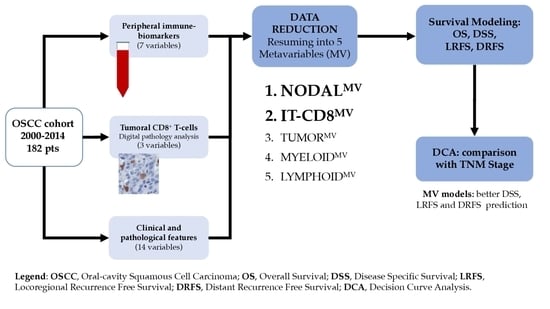
Metavariables Resuming Host Immune Features and Nodal Involvement Are Associated with Oncological Outcomes in Oral Cavity Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Cohort
2.2. Blood Samples
2.3. Tissues
2.4. Digital Pathology Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Findings of the OSCC Cohort
3.2. Clinical Relevance of CD8 Immune Contexture and Peripheral Blood Biomarkers in OSCC
3.3. Identification of the Meta-Variables in OSCC
3.4. NODALMV Is Highly Related to OS and DSS
3.5. Immune MV as Predictor of Loco-Regional and Distant Failure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015, 136, e359–e386. [Google Scholar] [CrossRef]
- Ghazawi, F.M.; Lu, J.; Savin, E.; Zubarev, A.; Chauvin, P.; Sasseville, D.; Zeitouni, A.; Litvinov, I.V. Epidemiology and Patient Distribution of Oral Cavity and Oropharyngeal SCC in Canada. J. Cutan. Med. Surg. 2020, 24, 340–349. [Google Scholar] [CrossRef]
- Conway, D.I.; Purkayastha, M.; Chestnutt, I.G. The changing epidemiology of oral cancer: Definitions, trends, and risk factors. Br. Dent. J. 2018, 225, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.P.; Cendon, R.A.; Farr, H.W.; Strong, E.W. Carcinoma of the oral cavity. Factors affecting treatment failure at the primary site and neck. Am. J. Surg. 1976, 132, 504–507. [Google Scholar] [CrossRef]
- Gil, Z.; Carlson, D.L.; Boyle, J.O.; Kraus, D.H.; Shah, J.P.; Shaha, A.R.; Singh, B.; Wong, R.J.; Patel, S.G. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer 2009, 115, 5700–5710. [Google Scholar] [CrossRef]
- Patel, S.G.; Amit, M.; Yen, T.C.; Liao, C.T.; Chaturvedi, P.; Agarwal, J.P.; Kowalski, L.P.; Ebrahimi, A.; Clark, J.R.; Cernea, C.R.; et al. Lymph node density in oral cavity cancer: Results of the International Consortium for Outcomes Research. Br. J. Cancer 2013, 109, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Shiono, S.; Kawamura, M.; Sato, T.; Okumura, S.; Nakajima, J.; Yoshino, I.; Ikeda, N.; Horio, H.; Akiyama, H.; Kobayashi, K. Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann. Thorac. Surg. 2009, 88, 856–860. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, A.K.; Vaish, R.; Kapre, N.; Dandekar, M.; Gupta, S.; Hawaldar, R.; Agarwal, J.P.; Pantvaidya, G.; Chaukar, D.; Deshmukh, A.; et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N. Engl. J. Med. 2015, 373, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Garrel, R.; Poissonnet, G.; Moyà Plana, A.; Fakhry, N.; Dolivet, G.; Lallemant, B.; Sarini, J.; Vergez, S.; Guelfucci, B.; Choussy, O.; et al. Equivalence Randomized Trial to Compare Treatment on the Basis of Sentinel Node Biopsy Versus Neck Node Dissection in Operable T1-T2N0 Oral and Oropharyngeal Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 4010–4018. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G.J.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet. Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulières, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Bellmunt, J.; De Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef] [Green Version]
- Brockstein, B.E.; Vokes, E.E. Treatment of metastatic and recurrent head and neck cancer. In Treatment of Metastatic and Recurrent Head and Neck Cancer; Post, T.W., Posner, M.R., Shah, S., Eds.; UpToDate: Waltham, MA, USA, 2021. [Google Scholar]
- Brandwein-Gensler, M.; Teixeira, M.S.; Lewis, C.M.; Lee, B.; Rolnitzky, L.; Hille, J.J.; Genden, E.; Urken, M.L.; Wang, B.Y. Oral squamous cell carcinoma: Histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am. J. Surg Pathol. 2005, 29, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Brandwein-Gensler, M.; Smith, R.V.; Wang, B.; Penner, C.; Theilken, A.; Broughel, D.; Schiff, B.; Owen, R.P.; Smith, J.; Sarta, C.; et al. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am. J. Surg Pathol. 2010, 34, 676–688. [Google Scholar] [CrossRef]
- Troiano, G.; Rubini, C.; Togni, L.; Caponio, V.C.A.; Zhurakivska, K.; Santarelli, A.; Cirillo, N.; Lo Muzio, L.; Mascitti, M. The immune phenotype of tongue squamous cell carcinoma predicts early relapse and poor prognosis. Cancer Med. 2020, 9, 8333–8344. [Google Scholar] [CrossRef] [PubMed]
- Borsetto, D.; Tomasoni, M.; Payne, K.; Polesel, J.; Deganello, A.; Bossi, P.; Tysome, J.R.; Masterson, L.; Tirelli, G.; Tofanelli, M.; et al. Prognostic Significance of CD4+ and CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Cancers 2021, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xie, N.; Liu, H.; Wan, Y.; Zhu, Y.; Zhang, M.; Tao, Y.; Zhou, H.; Liu, X.; Hou, J.; et al. The prognostic role of tumour-infiltrating lymphocytes in oral squamous cell carcinoma: A meta-analysis. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2019, 48, 788–798. [Google Scholar] [CrossRef]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef]
- Bardash, Y.; Olson, C.; Herman, W.; Khaymovich, J.; Costantino, P.; Tham, T. Platelet-Lymphocyte Ratio as a Predictor of Prognosis in Head and Neck Cancer: A Systematic Review and Meta-Analysis. Oncol. Res. Treat. 2019, 42, 665–677. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Y.; Zhou, L.; Dai, Y.; Hu, G. High pretreatment neutrophil-to-lymphocyte ratio as a predictor of poor survival prognosis in head and neck squamous cell carcinoma: Systematic review and meta-analysis. Head Neck 2019, 41, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-K.; Kim, M.W.; Choi, I.S.; Moon, U.Y.; Kim, M.-J.; Sohn, I.; Kim, S.; Jeong, H.-S. Optimal cutoff of pretreatment neutrophil-to-lymphocyte ratio in head and neck cancer patients: A meta-analysis and validation study. BMC Cancer 2018, 18, 969. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Altman, D.G.; Sauerbrei, W. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat. Med. 2006, 25, 127–141. [Google Scholar] [CrossRef]
- Chan, J.Y.; Tan, G.F.; Yeong, J.; Ong, C.W.; Ng, D.Y.X.; Lee, E.; Koh, J.; Ng, C.C.-Y.; Lee, J.Y.; Liu, W.; et al. Clinical implications of systemic and local immune responses in human angiosarcoma. NPJ Precis. Oncol. 2021, 5, 11. [Google Scholar] [CrossRef]
- Mattavelli, D.; Lombardi, D.; Missale, F.; Calza, S.; Battocchio, S.; Paderno, A.; Bozzola, A.; Bossi, P.; Vermi, W.; Piazza, C.; et al. Prognostic Nomograms in Oral Squamous Cell Carcinoma: The Negat Impact of Low Neutrophil to Lymph Ratio. Front. Oncol. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lonardi, S.; Missale, F.; Calza, S.; Bugatti, M.; Vescovi, R.; Debora, B.; Uppaluri, R.; Egloff, A.M.; Mattavelli, D.; Lombardi, D.; et al. Tumor-associated neutrophils (TANs) in human carcinoma-draining lymph nodes: A novel TAN compartment. Clin. Transl. Immunol. 2021, 10, e1252. [Google Scholar] [CrossRef]
- Walsh, S.R.; Cook, E.J.; Goulder, F.; Justin, T.A.; Keeling, N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J. Surg Oncol. 2005, 91, 181–184. [Google Scholar] [CrossRef]
- Takenaka, Y.; Oya, R.; Kitamiura, T.; Ashida, N.; Shimizu, K.; Takemura, K.; Yamamoto, Y.; Uno, A. Platelet count and platelet-lymphocyte ratio as prognostic markers for head and neck squamous cell carcinoma: Meta-analysis. Head Neck 2018, 40, 2714–2723. [Google Scholar] [CrossRef]
- Galon, J.; Pages, F.; Marincola, F.M.; Angell, H.K.; Thurin, M.; Lugli, A.; Zlobec, I.; Berger, A.; Bifulco, C.; Botti, G.; et al. Cancer classification using the Immunoscore: A worldwide task force. J. Transl. Med. 2012, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E. Multivariable Modeling Strategies. Regression Modeling Strategies, 2nd ed.; Springer: Cham, Switzerland, 2015; pp. 63–102. [Google Scholar]
- Chavent, M.; Kuentz-Simonet, V.; Liquet, B.; Saracco, J. ClustOfVar: An R Package for the Clustering of Variables. J. Stat. Softw. 2011, 50, 1–16. [Google Scholar]
- Vigneau, E.; Qannari, E.M. Clustering of Variables around Latent Components. Commun. Stat.-Simul. Comput. 2003, 32, 1131–1150. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Durrleman, S.; Simon, R. Flexible regression models with cubic splines. Stat. Med. 1989, 8, 551–561. [Google Scholar] [CrossRef]
- Harrell, F.E.J.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Pfeiffer, R.M.; Gail, M.H. Estimating the decision curve and its precision from three study designs. Biom. J. 2020, 62, 764–776. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Elkin, E.B.; Gonen, M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med. Inform. Decis. Mak. 2008, 8, 53. [Google Scholar] [CrossRef] [Green Version]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. Int. J. Soc. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Breheny, P.; Burchett, W. Package ‘visreg’: Visualization of Regression Models. R J. 2017, 9, 56–71. [Google Scholar] [CrossRef]
- Cerezo, L.; Millán, I.; Torre, A.; Aragón, G.; Otero, J. Prognostic factors for survival and tumor control in cervical lymph node metastases from head and neck cancer. A multivariate study of 492 cases. Cancer. 1992, 69, 1224–1234. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Ho, A.S.; Kim, S.; Tighiouart, M.; Gudino, C.; Mita, A.; Scher, K.S.; Laury, A.; Prasad, R.; Shiao, S.L.; Van Eyk, J.E.; et al. Metastatic Lymph Node Burden and Survival in Oral Cavity Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3601–3609. [Google Scholar] [CrossRef] [Green Version]
- Mirian, C.; Gerds, T.A.; Pedersen, M.M.; de Ridder, M.; Balm, A.; Mattavelli, D.; Piazza, C.; Jensen, L.R.; Balasubramanian, D.; Subramaniam, N.; et al. Metrics of pN-staging in oral squamous cell carcinoma: An analysis of 1905 patients. Eur. J. Cancer. 2021, 150, 33–41. [Google Scholar] [CrossRef]
- Papaioannou, E.; Sakellakis, M.; Melachrinou, M.; Tzoracoleftherakis, E.; Kalofonos, H.; Kourea, E. A Standardized Evaluation Method for FOXP3+ Tregs and CD8+ T-cells in Breast Carcinoma: Association with Breast Carcinoma Subtypes, Stage and Prognosis. Anticancer Res. 2019, 39, 1217–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperlich, A.; Balmert, A.; Doll, D.; Bauer, S.; Franke, F.; Keller, G.; Wilhelm, D.; Mur, A.; Respondek, M.; Friess, H.; et al. Genetic and immunological biomarkers predict metastatic disease recurrence in stage III colon cancer. BMC Cancer 2018, 18, 998. [Google Scholar] [CrossRef] [Green Version]
- Fujii, H.; Arakawa, A.; Utsumi, D.; Sumiyoshi, S.; Yamamoto, Y.; Kitoh, A.; Ono, M.; Matsumura, Y.; Kato, M.; Konishi, K.; et al. CD8+ tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int. J. Cancer 2014, 134, 2393–2402. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Q.; Chen, L.; Mao, Y.P.; Li, Y.Q.; Jiang, W.; Xu, S.-Y.; Zhang, Y.; Chen, Y.P.; Li, X.-M.; He, Q.M.; et al. Prognostic value of immune score in nasopharyngeal carcinoma using digital pathology. J. Immunother. Cancer 2020, 8, e000334. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Azuma, K.; Kawahara, A.; Akiba, J.; Kakuma, T.; Chitose, S.; Umeno, H. Pre-treatment CD8(+) tumour-infiltrating lymphocyte density predicts distant metastasis after definitive treatment in patients with stage III/IV hypopharyngeal squamous cell carcinoma. Clin. Otolaryngol. Off. J. ENT-UK Off. J. Neth. Soc. Oto-Rhino-Laryngol. Cerv.-Fac. Surg. 2018, 43, 1312–1320. [Google Scholar] [CrossRef]
- Wang, J.; Tian, S.; Sun, J.; Zhang, J.; Lin, L.; Hu, C. The presence of tumour-infiltrating lymphocytes (TILs) and the ratios between different subsets serve as prognostic factors in advanced hypopharyngeal squamous cell carcinoma. BMC Cancer 2020, 20, 731. [Google Scholar] [CrossRef]
- Balermpas, P.; Michel, Y.; Wagenblast, J.; Seitz, O.; Weiss, C.; Rödel, F.; Rödel, C.; Fokas, E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br. J. Cancer 2014, 110, 501–509. [Google Scholar] [CrossRef]
- Chen, W.Y.; Wu, C.T.; Wang, C.W.; Lan, K.H.; Liang, H.K.; Huang, B.S.; Chang, Y.L.; Kuo, S.H.; Cheng, A.L. Prognostic significance of tumor-infiltrating lymphocytes in patients with operable tongue cancer. Radiat. Oncol. 2018, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Garner, H.; de Visser, K.E. Immune crosstalk in cancer progression and metastatic spread: A complex conversation. Nat. Rev. Immunol. 2020, 20, 483–497. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Philip, M.; Schietinger, A. CD8(+) T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2021. [Google Scholar] [CrossRef]
- Devaud, C.; John, L.B.; Westwood, J.A.; Darcy, P.K.; Kershaw, M.H. Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology 2013, 2, e25961. [Google Scholar] [CrossRef] [Green Version]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.; Fan, J.; Wang, L.; Thompson, L.F.; Liu, A.; Daniel, B.J.; Shin, T.; Curiel, T.J.; Zhang, B. CD73 on tumor cells impairs antitumor T-cell responses: A novel mechanism of tumor-induced immune suppression. Cancer Res. 2010, 70, 2245–2255. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; O’Malley, M.; Urban, J.; Sampath, P.; Guo, Z.S.; Kalinski, P.; Thorne, S.H.; Bartlett, D.L. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol. Ther. 2011, 19, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wrzesinski, S.H.; Stern, E.; Look, M.; Criscione, J.; Ragheb, R.; Jay, S.M.; Demento, S.L.; Agawu, A.; Licona Limon, P.; et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 2012, 11, 895–905. [Google Scholar] [CrossRef]
- John, L.B.; Howland, L.J.; Flynn, J.K.; West, A.C.; Devaud, C.; Duong, C.P.; Stewart, T.J.; Westwood, J.A.; Guo, Z.S.; Bartlett, D.L.; et al. Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer. Cancer Res. 2012, 72, 1651–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoenfeld, J.D.; Hanna, G.J.; Jo, V.Y.; Rawal, B.; Chen, Y.-H.; Catalano, P.S.; Lako, A.; Ciantra, Z.; Weirather, J.L.; Criscitiello, S.; et al. Neoadjuvant Nivolumab or Nivolumab Plus Ipilimumab in Untreated Oral Cavity Squamous Cell Carcinoma: A Phase 2 Open-Label Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1563–1570. [Google Scholar] [CrossRef]
- Uppaluri, R.; Zolkind, P.; Lin, T.; Nussenbaum, B.; Jackson, R.S.; Rich, J.; Pipkorn, P.; Paniello, R.; Thorstad, W.; Michel, L.; et al. Neoadjuvant pembrolizumab in surgically resectable, locally advanced HPV negative head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2017, 35, 6012. [Google Scholar] [CrossRef]
- Zuur, L.; Vos, J.L.; Elbers, J.B.; Krijgsman, O.; Qiao, X.; van der Leun, A.; Smit, L.; van den Brekel, M.W.; Tan, B.; Jasperse, B.; et al. LBA40 Neoadjuvant nivolumab and nivolumab plus ipilimumab induce (near-) complete responses in patients with head and neck squamous cell carcinoma: The IMCISION trial. Ann. Oncol. 2020, 31, S1169. [Google Scholar] [CrossRef]
- Zuur, C.L.; Elbers, J.B.W.; Vos, J.L.; van der Leun, A.; Qiao, X.; Karakullukcu, B.; van den Brekel, M.W.M.; Tan, B.; Jasperse, B.; Vogel, W.V.; et al. Feasibility and toxicity of neoadjuvant nivolumab with or without ipilimumab prior to extensive (salvage) surgery in patients with advanced head and neck cancer (the IMCISION trial, NCT03003637). J. Clin. Oncol. 2019, 37, 2575. [Google Scholar] [CrossRef]
- Horton, J.D.; Knochelmann, H.; Armeson, K.; Kaczmar, J.M.; Paulos, C.; Neskey, D. Neoadjuvant presurgical PD-1 inhibition in oral cavity squamous cell carcinoma. J. Clin. Oncol. 2019, 37, 2574. [Google Scholar] [CrossRef]
- Kleinovink, J.W.; Fransen, M.F.; Löwik, C.W.; Ossendorp, F. Photodynamic-Immune Checkpoint Therapy Eradicates Local and Distant Tumors by CD8(+) T Cells. Cancer Immunol. Res. 2017, 5, 832–838. [Google Scholar] [CrossRef] [Green Version]
- Civantos, F.J.; Karakullukcu, B.; Biel, M.; Silver, C.E.; Rinaldo, A.; Saba, N.F.; Takes, R.P.; Vander Poorten, V.; Ferlito, A. A Review of Photodynamic Therapy for Neoplasms of the Head and Neck. Adv. Ther. 2018, 35, 324–340. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, J.; Delaere, P.; Vander Poorten, V. Photodynamic therapy in head and neck cancer: Indications, outcomes, and future prospects. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 136–141. [Google Scholar] [CrossRef]






| Variable | Overall (N = 182) | Variable | Overall (N = 182) |
|---|---|---|---|
| Age | Margins | ||
| Mean (SD) | 63.6 (13.1) | Positive | 32 (17.6%) |
| Median (Min, Max) | 64.0 (26.0, 93.0) | Close | 54 (29.7%) |
| Sex | Negative | 96 (52.7%) | |
| Male | 115 (63.2%) | Treatment | |
| Female | 67 (36.8%) | Surgery | 78 (42.9%) |
| pT category (8th Ed.) | Surgery+RT | 61 (33.5%) | |
| pT1 | 23 (12.6%) | Surgery+CRT | 43 (23.6%) |
| pT2 | 41 (22.5%) | WBC (109/L) | |
| pT3 | 85 (46.7%) | Mean (SD) | 7.41 (1.94) |
| pT4 | 33 (18.1%) | Median (Min, Max) | 7.39 (2.77, 13.7) |
| pN category (8th Ed.) | Lymphocytes (109/L) | ||
| pN0 | 96 (52.7%) | Mean (SD) | 1.82 (0.535) |
| pN1 | 24 (13.2%) | Median (Min, Max) | 1.78 (0.690, 3.69) |
| pN2a | 6 (3.3%) | Neutrophils (109/L) | |
| pN2b | 16 (8.8%) | Mean (SD) | 4.79 (1.64) |
| pN2c | 2 (1.1%) | Median (Min, Max) | 4.76 (1.04, 10.1) |
| pN3b | 38 (20.9%) | Monocytes (109/L) | |
| Npos | Mean (SD) | 0.585 (0.231) | |
| pN0 | 97 (53.3%) | Median (Min, Max) | 0.550 (0.160, 1.53) |
| pN+ | 85 (46.7%) | PLT (109/L) | |
| ENE | Mean (SD) | 228 (68.6) | |
| No | 140 (76.9%) | Median (Min, Max) | 220 (51.0, 431) |
| Yes | 42 (23.1%) | NLR | |
| Nodal ratio | Mean (SD) | 2.83 (1.31) | |
| Mean (SD) | 0.0326 (0.0558) | Median (Min, Max) | 2.53 (0.799, 7.55) |
| Median (Min, Max) | 0 (0, 0.320) | PLR | |
| Total Number Positive Nodes | Mean (SD) | 134 (52.7) | |
| Mean (SD) | 1.54 (2.84) | Median (Min, Max) | 129 (31.1, 315) |
| Median (Min, Max) | 0 (0, 18.0) | CD8 density Total (cells/mm2) | |
| LVI | Mean (SD) | 418 (403) | |
| No | 130 (71.4%) | Median (Min, Max) | 279 (16, 2180) |
| Yes | 52 (28.6%) | CD8 density CT (cells/mm2) | |
| PNI | Mean (SD) | 333 (406) | |
| No | 92 (50.5%) | Median (Min, Max) | 170 (5, 2270) |
| Yes | 90 (49.5%) | CD8 density IM (cells/mm2) | |
| Differentiation | Mean (SD) | 520 (429) | |
| G1 | 18 (9.9%) | Median (Min, Max) | 394 (24, 2340) |
| G2 | 87 (47.8%) | ||
| G3 | 77 (42.3%) | ||
| Bone invasion | |||
| No | 148 (81.3%) | ||
| Cortical | 16 (8.8%) | ||
| Medullary | 18 (9.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Missale, F.; Bugatti, M.; Mattavelli, D.; Lonardi, S.; Lombardi, D.; Nicolai, P.; Piazza, C.; Battocchio, S.; Bozzola, A.M.; Calza, S.; et al. Metavariables Resuming Host Immune Features and Nodal Involvement Are Associated with Oncological Outcomes in Oral Cavity Squamous Cell Carcinoma. Cells 2021, 10, 2203. https://doi.org/10.3390/cells10092203
Missale F, Bugatti M, Mattavelli D, Lonardi S, Lombardi D, Nicolai P, Piazza C, Battocchio S, Bozzola AM, Calza S, et al. Metavariables Resuming Host Immune Features and Nodal Involvement Are Associated with Oncological Outcomes in Oral Cavity Squamous Cell Carcinoma. Cells. 2021; 10(9):2203. https://doi.org/10.3390/cells10092203
Chicago/Turabian StyleMissale, Francesco, Mattia Bugatti, Davide Mattavelli, Silvia Lonardi, Davide Lombardi, Piero Nicolai, Cesare Piazza, Simonetta Battocchio, Anna Maria Bozzola, Stefano Calza, and et al. 2021. "Metavariables Resuming Host Immune Features and Nodal Involvement Are Associated with Oncological Outcomes in Oral Cavity Squamous Cell Carcinoma" Cells 10, no. 9: 2203. https://doi.org/10.3390/cells10092203
APA StyleMissale, F., Bugatti, M., Mattavelli, D., Lonardi, S., Lombardi, D., Nicolai, P., Piazza, C., Battocchio, S., Bozzola, A. M., Calza, S., & Vermi, W. (2021). Metavariables Resuming Host Immune Features and Nodal Involvement Are Associated with Oncological Outcomes in Oral Cavity Squamous Cell Carcinoma. Cells, 10(9), 2203. https://doi.org/10.3390/cells10092203