Flecainide Paradoxically Activates Cardiac Ryanodine Receptor Channels under Low Activity Conditions: A Potential Pro-Arrhythmic Action
Abstract
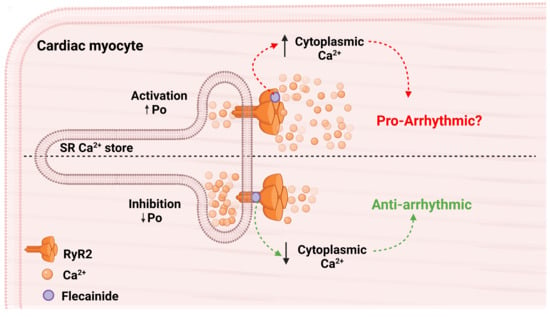
:1. Introduction
2. Materials and Methods
2.1. Harvesting of Mouse Hearts
2.2. Isolation of the RyR2 SR Vesicle Preparation
2.3. Single Channel Lipid Bilayer Recordings
2.4. Single Channel Lipid Bilayer Electrophysiology and Analysis
2.5. Statistics
3. Results
3.1. Overall Actions of Flecainide on WT and P2328S Channels
3.2. Normalisation Reveals a Consistent Increase in Channel Activity with Flecainide
3.3. The Control Activity of Individual Channels Determines the Action of Flecainide
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Lehnart, S.E.; Wehrens, X.H.T.; Laitinen, P.J.; Reiken, S.R.; Deng, S.-X.; Cheng, Z.; Landry, D.W.; Kontula, K.; Swan, H.; Marks, A.R. Sudden Death in Familial Polymorphic Ventricular Tachycardia Associated With Calcium Release Channel (Ryanodine Receptor) Leak. Circulation 2004, 109, 3208–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerrone, M.; Colombi, B.; Santoro, M.; di Barletta, M.R.; Scelsi, M.; Villani, L.; Napolitano, C.; Priori, S.G. Bidirectional Ventricular Tachycardia and Fibrillation Elicited in a Knock-In Mouse Model Carrier of a Mutation in the Cardiac Ryanodine Receptor. Circ. Res. 2005, 96, e77–e82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumitomo, N.; Sakurada, H.; Taniguchi, K.; Matsumura, M.; Abe, O.; Miyashita, M.; Kanamaru, H.; Karasawa, K.; Ayusawa, M.; Fukamizu, S.; et al. Association of Atrial Arrhythmia and Sinus Node Dysfunction in Patients With Catecholaminergic Polymorphic Ventricular Tachycardia. Circ. J. 2007, 71, 1606–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvage, S.C.; Gallant, E.M.; Beard, N.A.; Ahmad, S.; Valli, H.; Fraser, J.A.; Huang, C.L.-H.; Dulhunty, A.F. Ion channel gating in cardiac ryanodine receptors from the arrhythmic RyR2-P2328S mouse. J. Cell Sci. 2019, 132, jcs229039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvage, S.C.; Chandrasekharan, K.H.; Jeevaratnam, K.; Dulhunty, A.F.; Thompson, A.J.; Jackson, A.P.; Huang, C.L.H. Multiple targets for flecainide action: Implications for cardiac arrhythmogenesis. Br. J. Pharmacol. 2018, 175, 1260–1278. [Google Scholar] [CrossRef] [Green Version]
- Salvage, S.C.; King, J.H.; Chandrasekharan, K.H.; Jafferji, D.I.G.G.; Guzadhur, L.; Matthews, H.R.; Huang, C.L.-H.; Fraser, J.A. Flecainide exerts paradoxical effects on sodium currents and atrial arrhythmia in murine RyR2-P2328S hearts. Acta Physiol. 2015, 214, 361–375. [Google Scholar] [CrossRef] [Green Version]
- Goddard, C.A.; Ghais, N.S.; Zhang, Y.; Williams, A.J.; Colledge, W.H.; Grace, A.A.; Huang, C.L.-H. Physiological consequences of the P2328S mutation in the ryanodine receptor ( RyR2) gene in genetically modified murine hearts. Acta Physiol. 2008, 194, 123–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meli, A.C.; Refaat, M.M.; Dura, M.; Reiken, S.; Wronska, A.; Wojciak, J.; Carroll, J.; Scheinman, M.M.; Marks, A.R. A novel ryanodine receptor mutation linked to sudden death increases sensitivity to cytosolic calcium. Circ. Res. 2011, 109, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laitinen, P.J.; Brown, K.M.; Piippo, K.; Swan, H.; Devaney, J.M.; Brahmbhatt, B.; Donarum, E.A.; Marino, M.; Tiso, N.; Viitasalo, M.; et al. Mutations of the Cardiac Ryanodine Receptor (RyR2) Gene in Familial Polymorphic Ventricular Tachycardia. Circulation 2001, 103, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Kupershmidt, S.; Zhang, R.; Stepanovic, S.; Roden, D.M.; Wilde, A.A.M.; Anderson, M.E.; Balser, J.R. A calcium sensor in the sodium channel modulates cardiac excitability. Nature 2002, 415, 442–447. [Google Scholar] [CrossRef]
- Casini, S.; Verkerk, A.O.; van Borren, M.M.G.J.; van Ginneken, A.C.G.; Veldkamp, M.W.; de Bakker, J.M.T.; Tan, H.L. Intracellular calcium modulation of voltage-gated sodium channels in ventricular myocytes. Cardiovasc. Res. 2009, 81, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Ning, F.; Luo, L.; Ahmad, S.; Valli, H.; Jeevaratnam, K.; Wang, T.; Guzadhur, L.; Yang, D.; Fraser, J.A.; Huang, C.L.-H.; et al. The RyR2-P2328S mutation downregulates Nav1.5 producing arrhythmic substrate in murine ventricles. Pflugers Arch. Eur. J. Physiol. 2016, 468, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wu, J.; Jeevaratnam, K.; King, J.H.; Guzadhur, L.; Ren, X.; Grace, A.A.; Lei, M.; Huang, C.L.-H.; Fraser, J.A. Conduction Slowing Contributes to Spontaneous Ventricular Arrhythmias in Intrinsically Active Murine RyR2-P2328S Hearts. J. Cardiovasc. Electrophysiol. 2013, 24, 210–218. [Google Scholar] [CrossRef]
- King, J.H.; Zhang, Y.; Lei, M.; Grace, A.A.; Huang, C.L.-H.; Fraser, J.A. Atrial arrhythmia, triggering events and conduction abnormalities in isolated murine RyR2-P2328S hearts. Acta Physiol. Oxford Engl. 2012, 207, 308–323. [Google Scholar] [CrossRef]
- Watanabe, H.; Chopra, N.; Laver, D.; Hwang, H.S.; Davies, S.S.; Roach, D.E.; Duff, H.J.; Roden, D.M.; Wilde, A.A.M.; Knollmann, B.C. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat. Med. 2009, 15, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Bannister, M.L.; Thomas, N.L.; Sikkel, M.B.; Mukherjee, S.; Maxwell, C.; MacLeod, K.T.; George, C.H.; Williams, A.J. The mechanism of flecainide action in CPVT does not involve a direct effect on RyR2. Circ. Res. 2015, 116, 1324–1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikkel, M.B.; Collins, T.P.; Rowlands, C.; Shah, M.; O’Gara, P.; Williams, A.J.; Harding, S.E.; Lyon, A.R.; MacLeod, K.T. Flecainide reduces Ca2+ spark and wave frequency via inhibition of the sarcolemmal sodium current. Cardiovasc. Res. 2013, 98, 286–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copello, J.A.; Barg, S.; Onoue, H.; Fleischer, S. Heterogeneity of Ca2+ gating of skeletal muscle and cardiac ryanodine receptors. Biophys. J. 1997, 73, 141–156. [Google Scholar] [CrossRef] [Green Version]
- Walweel, K.; Molenaar, P.; Imtiaz, M.S.; Denniss, A.; dos Remedios, C.; van Helden, D.F.; Dulhunty, A.F.; Laver, D.R.; Beard, N.A. Ryanodine receptor modification and regulation by intracellular Ca2+ and Mg2+ in healthy and failing human hearts. J. Mol. Cell. Cardiol. 2017, 104, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehra, D.; Imtiaz, M.S.; Van Helden, D.F.; Knollmann, B.C.; Laver, D.R. Multiple modes of ryanodine receptor 2 inhibition by flecainide. Mol. Pharmacol. 2014, 86, 696–706. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.; Culley, D.; Waring, S.; Lamb, G.D.; Easton, C.; Casarotto, M.G.; Dulhunty, A.F. Peptide mimetic compounds can activate or inhibit cardiac and skeletal ryanodine receptors. Life Sci. 2020, 260. [Google Scholar] [CrossRef]
- Dulhunty, A.F.; Laver, D.; Curtis, S.M.; Pace, S.; Haarmann, C.; Gallant, E.M. Characteristics of irreversible ATP activation suggest that native skeletal ryanodine receptors can be phosphorylated via an endogenous CaMKII. Biophys. J. 2001, 81, 3240–3252. [Google Scholar] [CrossRef] [Green Version]
- Eager, K.R.; Dulhunty, A.F. Activation of the cardiac ryanodine receptor by sulfhydryl oxidation is modified by Mg2+ and ATP. J. Membr. Biol. 1998, 163, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.D.; Janczura, M.; Cho, E.; Dulhunty, A.F.; Beard, N.A. Multiple actions of the anthracycline daunorubicin on cardiac ryanodine receptors. Mol. Pharmacol. 2011, 80, 538–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, W.; Liu, G.; Allen, P.D.; Pessah, I.N. Transmembrane redox sensor of ryanodine receptor complex. J. Biol. Chem. 2000, 275, 35902–35907. [Google Scholar] [CrossRef] [Green Version]
- Sitsapesan, R. Similarities in the effects of DIDS, DBDS and suramin on cardiac ryanodine receptor function. J. Membr. Biol. 1999, 168, 159–168. [Google Scholar] [CrossRef]
- Hilliard, F.A.; Steele, D.S.; Laver, D.; Yang, Z.; Le Marchand, S.J.; Chopra, N.; Piston, D.W.; Huke, S.; Knollmann, B.C. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J. Mol. Cell. Cardiol. 2010, 48, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Györke, I.; Hester, N.; Jones, L.R.; Györke, S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 2004, 86, 2121–2128. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Hanna, A.D.; Beard, N.A.; Dulhunty, A.F. Unique isoform-specific properties of calsequestrin in the heart and skeletal muscle. Cell Calcium 2009, 45, 474–484. [Google Scholar] [CrossRef]
- Fill, M.; Gillespie, D. Ryanodine receptor open times are determined in the closed state. Biophys. J. 2018, 115, 1160–1165. [Google Scholar] [CrossRef] [Green Version]
- Zahradníková, A.; Valent, I.; Zahradník, I. Frequency and release flux of calcium sparks in rat cardiac myocytes: A relation to RYR gating. J. Gen. Physiol. 2010, 136, 101–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryshtal, D.O.; Blackwell, D.J.; Egly, C.L.; Smith, A.N.; Batiste, S.M.; Johnston, J.N.; Laver, D.R.; Knollmann, B.C. RYR2 channel inhibition is the principal mechanism of flecainide action in CPVT. Circ. Res. 2021, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Padfield, G.; Al-Ahmari, L.; Lieve, K.; Al-Ahmari, T.; Roston, T.; Wilde, A.; Krahn, A.; Sanatani, S. Flecainide monotherapy is an option for selected patients with catecholaminergic polymorphic ventricular tachycardia intolerant of β-blockade. Heart Rhythm. 2016, 13, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Roston, T.M.; Vinocur, J.M.; Maginot, K.R.; Mohammed, S.; Salerno, J.C.; Etheridge, S.P.; Cohen, M.; Hamilton, R.M.; Pflaumer, A.; Kanter, R.J.; et al. Catecholaminergic polymorphic ventricular tchycardia in children: Analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ. Arrhythmia Electrophysiol. 2015, 8, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Zhao, N.; Zhong, S.; Wang, Y.; Li, J. Safety and efficacy of flecainide for patients with catecholaminergic polymorphic ventricular tachycardia. Medicine 2019, 98, e16961. [Google Scholar] [CrossRef] [PubMed]
- Echt, D.S.; Ruskin, J.N. Use of Flecainide for the treatment of atrial fibrillation. Am. J. Cardiol. 2020, 125, 1123–1133. [Google Scholar] [CrossRef] [Green Version]
- Des Georges, A.; Clarke, O.B.; Zalk, R.; Yuan, Q.; Condon, K.J.; Grassucci, R.A.; Hendrickson, W.A.; Marks, A.R.; Frank, J. Structural basis for gating and activation of RyR1. Cell 2016, 167, 145.e17–157.e17. [Google Scholar] [CrossRef] [Green Version]
- Dhindwal, S.; Lobo, J.; Cabra, V.; Santiago, D.J.; Nayak, A.R.; Dryden, K.; Samsó, M. A cryo-EM-based model of phosphorylation- and FKBP12.6-mediated allosterism of the cardiac ryanodine receptor. Sci. Signal. 2017, 10, eaai8842. [Google Scholar] [CrossRef]
- Blayney, L.; Beck, K.; MacDonald, E.; D’Cruz, L.; Nomikos, M.; Griffiths, J.; Thanassoulas, A.; Nounesis, G.; Lai, F.A. ATP interacts with the CPVT mutation-associated central domain of the cardiac ryanodine receptor. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4426–4432. [Google Scholar] [CrossRef]




| [Flec] | 0 µM | 0.5 µM | 1.0 µM | 5 µM | 10 µM | 50 µM | 100 µM |
|---|---|---|---|---|---|---|---|
| WILD TYPE | |||||||
| Po | 0.147 ± 0.022 | 0.120 ± 0.024 | 0.142 ± 0.034 | 0.067 ± 0.032 * | 0.039 ± 0.004 * | 0.011 ± 0.005 * | nd |
| To (ms) | 4.18 ± 0.34 | 2.43 ± 0.05 | 3.14 ± 0.69 | 3.71 ± 2.28 | 1.37 ± 0.17 | 0.73 ± 0.22 * | nd |
| Tc (ms) | 26.65 ± 8.23 | 23.26 ± 8.62 | 25.17 ± 13.28 | 48.75 ± 11.93 | 35.39 ± 7.75 | 70.5 ± 11.6 * | nd |
| Fo (s−1) | 49.32 ± 19.67 | 62.34 ± 22.43 | 52.41 ± 17.40 | 22.57 ± 7.20 | 28.35 ± 5.70 | 14.40 ± 2.31 | nd |
| P2328S | |||||||
| Po | 0.241 ± 0.044 # | 0.204 ± 0.065 | 0.227 ± 0.079 # | 0.228 ± 0.083 | 0.201 ± 0.087 # | 0.133 ± 0.040 *,# | 0.116 ± 0.049 |
| To (ms) | 4.08 ± 0.72 | 4.01 ± 1.22 | 3.44 ± 1.09 | 3.98 ± 1.07 | 1.97 ± 0.40 * | 1.23 ± 0.14 * | 1.17 ± 0.13 * |
| Tc (ms) | 15.07 ± 2.78 # | 19.39 ± 4.70 | 24.55 ± 12.83 | 20.92 ± 7.86 # | 11.43 ± 3.62 # | 10.16 ± 2.83 # | 10.50 ± 3.91 |
| Fo (s−1) | 60.58 ± 6.25 | 61.72 ± 14.74 | 66.00 ± 10.24 | 49.98 ± 6.78 # | 92.89 ± 18.95 *,# | 105.80 ± 26.53 *,# | 95.76 ± 31.07 * |
| [Flec] | 0 µM | 0.5 µM | 1.0 µM | 5 µM | 10 µM | 50 µM | 100 µM |
|---|---|---|---|---|---|---|---|
| WILD TYPE | |||||||
| Po | 0.009 ± 0.003 | 0.009 ± 0.002 | 0.015 ± 0.012 | 0.023 ± 0.012 | 0.011 ± 0.003 | 0.007 ± 0.002 | nd |
| To (ms) | 2.01 ± 0.31 | 1.63 ± 0.31 | 1.86 ± 0.18 | 4.43 ± 2.66 | 1.50 ± 0.34 | 1.67 ± 0.19 | nd |
| Tc (ms) | 387.4 ± 69.7 | 254.7 ± 57.6 | 249.1 ± 61.0 | 335.6 ± 71.4 | 355.3 ± 116.2 | 413.8 ± 154.7 | nd |
| Fo (s−1) | 4.03 ± 0.81 | 5.84 ± 1.24 | 8.82 ± 1.79 * | 7.73 ± 2.52 | 6.54 ± 1.61 | 4.61 ± 1.34 | nd |
| P2328S | |||||||
| Po | 0.017 ± 0.005 # | 0.028 ± 0.010 # | 0.026 ± 0.013 | 0.025 ± 0.010 | 0.028 ± 0.018 | 0.019 ± 0.011 | 0.010 ± 0.011 |
| To (ms) | 2.08 ± 0.28 | 2.09 ± 0.41 | 2.03 ± 0.52 | 1.46 ± 0.05 | 1.15 ± 0.14 * | 1.67 ± 0.29 | 1.64 ± 0.57 |
| Tc (ms) | 192.4 ± 27.16 # | 113.8 ± 20.71 *,# | 176.3 ± 43.20 | 152.6 ± 52.76 # | 154.4 ± 69.40 | 207.8 ± 114.9 | 331.2 ± 267.5 |
| Fo (s−1) | 7.172 ± 0.935 # | 12.79 ± 2.117 *,# | 9.96 ± 1.570 | 16.26 ± 3.074 *,# | 20.54 ± 7.924 *,# | 10.92 ± 4.150 *,# | 7.715 ± 6.665 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvage, S.C.; Gallant, E.M.; Fraser, J.A.; Huang, C.L.-H.; Dulhunty, A.F. Flecainide Paradoxically Activates Cardiac Ryanodine Receptor Channels under Low Activity Conditions: A Potential Pro-Arrhythmic Action. Cells 2021, 10, 2101. https://doi.org/10.3390/cells10082101
Salvage SC, Gallant EM, Fraser JA, Huang CL-H, Dulhunty AF. Flecainide Paradoxically Activates Cardiac Ryanodine Receptor Channels under Low Activity Conditions: A Potential Pro-Arrhythmic Action. Cells. 2021; 10(8):2101. https://doi.org/10.3390/cells10082101
Chicago/Turabian StyleSalvage, Samantha C., Esther M. Gallant, James A. Fraser, Christopher L.-H. Huang, and Angela F. Dulhunty. 2021. "Flecainide Paradoxically Activates Cardiac Ryanodine Receptor Channels under Low Activity Conditions: A Potential Pro-Arrhythmic Action" Cells 10, no. 8: 2101. https://doi.org/10.3390/cells10082101
APA StyleSalvage, S. C., Gallant, E. M., Fraser, J. A., Huang, C. L. -H., & Dulhunty, A. F. (2021). Flecainide Paradoxically Activates Cardiac Ryanodine Receptor Channels under Low Activity Conditions: A Potential Pro-Arrhythmic Action. Cells, 10(8), 2101. https://doi.org/10.3390/cells10082101