Sperm Accumulation Induced by the Female Reproductive Fluid: Putative Evidence of Chemoattraction Using a New Tool
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Fish Maintenance
2.2. Design of the Sperm Choice-Chamber
2.3. FRF Collection
2.4. Sperm Collection from Males
2.5. Test of the Sperm Choice-Chamber
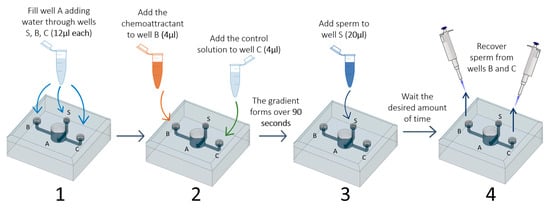
- Add water through the three smaller wells (S, B, and C; 12 µL each) using a micropipette (P10 or P20). This step fills chamber A and avoids air bubble formation in the channels;
- Four microliters of each of the chosen solutions (FRF as chemoattractant and water as control) is added to wells B and C. At this point, the two solutions will start to gradually mix with the water already present in the device. The FRF gradient will gradually form over 90 s, starting from the well, through its channel (channel B or C), to the central well (A);
- Twenty microliters of sperm pool solution is gently added to the sperm well S;
- After the desired amount of time (TA), sperm can be collected at the desire sampling volume (SV) through the two collection wells (B and C) using a micropipette (P10 or P20);
- The number of sperm collected are counted to assess sperm accumulation.
2.6. Repeatability Test
2.7. Ejaculate Quality Assay
2.8. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gasparini, C.; Pilastro, A.; Evans, J.P. The role of female reproductive fluid in sperm competition. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2020, 375, 20200077. [Google Scholar] [CrossRef] [PubMed]
- Pitnick, S.; Wolfner, M.F.; Dorus, S. Post-ejaculatory modifications to sperm (PEMS). Biol. Rev. 2020, 95, 365–392. [Google Scholar] [CrossRef] [PubMed]
- Lillie, F.R. The Production of Sperm Iso-Agglutinins by Ova. Science 1912, 36, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Yeates, S.E.; Diamond, S.E.; Einum, S.; Emerson, B.C.; Holt, W.V.; Gage, M.J.G. Cryptic choice of conspecific sperm controlled by the impact of ovarian fluid on sperm swimming behavior. Evolution 2013, 67, 3523–3536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.P.; Sherman, C.D.H. Sexual Selection and the Evolution of Egg-Sperm Interactions in Broadcast-Spawning Invertebrates. Biol. Bull. 2013, 224, 166–183. [Google Scholar] [CrossRef]
- Fabro, G.; Rovasio, R.A.; Civalero, S.; Frenkel, A.; Caplan, S.R.; Eisenbach, M.; Giojalas, L.C. Chemotaxis of capacitated rabbit spermatozoa to follicular fluid revealed by a novel directionality-based assay. Biol. Reprod. 2002, 67, 1565–1571. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, J.L.; Willis, C.; Devigili, A.; Young, A.; Carroll, M.; Hunter, H.R.; Brison, D.R. Chemical signals from eggs facilitate cryptic female choice in humans. Proc. R. Soc. B Biol. Sci. 2020, 287, 20200805. [Google Scholar] [CrossRef]
- Eisenbach, M.; Tur-Kaspa, I. Human sperm chemotaxis is not enigmatic anymore. Fertil. Steril. 1994, 62, 233–235. [Google Scholar] [CrossRef]
- Alonzo, S.H.; Stiver, K.A.; Marsh-Rollo, S.E. Ovarian fluid allows directional cryptic female choice despite external fertilization. Nat. Commun. 2016, 7, 12452. [Google Scholar] [CrossRef] [Green Version]
- Oliver, M.; Evans, J.P. Chemically moderated gamete preferences predict offspring fitness in a broadcast spawning invertebrate. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140148. [Google Scholar] [CrossRef]
- Vieira, L.A.; Diana, A.; Soriano-Ubeda, C.; Matas, C. Selection of Boar Sperm by Reproductive Biofluids as Chemoattractants. Animals 2020, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Ward, G.E.; Brokaw, C.J.; Garbers, D.L.; Vacquier, V.D. Chemotaxis of Arbacia-Punctulata Spermatozoa to Resact, a Peptide from the Egg Jelly Layer. J. Cell Biol. 1985, 101, 2324–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohmer, M.; Van, Q.; Weyand, I.; Hagen, V.; Beyermann, M.; Matsumoto, M.; Hoshi, M.; Hildebrand, E.; Kaupp, U.B. Ca2+ spikes in the flagellum control chemotactic behavior of sperm. EMBO J. 2005, 24, 2741–2752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, M.; Murata, M.; Inaba, K.; Morisawa, M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc. Natl. Acad. Sci. USA 2002, 99, 14831–14836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, J.H.; Xiang, X.Y.; Ziegert, T.; Kittelson, A.; Rawls, A.; Bieber, A.L.; Chandler, D.E. Allurin, a 21-kDa sperm chemoattractant from Xenopus egg jelly, is related to mammalian sperm-binding proteins. Proc. Natl. Acad. Sci. USA 2001, 98, 11205–11210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenbach, M.; Giojalas, L.C. Sperm guidance in mammals—An unpaved road to the egg. Nat. Rev. Mol. Cell Biol. 2006, 7, 276–285. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, B.; Fu, Q.; Wu, J.; Liu, R. A fully integrated biomimetic microfluidic device for evaluation of sperm response to thermotaxis and chemotaxis. Lab Chip 2021, 21, 310–318. [Google Scholar] [CrossRef]
- Xie, L.; Ma, R.; Han, C.; Su, K.; Zhang, Q.; Qiu, T.; Wang, L.; Huang, G.; Qiao, J.; Wang, J.; et al. Integration of sperm motility and chemotaxis screening with a microchannel-based device. Clin. Chem. 2010, 56, 1270–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.J.; Maeng, J.H.; Lee, B.C.; Lee, S.; Hwang, S.Y.; Ahn, Y. Separation of progressive motile sperm from mouse semen using on-chip chemotaxis. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2012, 28, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, Y.J.; Maeng, J.H.; Hwang, S.Y.; Ahn, Y. Design, Fabrication, and Testing of a Microfluidic Device for Thermotaxis and Chemotaxis Assays of Sperm. SLAS Technol. 2018, 23, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Burnett, L.A.; Tholl, N.; Chandler, D.E. Two Types of Assays for Detecting Frog Sperm Chemoattraction. J. Vis. Exp. 2011, 58, 3407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.P.; Garcia-Gonzalez, F.; Almbro, M.; Robinson, O.; Fitzpatrick, J.L. Assessing the potential for egg chemoattractants to mediate sexual selection in a broadcast spawning marine invertebrate. Proc. R. Soc. B Biol. Sci. 2012, 279, 2855–2861. [Google Scholar] [CrossRef]
- Poli, F.; Immler, S.; Gasparini, C. Effects of ovarian fluid on sperm traits and its implications for cryptic female choice in zebrafish. Behav. Ecol. 2019, 30, 1298–1305. [Google Scholar] [CrossRef]
- Purchase, C.F.; Rooke, A.C. Freezing ovarian fluid does not alter how it affects fish sperm swimming performance: Creating a cryptic female choice ’spice rack’ for use in split-ejaculate experimentation. J. Fish Biol. 2020, 96, 693–699. [Google Scholar] [CrossRef]
- Jing, R.Y.; Huang, C.J.; Bai, C.L.; Tanguay, R.; Dong, Q.X. Optimization of activation, collection, dilution, and storage methods for zebrafish sperm. Aquaculture 2009, 290, 165–171. [Google Scholar] [CrossRef]
- Jokiniemi, A.; Magris, M.; Ritari, J.; Kuusipalo, L.; Lundgren, T.; Partanen, J.; Kekalainen, J. Post-copulatory genetic matchmaking: HLA-dependent effects of cervical mucus on human sperm function. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201682. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Schielzeth, H. Repeatability for Gaussian and non-Gaussian data: A practical guide for biologists. Biol. Rev. 2010, 85, 935–956. [Google Scholar] [CrossRef]
- Stoffel, M.; Nakagawa, S.; Schielzeth, H. Repeatability Estimation for Gaussian and Non-Gaussian Data. 2019. Available online: https://CRAN.R-project.org/package=rptR (accessed on 30 May 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Scheipl; Peter Green, F.; Fox, J.; et al. Linear Mixed-Effects Models using ’Eigen’ and S4 (lme4). 2020. Available online: https://cran.r-project.org/web/packages/lme4/index.html (accessed on 30 May 2021).
- Kirkman-Brown, J.C.; Smith, D.J. Sperm motility: Is viscosity fundamental to progress? Mol. Hum. Reprod. 2011, 17, 539–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenbach, M. Sperm chemotaxis. Rev. Reprod. 1999, 4, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, U.B. 100 years of sperm chemotaxis. J. Gen. Physiol. 2012, 140, 583–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, A.; Wood, C.D.; Nishigaki, T.; Carneiro, J.; Darszon, A. Tuning sperm chemotaxis. Biochem. Soc. Trans. 2010, 38, 1270–1274. [Google Scholar] [CrossRef] [Green Version]
- Kaupp, U.B.; Kashikar, N.D.; Weyand, I. Mechanisms of sperm chemotaxis. Annu. Rev. Physiol. 2008, 70, 93–117. [Google Scholar] [CrossRef]
- Friedrich, B.M.; Julicher, F. Chemotaxis of sperm cells. Proc. Natl. Acad. Sci. USA 2007, 104, 13256–13261. [Google Scholar] [CrossRef] [Green Version]
- Lange, S.; Friedrich, B.M. Fertilization in the sea: Sperm chemotaxis in physiological shear flows. ArXiv 2019, arXiv:1912.09112. [Google Scholar]
- Hussain, Y.H.; Guasto, J.S.; Zimmer, R.K.; Stocker, R.; Riffell, J.A. Sperm chemotaxis promotes individual fertilization success in sea urchins. J. Exp. Biol. 2016, 219, 1458–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero-Campo, P.; Buffone, M.G.; Benencia, F.; Conejo-Garcia, J.R.; Rinaudo, P.F.; Gerton, G.L. A role for the chemokine receptor CCR6 in mammalian sperm motility and chemotaxis. J. Cell. Physiol. 2014, 229, 68–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, M.; Hiradate, Y.; Sensui, N.; Cosson, J.; Morisawa, M. Species-specificity of sperm motility activation and chemotaxis: A study on ascidian species. Biol. Bull. 2013, 224, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, U.B.; Hildebrand, E.; Weyand, I. Sperm chemotaxis in marine invertebrates--molecules and mechanisms. J. Cell. Physiol. 2006, 208, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Friedrich, B.M. Sperm chemotaxis in marine species is optimal at physiological flow rates according theory of filament surfing. PLoS Comput. Biol. 2021, 17, e1008826. [Google Scholar] [CrossRef] [PubMed]
- Farley, G.S.; Levitan, D.R. The role of jelly coats in sperm-egg encounters, fertilization success, and selection on egg size in broadcast spawners. Am. Nat. 2001, 157, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, T.; Behr, B.; Demirci, U. Microfluidic device based selective sorting of motile human sperm for IUI application: A preliminary study. Hum. Reprod. 2016, 31, 83. [Google Scholar]
- Chang, H.; Kim, B.J.; Kim, Y.S.; Suarez, S.S.; Wu, M. Different migration patterns of sea urchin and mouse sperm revealed by a microfluidic chemotaxis device. PLoS ONE 2013, 8, e60587. [Google Scholar] [CrossRef] [PubMed]
- Villanueva-Diaz, C.; Arias-Martinez, J.; Bustos-Lopez, H.; Vadillo-Ortega, F. Novel model for study of human sperm chemotaxis. Fertil. Steril. 1992, 58, 392–395. [Google Scholar] [CrossRef]
- Lai, D.; Takayama, S.; Smith, G.D. Recent microfluidic devices for studying gamete and embryo biomechanics. J. Biomech. 2015, 48, 1671–1678. [Google Scholar] [CrossRef] [Green Version]
- Zadmajid, V.; Myers, J.N.; Sorensen, S.R.; Butts, I.A.E. Ovarian fluid and its impacts on spermatozoa performance in fish: A review. Theriogenology 2019, 132, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, M.; Medem, F.G.; Kuhn, R.; Bielig, H.-J. Untersuchungen über die Befruchtungsstoffe der Regenbogenforell. Z. Nat. B 1947, 2, 330–349. [Google Scholar] [CrossRef]
- Kholodnyy, V.; Gadelha, H.; Cosson, J.; Boryshpolets, S. How do freshwater fish sperm find the egg? The physicochemical factors guiding the gamete encounters of externally fertilizing freshwater fish. Rev. Aquac. 2020, 12, 1165–1192. [Google Scholar] [CrossRef] [Green Version]
- Rosengrave, P.; Gemmell, N.J.; Metcalf, V.; McBride, K.; Montgomerie, R. A mechanism for cryptic female choice in chinook salmon. Behav. Ecol. 2008, 19, 1179–1185. [Google Scholar] [CrossRef] [Green Version]
- Kaupp, U.B.; Solzin, J.; Hildebrand, E.; Brown, J.E.; Helbig, A.; Hagen, V.; Beyermann, M.; Pampaloni, F.; Weyand, I. The signal flow and motor response controling chemotaxis of sea urchin sperm. Nature 2003, 5, 109–117. [Google Scholar] [CrossRef]
- Guidobaldi, H.A.; Teves, M.E.; Uñates, D.R.; Anastasía, A.; Giojalas, L.C. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. PLoS ONE 2008, 3, e3040. [Google Scholar] [CrossRef]
- Gakamsky, A.; Schechtman, E.; Caplan, S.R.; Eisenbach, M. Analysis of chemotaxis when the fraction of responsive cells is small--application to mammalian sperm guidance. Int. J. Dev. Biol. 2008, 52, 481–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosalvez, J.; Lopez-Fernandez, C.; Hermoso, A.; Fernandez, J.L.; Kjelland, M.E. Sperm DNA fragmentation in zebrafish (Danio rerio) and its impact on fertility and embryo viability—Implications for fisheries and aquaculture. Aquaculture 2014, 433, 173–182. [Google Scholar] [CrossRef]
- Perez-Cerezales, S.; Martinez-Paramo, S.; Beirao, J.; Herraez, M.P. Fertilization capacity with rainbow trout DNA-damaged sperm and embryo developmental success. Reproduction 2010, 139, 989–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devigili, A.; Cattelan, S.; Gasparini, C. Sperm Accumulation Induced by the Female Reproductive Fluid: Putative Evidence of Chemoattraction Using a New Tool. Cells 2021, 10, 2472. https://doi.org/10.3390/cells10092472
Devigili A, Cattelan S, Gasparini C. Sperm Accumulation Induced by the Female Reproductive Fluid: Putative Evidence of Chemoattraction Using a New Tool. Cells. 2021; 10(9):2472. https://doi.org/10.3390/cells10092472
Chicago/Turabian StyleDevigili, Alessandro, Silvia Cattelan, and Clelia Gasparini. 2021. "Sperm Accumulation Induced by the Female Reproductive Fluid: Putative Evidence of Chemoattraction Using a New Tool" Cells 10, no. 9: 2472. https://doi.org/10.3390/cells10092472
APA StyleDevigili, A., Cattelan, S., & Gasparini, C. (2021). Sperm Accumulation Induced by the Female Reproductive Fluid: Putative Evidence of Chemoattraction Using a New Tool. Cells, 10(9), 2472. https://doi.org/10.3390/cells10092472