cerebellar cortex. Histochem. Cell Biol. 2008, 130, 601–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marzban, H.; Del Bigio, M.R.; Alizadeh, J.; Ghavami, S.; Zachariah, R.M.; Rastegar, M. Cellular commitment in the developing cerebellum. Front. Cell. Dev. 2015, 8, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Consalez, G.G.; Goldowitz, D.; Casoni, F.; Hawkes, R. Origins, development, and compartmentation of the granule cell of the cerebellum. Front. Neural Circuits 2021, 14, 611841. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.Y.; Wan, G.X.; Cheng, A.S.K.; Cheung, K.K. In search of molecular markers for cerebellar neurons. Int. J. Mol. Sci. 2021, 22, 1850. [Google Scholar] [CrossRef] [PubMed]
- Leto, K.; Arancillo, M.; Becker, E.B.; Buffo, A.; Chiang, C.; Ding, B.; Dobyns, W.B.; Dusart, I.; Haldipur, P.; Hatten, M.E.; et al. Consensus paper: Cerebellar development. Cerebellum 2016, 15, 789–828. [Google Scholar] [CrossRef]
- Nakamura, H.; Sato, T.; Suzuki-Hirano, A. Isthmus organizer for mesencephalon and metencephalon. Dev. Growth Differ. 2008, 50, S113–S118. [Google Scholar] [CrossRef]
- Joyner, A.L.; Liu, A.; Millet, S. Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr. Opin. Cell Biol. 2000, 12, 736–741. [Google Scholar] [CrossRef]
- Carletti, B.; Rossi, F. Neurogenesis in the cerebellum. Neuroscientist 2008, 14, 91–100. [Google Scholar] [CrossRef]
- Beckinghausen, J.; Sillitoe, R.V. Insights into cerebellar development and connectivity. Neurosci. Lett. 2019, 688, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, C. Cellular and genetic regulation of the development of the cerebellar system. Prog. Neurobiol. 2004, 72, 295–339. [Google Scholar] [CrossRef]
- Orvis, G.D.; Hartzell, A.L.; Smith, J.B.; Barraza, L.H.; Wilson, S.L.; Szulc, K.U.; Turnbull, D.H.; Joyner, A.L. The engrailed homeobox genes are required in multiple cell lineages to coordinate sequential formation of fissures and growth of the cerebellum. Dev. Biol. 2012, 367, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wassef, M.; Joyner, A.L. Early mesencephalon/metencephalon patterning and development of the cerebellum. Perspect Dev. Neurobiol. 1997, 5, 3–16. [Google Scholar]
- Carter, R.A.; Bihannic, L.; Rosencrane, C.; Hadley, J.L.; Tong, Y.; Phoenix, T.N.; Natarajan, S.; Easton, J.; Northcott, P.A.; Gawad, C. A single-cell transcriptional atlas of the developing murine cerebellum. Curr. Biol. 2018, 28, 2910–2920. [Google Scholar] [CrossRef] [Green Version]
- Chédotal, A. Should I stay or should I go? Becoming a granule cell. Trends Neurosci. 2010, 33, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Manto, M. Toxic agents causing cerebellar ataxias. Handb. Clin. Neurol. 2012, 103, 201–213. [Google Scholar]
- Takahashi, T.; Nowakowski, R.S.; Caviness, V.S. BrdU as an S-phase marker for quantitative studies of cytokinetic behavior in the murine cerebral ventricular zone. J. Neurocytol. 1992, 21, 185–197. [Google Scholar] [CrossRef]
- Takahashi, T.; Goto, T.; Miyama, S.; Nowakowski, R.S.; Caviness, V.S. Sequence of neuron origin and neocortical laminar fate: Relation to cell cycle of origin in the development murine cerebral wall. J. Neurocytol. 1999, 19, 10357–10371. [Google Scholar] [CrossRef]
- Hancock, A.; Priester, C.; Kidder, E.; Keith, J.R. Does 5-bromo-2′-deoxyuridine (BrdU) disrupt cell proliferation and Neuronal maturation in the adult rat hippocampus in vivo? Behav. Brain Res. 2009, 199, 218–221. [Google Scholar] [CrossRef] [Green Version]
- Yoshihara, T.; Hibi, S.; Tsunamoto, K.; Todo, S.; Imashuku, S. Increased levels of alfa V-associated integrins in association with growth inhibition of cultured tumor cells by bromodeoxyuridine. Anticancer Res. 1997, 17, 833–838. [Google Scholar]
- Masterson, J.C.; O’Dea, S. 5-bromo-2-deoxyuridine activates DNA damage signaling responses and induces a senescence-like phenotype in p16-null lung cancer cells. Anticancer Drugs 2007, 18, 1053–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauder, J.M. The effects of early hypo- and hyperthyroidism on the development of rat cerebellar cortex. III. Kinetics of cell proliferation in the external granular layer. Brain Res. 1977, 126, 31–51. [Google Scholar] [CrossRef]
- Bayer, S.A.; Altman, J. Directions in neurogenetic gradients and patterns of anatomical connections in the telencephalon. Prog. Neurobiol. 1987, 29, 57–106. [Google Scholar] [CrossRef]
- Torii, M.; Sasaki, M.; Chang, Y.-W.; Ishii, S.; Waxman, S.G.; Kocsis, J.D.; Rakic, P.; Hashimoto-Torii, K. Detection of vulnerable neurons damaged by environmental insults in utero. Proc. Natl. Acad. Sci. USA 2017, 114, 2367–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowakowski, R.S.; Hayes, N.L. New neurons: Extraordinary evidence or extraordinary conclusion? Science 2000, 288, 771. [Google Scholar] [CrossRef] [Green Version]
- Rakic, P. Neurogenesis in adult primate neocortex: An evaluation of the evidence. Nat. Rev. Neurosci. 2002, 3, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P. Adult neurogenesis in mammals: An identity crisis. J. Neurosci. 2002, 22, 614–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martí-Clúa, J. Developmental timetables and gradients of neurogenesis in cerebellar Purkinje cells and deep glutaminergic neurons: A comparative study between the mouse and the rat. Anat. Rec. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martí-Clúa, J. Incorporation of 5-Bromo-2′-deoxyuridine into DNA and Proliferative Behavior of Cerebellar Neuroblasts: All That Glitters Is Not Gold. Cells 2021, 10, 1453. https://doi.org/10.3390/cells10061453
Martí-Clúa J. Incorporation of 5-Bromo-2′-deoxyuridine into DNA and Proliferative Behavior of Cerebellar Neuroblasts: All That Glitters Is Not Gold. Cells. 2021; 10(6):1453. https://doi.org/10.3390/cells10061453
Chicago/Turabian StyleMartí-Clúa, Joaquín. 2021. "Incorporation of 5-Bromo-2′-deoxyuridine into DNA and Proliferative Behavior of Cerebellar Neuroblasts: All That Glitters Is Not Gold" Cells 10, no. 6: 1453. https://doi.org/10.3390/cells10061453
APA StyleMartí-Clúa, J. (2021). Incorporation of 5-Bromo-2′-deoxyuridine into DNA and Proliferative Behavior of Cerebellar Neuroblasts: All That Glitters Is Not Gold. Cells, 10(6), 1453. https://doi.org/10.3390/cells10061453