1. Introduction
For the defense of invaded pathogens, neutrophils are rapidly recruited from the blood to infected tissue sites. Granulocytic neutrophils are the first immune cells arriving at the site of infection via postcapillary venules [
1]. The migration of neutrophils towards the infected or inflamed site within the tissue can be divided into different phases [
2,
3]. Initially, neutrophils roll along the vessel wall, followed by their attachment to the endothelial cells, subsequent neutrophil arrest and crawling, before they transmigrate through the vessel wall into the tissue [
1].
For a long time, it had been accepted that neutrophils undergo apoptosis at the site of infection and get cleared by macrophages. However, evidence is accumulating that a considerable proportion of the neutrophils that entered inflamed tissue sites are capable to migrate back to the blood circulation [
2]. This reverse migration is usually mediated by chemo-attractants [
2]. There is evidence that Leukotriene B4 (LTB
4) is not only involved in the migration towards the tissue but also in the reverse migration out of the tissue [
2]. Likewise, the atypical receptor ACKR1 was shown to be involved in this process [
4].
In addition to migrating back to blood circulation, it was also suggested that neutrophils can exit from inflamed sites via afferent lymphatic vessels [
2]. The presence of neutrophils inside lymphatic vessels was shown earlier by microscopy, indicating the migration of neutrophils via lymphatic vessels towards draining lymph nodes [
5,
6,
7,
8]. Additionally, it was shown that neutrophil migration was restricted to the ipsilateral lymph nodes draining the site of infection but not to the contralateral site. These observations favor the hypothesis that neutrophils enter lymph nodes via lymphatics, rather than blood vessels [
5,
7,
9]. In contrast, recent studies excluded neutrophil lymph node entry via lymphatics, as neutrophils were only found within blood but not lymphatic vessels [
10].
In general, neutrophil reverse migration via lymphatics has not been unambiguously characterized. There is evidence that neutrophil migration from the inflamed tissue to the draining lymph node is dependent on the chemokine receptor CCR7 [
11]. In that model CCR7 on neutrophils recognizes high levels of the chemokines CCL19 and CCL21, which are produced by fibroblastic reticular cells inside the T cell zone of the lymph node [
3,
11]. In contrast, Hampton and colleagues showed lately in an
S. aureus infection model that neutrophils are capable of migrating to the lymph node independent of CCR7 [
6,
12]. A recent study could confirm CCR7-independent neutrophil migration to draining lymph nodes during
S. aureus infection [
10]. The model of a CCR7-independent migration of neutrophils from the infection site via afferent lymphatics to the draining lymph node seems rather plausible since this chemokine receptor is hardly expressed on any neutrophil subset [
13]. Furthermore, CD11b (Integrin α-M) and the chemokine receptor CXCR4 were recently suggested to contribute to neutrophil lymph node homing via lymphatics [
6,
12]. Blocking of CD11b or CXCR4 [
13] resulted in lower neutrophil numbers in the draining lymph nodes upon
S. aureus infection [
6].
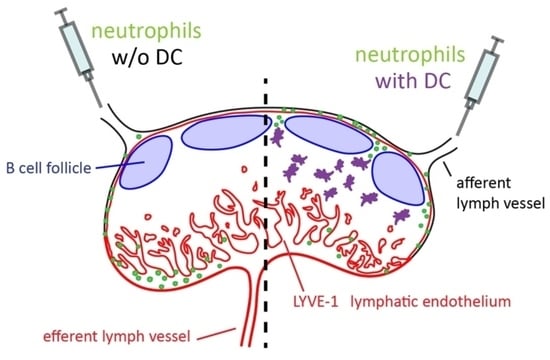
To circumvent the requirements that control neutrophil lymphatic entry at peripheral tissue sites we adoptively transferred neutrophils by intra lymphatic injection into the afferent lymphatic vessel that drains towards the popliteal lymph node in mice. We show that both resting as well as activated neutrophils largely locate to the medullary sinuses and are largely excluded from the deep T cell zone. However, when delivered together with dendritic cells neutrophils get access into the interfollicular area.
2. Materials and Methods
2.1. Mice
C57BL/6 N Crl (C57BL/6; Charles River, (Sulzfeld, Germany) were kept in the central animal facility at Hannover Medical School under specific pathogen-free conditions and were used at the ages of between 7 and 14 weeks. All animal experiments were approved by the Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit (LAVES; 33.12-42502-04-17/2660; 24 November 2017).
2.2. Immunization
In immunization and infection models, mice were subcutaneously (s.c.) injected with different pathogens or pathogen-derived components, to induce an immune response in the popliteal lymph node. Mice were anesthetized with a single intraperitoneal injection of 50 mg/kg body weight Ketamin and 10 mg/kg body weight Xylazin, and pathogens were s.c. injected in the footpad of the hind leg using a volume of 30 µL PBS four hours to 1 day before intra-lymphatic (i.l.) cell delivery. Per s.c. footpad injection 107 CFU of heat-inactivated Pseudomonas (P.) aeruginosa, or 1 × 106 IU MVA (Modified Vaccinia virus Ankara–Virus) were applied. Lipopolysaccharide (LPS) was injected s.c. at a concentration of 0.3 mg/kg body weight in 30 µL PBS to activate lymph nodes. In other experiments, 5 mg/kg body weight of the tripeptide fMLP (N-Formylmethionyl-leucyl-phenylalanine) were used for the s.c. injection. One day before cell isolation, donor mice were intranasally infected with 1 × 108 IU MVA for the generation of in vivo-activated neutrophils.
2.3. Intra-Lymphatic Injection
For the i.l. cell transfer, mice were anesthetized by intraperitoneal injection of 100 mg/kg body weight Ketamin and 10 mg/kg body weight Xylazin. Subsequently, hind legs were shaved and a short longitudinal skin incision above the vena saphena allowed the visualization of both lymphatic vessels left and right to the vena saphena. Borosilicate glass capillaries (outer diameter: 1.5 mm; inner diameter: 1.17 mm; Harvard Apparatus, Holliston, MA, USA) were pulled either manually or with the P-1000 Flaming/Brown micropipette puller (Sutter Instrument, Novato, CA, USA), and ground using the EG-44/EG-45 micropipette grinder (Narishige, London, UK). Cell suspensions with a volume of 5 to 10 µL (50,000–75,000 cells) were injected at a maximum pressure of 35 kPa in pulses of 90 to 120 s. The injection of neutrophils was performed using kininogen-coated glass capillaries (10 µg/mL) [
14]. For the injection procedure, a PLI-100/PLI-100A microinjector (Harvard Apparatus), as well as the micromanipulator (MN-151; Narishige) to stabilize the glass capillaries, were used. During the entire surgical procedure, skin incisions were kept moist by application of PBS. Mice were monitored and kept warm to preserve a normal body temperature.
2.4. Isolation of Neutrophils
Neutrophils were isolated from the bone marrow of untreated mice or from the blood and lungs of the MVA-infected mice. Femur and tibia of the murine hind legs were removed, the ends were cut open and bone marrow cells were harvested by a short centrifugation step (30 s at 5500 rpm). Erythrocytes were lysed for 3 min on ice in Erylysis-buffer (168 mM ammonium chloride, 10 mM potassium bicarbonate and 1.095 mM Disodium ethylenediaminetetraacetate dehydrate in water; pH 7.3). Cells were then separated accordingly using the untouched Neutrophil Isolation Kit. In vivo-activated neutrophils were isolated from the lungs and blood of mice that were intranasally immunized with MVA. Blood was collected in sodium citrate, erythrocytes were lysed and neutrophils isolated by MACS as described above. Lungs were flushed with PBS and digested for 1 h at 37 °C (100 rpm) in red DMEM containing 100 µg/mL Liberase and 0.3 mg/mL DNase. After the lysis of erythrocytes, lung cells were blocked for 15 min on ice with 5% (v/v) rat serum and 10% (v/v) of an antibody against CD16 (FcγRIII)/CD32 (FcγRII) (clone: 2.4G2). Next, cells were stained with FITC-conjugated anti-CD11b (clone: M1/70.15; Invitrogen, Waltham, MA, USA) as well as PE-conjugated anti-Ly6G (clone: 1A8; Biolegend, San Diego, CA, USA) for 30–40 min on ice. Subsequently, FITC+ PE+ cells (neutrophils) were sorted using a FACS Aria Fusion at Hannover Medical School Central Cell Sorting Core Facility. Depending on availability, neutrophils were isolated wild-type mice and labeled with fluorescent dyes or from transgenic mice expressing a cyan fluorescent protein (CFP).
2.5. Generation of Dendritic Cells
Dendritic cells were generated in vitro from isolated bone marrow cells of the femur and tibia of murine hind legs. Bone marrow cells were isolated as described above. A total of 2.5–3 × 106 bone marrow cells were cultured for 8 days in RPMI with 10% (v/v) heat-inactivated FCS, 100 Units/mL Penicillin, 100 µg/mL Streptomycin, 2 mM L-Glutamine, 0.00035% (v/v) β-mercaptoethanol and 5% (v/v) GM-CSF (from cell culture supernatant) in a 10 cm petri dish. The medium was renewed on days 3 and 6. On day 8, 5 × 106 immature DCs were activated overnight by removing GM-CSF-containing medium and adding fresh medium (without GM-CSF) supplied with 1 µg/mL LPS in a 10 cm cell culture dish for adherent cells.
2.6. Neutrophil Activation
MACS purified neutrophils were activated in vitro by incubation with heat-inactivated P. aeruginosa in a ratio of 1:3 (neutrophil:bacteria) for 60–120 min at 37 °C to trigger phagocytosis-induced activation. Activated neutrophils were washed to remove free bacteria and re-suspended in PBS/3% (v/v) FCS. Activated neutrophils were immediately delivered via i.l. injection.
2.7. Flow Cytometry
For flow cytometry, antibody staining of isolated cells from organs or cells from cell culture was performed in a volume of 100 µL with a maximum of 750,000 cells per staining reaction. Fc receptors were blocked for 15 min on ice by using 10% (v/v) of cell culture supernatant, containing an antibody against CD16 (FcγRIII)/CD32 (FcγRII) (clone: 2.4G2) diluted in PBS/3% (v/v) FCS and 5% (v/v) rat serum. Then, the antibody mixtures were added and incubated on ice for 20–30 min. Cells were washed once with PBS/3% (v/v) FCS. Cells were re-suspended in 50–80 µL PBS/3% (v/v) FCS and analyzed using an LSR II (BD Biosciences). All flow cytometer data files were analyzed with FlowJo7.5 software. The following antibodies were used: anti-CD11b dye (clone M1/70.15; PE, FITC from Invitrogen; clone M1/70; PE-Cy7 from Biolegend, eFl450 from eBioScience, San Diego, CA, USA); anti-Ly6G (clone 1A8; PE from Biolegend); anti-CD62L (clone MEL14; APC from Biolegend); AF488- and Pacific Blue-labelling were homemade.
2.8. Labelling of Cells with Fluorescent Cell Dyes
For adoptive transfer, cells were stained with the fluorescent dyes eFluor™ 450 or eFluor™ 670 (Invitrogen). Cells were adjusted to 2 × 107 cells/mL PBS and dye was added at a final dilution of 1.25 µM for 10 min at 37 °C. The labeling reaction was stopped by adding 4–5× volume of ice-cold PBS/10% (v/v) FCS. In some experiments, cells were labeled with CMFDA (5-chloromethylfluorescein diacetate; Life Technologies, Carlsbad, CA, USA). Approximately 1 × 107 cells/mL medium (e.g., RPMI) was pre-warmed in a water bath at 37 °C, and stained with 0.25 µM CMFDA for 15 min at 37 °C. Cells were washed and re-suspended at defined concentrations in PBS/3% (v/v) FCS for further use.
2.9. MACS
After lysis of erythrocytes, cells were separated according to the manufacturer’s instructions by using magnetic-activated cell sorting (MACS, Miltenyi Biotec, Bergisch Gladbach, Germany) and the “untouched” neutrophil Isolation kit for mice. With this kit, neutrophils were isolated applying the manufacturer’s antibody cocktail that binds to all cells except neutrophils. Next, antibodies bound to cells were linked to magnetic beads which were then separated via special separation columns. During this step, cells bound to magnetic antibodies were retained in the column while untouched neutrophils were able to flow through for collection and further use.
2.10. Immunohistology
Lymph nodes were removed from sacrificed mice and fixed in PBS, 2% PFA, 30% Sucrose at 4 °C overnight. Lymph nodes were washed in PBS for 3–10 min, embedded in Tissue Tek O.C.T. (Sakura Finetek, Umkirch, Germany) and sectioned in 8 µm thick slices using a cryotome (Leica CM 3050 S). Cryosectioned slides were stored at −20 °C, or after a short drying time directly used for histology staining. For this staining, slides were rehydrated for 5 min with 1× TBS-T (1 M Tris (Base, Mechelen, Belgium), 1.55 M NaCl; pH 7.5; 0.05% Tween20) and blocked for 15 min with 10% (v/v) rat serum as well as 10% (v/v) Fc-Block (cell culture supernatant containing the antibody clone 2.4G2; anti-CD16 (FcγRIII)/CD32 (FcγRII)) in TBS-T. After blocking, slides were stained with one or more of the following antibodies: anti-LYVE-1 (purified polyclonal from Acris followed by anti-rabbit-FITC or Cy5-labelled from Jackson or clone ALY7; eFl660 from eBioScience), anti-IgD (clone: HB250, Cy3-, Cy5- home labelled), anti-CD11b (clone M1/70; eFl450 from eBioScience) or anti-Ly6 G (clone 1A8; PE from Biolegend) for 45 min to 1 h. Cell nuclei were stained using either DAPI (4′,6-diamidino-2-phenylindole, 1 µg/mL, Sigma, St. Louis, MO, USA) or PI (propidium iodide, 10 µg/mL, Fluka, Munich, Germany) for 2 min. Immuno-stained slides were dried overnight in darkness at room temperature.
2.11. Fluorescence Microscopy and Analysis of Images
Composite images of lymph node cryosections were taken after immunofluorescence staining with the AxioCam MRm camera (Carl Zeiss, Jena, Germany) connected to an Axiovert fluorescence microscope (Carl Zeiss), using PlanApochromat objectives 10×/0.45 and 20×/0.75 (magnification/numerical aperture). The images were processed with AxioVision 4.8.2 software. Cell counts were performed on 3–4 sections per lymph node using Imaris ×64 8.3.1 (Bitplane, Zurich, Switzerland). In order to obtain the distribution of adoptively transferred neutrophils within the lymph node, neutrophils were allocated to three lymph node compartments (medulla, parenchyma and subcapsular sinus). The definition of subcapsular as well as medullary sinuses in popliteal lymph nodes was performed based on the immunofluorescent staining of LYVE-1. For the neutrophil migration distance measurement, the lymph node borders were outlined manually and neutrophils were semi-automatically tracked using the Imaris spot detection function. Migration distance measurements were performed using the shortest calculated distance of the cell from the subcapsular sinus by using a macro (by Tim Worbs, Institute of Immunology, (Hannover Medical School, Hannover, Germany) for ImageJ. For this, the plugins “analyze particles” and “line graph” from ImageJ were used to examine the coordinates of the manually outlined subcapsular sinus and tracked neutrophils. The macro “Visual Basic for Applications” in Microsoft Excel was used for listing the final distance results of the neutrophils. Migration distances were exclusively obtained from neutrophils that migrated into the lymph node parenchyma.
Two-photon microscopy movies of i.l. injected neutrophils in lymph nodes were acquired using a TriM Scope setup (La Vision Biotec, Bielefeld, Germany) as described [
15,
16]. After the transfer of neutrophils, mice were killed at different time points before an eFl660-conjugated LYVE-1 antibody (33.34 µg/mL) was injected to distinguish the medullary from the sinus region in the popliteal lymph node. Afterward, excised popliteal lymph nodes were glued into an imaging chamber, and an oxygen-supplied medium circulation with a temperature of 37 °C was connected to mimic natural conditions. In order to excite eGFP or eCFP (enhanced cyan fluorescent protein) neutrophils, Ti:Sa laser was tuned to 920 nm for a good visualization. For the excitation of eFl660, the optical parametric oscillator (OPO) was tuned to 1100 nm. Movies were taken with a scan field range between 300 × 300 µm and 500 × 500 µm for up to 60 min. Data acquired from 2-photon microscopy were analyzed using Imaris ×64 8.3.1 or Imaris ×64 7.7.2 software. All movies were median-filtered.
2.12. Statistical Analysis
A statistical significance test was performed for two groups of independent samples. GraphPad Prism4 (GraphPad Software, Inc., San Diego, CA, USA) was used to perform the two-tailed non-parametric Mann–Whitney test assuming no Gaussian distribution. Results with a p-value ≤0.05 were considered significant. The following symbols are used * p < 0.05; ** p < 0.01; *** p < 0.001. Data regarding the distribution of cells within the lymph node are represented as mean with SD. Migration distances are shown as the median.
4. Discussion
Immune cells gain access to lymph nodes via two different routes: from the blood via specialized high endothelial venules (HEVs) and from peripheral tissues via afferent lymphatics. These two pathways are not equally used by the different immune cell subsets. Recirculating naïve T cells [
17] and B cells are known to enter lymph nodes at high frequencies via HEV. In contrast, tissue-resident DCs, once activated migrate towards and subsequently into terminal lymphatic vessels, a process that is facilitated by the expression of the chemokine receptor CCR7 on DCs and its ligands CCL19 and CCL21 by lymphatic endothelial cells [
17,
23]. Within the terminal lymphatics, DCs migrate towards larger collecting vessels and once those are reached, they passively get transported with the lymph fluid into the subcapsular sinus of the draining lymph node. From there, DCs manage to exit by crawling through preformed pores in the subcapsular sinus floor and move with straight directionality into the T cell zone [
17]. Both exit from the subcapsular sinus as well as directional migration again depends on CCR7 and its ligands.
Recent studies suggest that, besides DCs and macrophages, also neutrophils might be able to take up antigen and migrate from the infected tissue site to draining lymph nodes [
5,
6,
12,
21,
24,
25]. However, the frequency of this process, the route by which neutrophils entered these lymph nodes and their localization within lymph nodes remain largely elusive. Therefore, the present study aimed to investigate the positioning of neutrophils in lymph nodes upon homing via lymphatic vessels. We used i.l. transfer of cells, a method developed earlier by our group, in combination with lymph node imaging to address some of these points. The results of this study revealed that within non-inflamed lymph nodes i.l. transferred neutrophils localized primarily to the medulla or the subcapsular sinus. Even following lymph node inflammation, neutrophils stayed primarily in the subcapsular sinus or within the medulla and only rarely entered the lymph node parenchyma. Interestingly, resting neutrophils entered to some degree the interfollicular area via the subcapsular sinus floor upon co-injection with DCs while in vivo-activated neutrophils were also capable to enter to some degree the interfollicular area even in the absence of DCs.
Neutrophils are known to circulate in the blood [
26] and to migrate into the tissue upon infection or inflammation. Based on the experimental setup applied in this study, adoptively transferred neutrophils were passively transported with the lymph flow into the subcapsular sinus of the draining lymph node. Some of the transferred cells stayed for several hours at this location, while others were found at later time points within the medulla. This behavior was similar to the situation observed before for naïve T cells within the first two hours of i.l. delivery. However, in contrast to the situation for naïve T cells that were able to translocate from the medullary region into the T cell zone during the following 2 h [
17] neutrophils failed to translocate into the deep T cell zone. Our earlier finding that i.l. transferred naïve, CCR7-deficient T cells were also excluded from the T cell zone suggested that expression of CCR7 is indispensable for this process. We therefore checked whether any of the neutrophil populations used in this study—resting or activated—express CCR7. However, as reported by others [
13], we failed to find any evidence that neutrophils express this lymph node homing chemokine receptor (data not shown). Furthermore, the absence of inflammatory stimuli and thus lack of inflammatory chemokines in non-inflamed lymph nodes could also contribute to impaired neutrophil migration into the lymph node parenchyma.
It was shown by others that Phorbol-12-myristate-13-acetate (PMA) serves as a potent chemical activator of neutrophils [
27]. We were able to confirm the potential of this agent to strongly stimulate neutrophils (data not shown). Neutrophil activation was also described upon recognition of various pathogenic particles or pathogen-derived molecules via pattern recognition receptors [
2]. Results obtained in the present work confirmed that all of the tested pathogens have the potential to activate neutrophils. Activated neutrophils are known to express high levels of CD11b [
26] which was also confirmed in the present study. Interestingly, Hampton et al. showed a CD11b-dependent migration of neutrophils via lymphatic vessels to lymph nodes [
6]. However, the distribution of neutrophils within these lymph nodes had not been investigated in that study. Results of the present study showed that in vitro-activated CD11b
high neutrophils migrated occasionally into the lymph node parenchyma. Nevertheless, the majority of these highly-activated i.l. transferred neutrophils were retained in the subcapsular sinus. Since PMA is known as a potent activator of granule release [
27], it seems possible that neutrophils attached to each other due to their strong activation and granule release. Therefore, strong in vitro-activation, which was revealed in the present study, might have contributed to rapid neutrophil accumulation within the subcapsular sinus. This accumulation of neutrophils or formation of cell aggregates might prevent further migration and thus neutrophils might get stuck within the subcapsular sinus for prolonged periods of time. The present study also showed that the localization within the lymph node compartments is similar for resting and activated neutrophils. As mentioned above, the lack of neutrophil chemo-attractants in non-inflamed lymph nodes might be a factor that prevents neutrophil translocation to the deep T cell zone. This model is in line with the idea that pre-activated cells, such as some of the neutrophils used in the present study, still require chemo-attractant signals provided by the environment in order to directional migrate within tissues.
Massive neutrophil lymph node infiltration upon s.c. infection with
P. aeruginosa was recently reported by others [
21,
22]. Kastenmüller et al. showed that endogenous neutrophils localized within the subcapsular sinus, the medulla as well as interfollicular areas. Results of the present study confirmed that presumably blood-derived neutrophils infiltrated the draining lymph node after the s.c. application of heat-inactivated
P. aeruginosa. Here, blood-derived neutrophils migrated into the lymph node parenchyma of
P. aeruginosa-activated lymph nodes, whereas intra-lymphatically transferred neutrophils failed in doing so. The reasons for these differences are currently not clear. Endogenous neutrophils are released from the bone marrow to the blood to migrate into infected tissue and some of them might reach the draining lymph node by reverse migration. In contrast, i.l. transferred neutrophils were isolated from the bone marrow and subsequently injected into the lymph vessel of the draining lymph node. Therefore, it seems likely that bone marrow neutrophils were not primed or “mature” enough to migrate into the lymph node parenchyma, as endogenous neutrophils could do after being activated at the site of infection. Another possibility could be that activating signals mediated from the lymphatic endothelial cells were not potent enough for i.l. delivered neutrophils to migrate into the lymph node parenchyma. Alternatively, the s.c. injection of heat-inactivated bacteria might lead to the induction of chemotactic molecules on HEV or other capillary vessels within the lymph node that allows broad access of neutrophils from the blood into the lymph node parenchyma without any further translocation.
Surprisingly, this study also revealed that i.l. neutrophils that were isolated from inflamed lungs are capable to enter interfollicular areas of lymph nodes. The presence of DCs neither affected their distribution within the lymph node nor facilitated their translocation deeper into the parenchyma. In contrast to bone marrow and blood neutrophils, the lung neutrophils used in the present study were highly activated, reflected by high levels of CD11b and the absence of CD62L expression. In particular, increased expression of the adhesion molecule CD11b and other factors present in activated but not in resting neutrophils such as proteases might facilitate their egress from the subcapsular sinus floor.
Data from the present study revealed a change in the localization of resting bone marrow and blood neutrophils upon co-transfer with DCs. Neutrophils co-injected with DCs positioned in the subcapsular sinus, and subsequently migrated to some extent into the lymph node parenchyma, whereas neutrophils injected alone were less proficient in exiting from the subcapsular sinus. This observation suggests that the presence of co-migrating DCs affects the behavior of bone marrow and blood neutrophils. We reported earlier that migrating DCs might induce changes in the subcapsular sinus floor that allowed direct homing of naïve T cells which usually entered the lymph node parenchyma in a retrograde manner via the medullary sinuses [
17]. This model would also help to explain the finding of the present study that co-delivered DCs supported neutrophils to enter the lymph node parenchyma via such potential changes. More recently we reported preformed pores within the subcapsular sinus floor [
28]. Such preformed pores could have been potentially expanded by co-migrating DCs, and therefore might also allow neutrophils to enter the lymph node parenchyma directly from the subcapsular sinus. Another explanation for neutrophil entry could be that co-injected DCs block the subcapsular sinus lumen and therefore trapped smaller cells, such as neutrophils in this area. This might give neutrophils more time to find potential entry pores in the subcapsular sinus floor to subsequently enter the lymph node parenchyma.
In the present study, we addressed the migration and positioning of isolated neutrophils in draining lymph nodes following their i.l. delivery. Although this process has an artificial component since it circumvents the need for neutrophils to enter from tissue via lymphatic capillaries into lymphatic vessels, it offers the opportunity to manipulate and dissect several aspects of neutrophil homing to lymph nodes and subsequent positioning. This approach revealed that lymph-derived neutrophils overall locate primarily to the subcapsular and medullary sinus system while they rarely entered the deep T cell zone. The absence of mouse neutrophils from this lymph node area goes along with the lack of detectable expression of surface CCR7 on neutrophils in the present study. However, intracellular stores of CCR7 in mouse neutrophils were described by others [
11] while experiments from the present study indicate that those, if present, did not functionally translocate to the cell surface in order to allow neutrophil entry into the T cell zone. The absence of neutrophils from the T cell zone goes along with the inability of murine neutrophils to activate T cells. The observations made in the mouse model are however different from the situation in humans. In the latter species, a subset of neutrophils expresses MHC-II and co-stimulatory molecules as well as the T zone homing chemokine receptor CCR7 [
29]. These molecules get in particular upregulated after exposure to antigen-immune globulin complexes and allow activation of CD4
+ T cells [
30]. In the human lymph node, these cells locate to the interfollicular area, a place known for rapid T cell activation [
30]. Together these data indicate that lymph node neutrophils serve a different function in different species.
In conclusion, the present study did not aim to address whether and under which conditions neutrophils are able to migrate from inflamed tissue via afferent lymphatics to draining lymph nodes. Instead, we used i.l. cell delivery to study whether the activation status of the neutrophils or that of the draining lymph node affects the positioning of the adoptively transferred cells. Although we observed variations to some degree most of the transferred cells were either found in the subcapsular sinus or in the medulla irrespective of their activation and only very few cells were found in the T or B cell areas. However, inflammatory stimuli led to substantial recruitment of neutrophils from blood into the lymph node parenchyma but the role of lymph-derived neutrophils in lymph node physiology further remains elusive.