Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor
Abstract
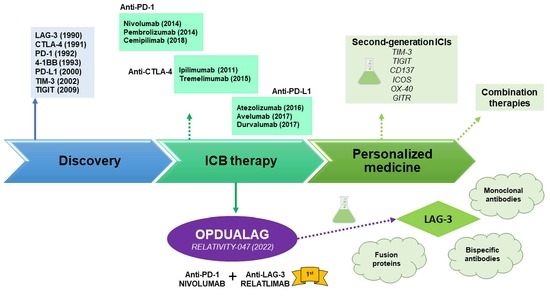
:1. Introduction: Brief History of Immunotherapy
2. Preclinical and Clinical Development of Opdualag
2.1. LAG-3 Molecular Function
2.2. LAG-3 Clinical Research
2.2.1. Anti-LAG-3 Monoclonal Antibodies
- BMS-986016 or relatlimab, developed by Bristol-Myers Squibb in 47 clinical trials, was the first anti-LAG-3 monoclonal antibody to be clinically developed and the first one to receive the FDA approval for its clinical use. It has 4 subunits, with 16 disulfide links and 2 N-glycosylation sites, with an average molecular weight of 145.3 kDa [78]. Phase I (7 trials), I/II (12 trials), II (26 trials), II/III (1 trial) and III (1 trial) preliminary results showed good tolerability, efficacy, toxicity and antitumour profiles alone or in combination with anti-PD-1/PD-L1 blockade immunotherapies, as a good alternative to overcome immunotherapy resistance [79,80,81]. For example, it restores T cell mediated responses and TNF-a, IFN-y and IL-2 cytokine release [82]. The phase III clinical trial that led to the LAG-3/PD-1 combination approval for melanoma treatment is further discussed in the next section.
- GSK2831781, derived from IMP731 Immunetep’s antibody, developed in monotherapy by GlaxoSmithKline in 3 clinical trials (2 phase I and 1 phase II) for psoriasis and ulcerative colitis. The ulcerative colitis phase II trial was terminated after an interim analysis [83], but phase I results show good tolerability, safety and inflammation regulation profiles [84].
- HLX26, developed by Fosun Pharma in 2 phase I clinical trials (NCT05078593 and NCT05400265), where its safety, tolerability, pharmacokinetic characteristics and preliminary efficacy are being evaluated alone and in combination with anti-PD-1 treatments in patients with solid tumors or lymphoma.
- IBI110, developed by Innovent Biologics in a phase I clinical trial alone and in combination with anti-PD-1 in patients with relapsed or refractory diffuse large B cell lymphoma (r/r DLBCL) (NCT05039658).
- INCAGN02385, is being developed by Incyte in 4 clinical trials (1 phase I, 1 phase I/II and 2 phase II) alone (NCT03538028) or in combination (NCT04370704, NCT05287113, NCT04586244) with anti-PD-1 and anti-TIM-3 immune checkpoint therapies. Preliminary data shows good tolerability profiles [85].
- LAG525 or IMP701 developed by Novartis in 5 clinical trials (1 phase I, 1 phase I/II and 3 phase II), alone or in combination with anti-PD-1 blockers. The structure of this antibody consists of 4 subunits, 16 disulfide bridges and 2 N-glycosylation sites, with an estimated molecular weight of 147 kDa [86]. Preliminary data demonstrate promising pharmacokinetics, antitumour activity and safety alone and in combination [87,88,89].
- MK-4830 or favezelimab, developed by Merck in 8 clinical trials (1 phase I, 5 phase I/II, 1 phase II and 1 phase III), alone or in combination with anti-PD-1, oxaliplatin, Leucovorin (Calcium Folinate), Fluorouracil [5-FU] and lenvatinib, showing manageable safety and tolerability alone and in combination. In fact, anti-LAG-3/anti-PD-1 combination showed a 6.3% objective response rate, better than the monotherapy treatment, with similar treatment-related adverse effects [90,91]. The structure of this antibody consists of 4 subunits, 16 disulfide bridges and 2 glycosylation sites, with an estimated molecular weight of 146 kDa [92].
- REGN3767 or fianlimab, developed by Regeneron Pharmaceuticals and Sanofi in 3 clinical trials (1 phase I, 1 phase II and 1 phase III), promotes T cell activation and T cell mediated cytotoxicity with good pharmacokinetics and toxicology profiles in vitro and in vivo [93]. The structure of the antibody is composed of 4 subunits, 16 sulfide bridges and 2 N-glycosilation sites [94]. Early efficacy and antitumor activity were also suggested in the preliminary clinical trials results. Its combination with cemiplimab also showed a good safety profile [95,96,97]. The combination with anti-PD-1, and cemiplimab is being evaluated in phase I (NCT03005782), II (NCT01042379) and III (NCT05352672) trials while it is being studied alone in the NCT03005782 phase I trial. Interestingly, anti-LAG-3 PET tracers (89Zr-DFO-REGN3767) are being clinically developed to establish the tracer biodistribution and dosimetry, monitoring the response to REGN3767 treatment (NCT05346276, NCT04706715, NCT04566978). However, these clinical trials are not being considered in this review as LAG-3 targeting clinical trials, because their main purpose is establishing PET scanning as a diagnostic method.
- Sym022, developed by Symphogen in 3 phase I clinical trials, is being evaluated for dose-escalation and dose-expansion alone (NCT03489369) or in combination (NCT03311412, NCT04641871) with anti-PD-1 and anti-TIM-3 immunotherapies. The treatment combination showed synergic antitumor activity in preclinical models [98,99].
- TSR-033, is being developed by Tesaro in 2 phase I clinical trials, alone and in combination with anti-PD-1 and anti-TIM-3 treatments (NCT03250832, NCT02817633). The combination with PD-1 blockers increases CD4 T cell activation and IL-2 production and cell proliferation [100]. Phase I preliminary data indicates good safety and tolerability.
2.2.2. Anti-LAG-3 Bispecific Antibodies
- ABL501 is being developed by ABL Bio in a phase I trial for the treatment of any progressive, locally advanced (unresectable) or metastatic solid tumor (NCT05101109). This bispecific antibody blocks PD-L1 and LAG-3 as a single agent. Dose-escalation analysis is being conducted. The dosing interval to be used in the dose-expansion part will be re-evaluated based on the emerging safety and pharmacokinetics data from the dose-escalation part of the study. It promotes enhanced human T cell activation in vitro and potentiates antitumor responses of T cells through DC activation [101,102].
- IBI323, a LAG-3/PD-L1 bispecific antibody, is being developed by Innovent Biologics in a phase I clinical trial alone and in combination with chemotherapy in patients with advanced malignancies. The purpose of this study is to evaluate IBI323 safety, tolerability and efficacy. It enhances tumor-specific immunity in vitro [103].
- MGD013 or Tebotelimab, a LAG-3/PD-1 bispecific DART® antibody, is being developed by MacroGenetics in 7 clinical trials (3 phase I, 1 phase I/II, 1 phase II and 2 phase II/III) in patients with unresectable or metastatic neoplasms (NCT03219268), patients with advanced or metastatic solid tumors who failed prior treatment (NCT04178460), melanoma (NCT04653038), liver cancer (NCT04212221), Head and Neck Cancer (NCT04634825, NCT04082364) and HER2+ Gastric/GEJ Cancer (NCT04082364), to evaluate its safety and efficacy, alone or in combination with margetuximab (anti-HER2), niraparib (a selective PARP1/2 inhibitor), Brivanib Alaninate (Multitargeted tyrosine kinase inhibitor) and enoblituzumab (Anti-B7-H3 antibody). Preliminary results showed good tolerability, safety and antitumour activity profiles [104].
- RO7247669, a LAG-3/PD-1 bispecific antibody, is being developed by Hoffmann-La Roche in 1 phase I and 1 phase II clinical trials in patients with advanced and/or metastatic solid tumors (NCT04140500) and advanced or metastatic squamous cell carcinoma of the oesophagus (NCT04785820), alone or in combination with a PD-1/TIM-3 bispecific antibody or an anti-PD-1 single agent.
- XmAb®22841 or pavunalimab, a LAG-3/CTLA-4 bispecific antibody, is being developed by Xencor in a phase I clinical trial (NCT03849469), alone and in combination with anti-PD-1 as a single agent in selected advanced solid tumors. It enhances antitumor activity, T cell activation, cytokine secretion and cell proliferation [105].
- EMB-02, a LAG-3/PD-1 bispecific antibody, is being developed as a single treatment agent by EpimAb Biotherapeutics in a phase I/II clinical trial (NCT04618393) in advanced solid tumors. Dose escalation followed by cohort expansion will be performed. In vivo preclinical data showed antitumor activity in anti-PD-1 resistant models.
- FS118, a LAG-3/PD-L1 bispecific antibody, is being developed as a single agent treatment by F-star Therapeutics in a phase I/II clinical trial (NCT03440437) in patients with advanced malignancies, to determine dosing and toxicity. It enhanced T cell activation and antitumor activity in vitro and in vivo [105,106,107]. Preliminary clinical trial data showed good pharmacodynamics and tolerability profiles. [108,109].
- CB213 Humabody®, a PD-1xLAG-3 antagonist developed by Crescendo Biologics Ltd., have recently entered a partnership with Cancer Research UK for its clinical development into a future phase I clinical trial ([110]). This bispecific molecule binds and blocks with high affinity PD1 and LAG-3 on PD-1+LAG-3+ T cells, induces ex vivo T cell proliferation of dysfunctional T cells from NSCLC patients, with superior activity than anti-PD-1 alone and suppress tumor growth in vivo [111].
2.2.3. LAG-3 Fusion Proteins
- IMP321, Eftilagimod Alpha or Efti, a LAG-3 soluble fusion protein, is being developed by Immutep in 14 clinical trials (9 phase I, 2 phase I/II and 3 phase II) for the treatment of advanced solid tumors, hepatitis B and flu. IMP321 is being developed as an adjuvant and immune modulator for cancer and vaccines against infectious diseases, as well as an anticancer treatment agent. It is being tested alone and in combination with chemotherapy (gemcitabine), anti-PD-L1, anti-PD-1, paclitaxel, hepatitis B antigen (without alum), a reference flu antigen, Melan-A VLP vaccine and melanoma tumor-specific peptides. Data shows that IMP321 enhances T cell activation and proliferation, humoral, effector and adaptive immunity, cytokine release, immunogenicity and antitumor activity, with good tolerability, efficacy and safety profiles [112,113,114,115,116,117,118,119,120].
- EOC202, a recombinant human LAG-3 fusion protein, is being developed by Taizhou EOC Pharma in a phase II clinical trial (NCT05322720) in HR+, HER2- advanced breast cancer with progression after endocrine therapy to evaluate the PFS for EOC202 combined with albumin-bound paclitaxel versus albumin-bound paclitaxel alone.
2.2.4. Anti-LAG-3 CAR-T Cells
2.3. Opdualag and Its Pathway towards the Clinic
3. Behind the Steps of LAG-3 towards the Clinic
- Immune activation at lymph nodes and peripheral tissues. The activation of T cells requires not only the antigen presentation through the MHC complex, but also a second costimulatory signal provided by APCs. T cells are initially primed at lymph nodes, although the interaction with other immune cell populations in peripheral tissues as well as in the tumor microenvironment may also provide immunoregulatory signals. These signals mediate the acquisition of effector functions or immunosuppressive phenotypes by T cells depending on the engagement of costimulatory or checkpoint receptors. In this case, immune checkpoint blockade would enhance T cell activation by APCs.
- Priming of immune tolerogenic phenotypes. Some immune checkpoint receptors induce tolerogenic phenotypes on antigen-presenting cells (APCs) and prime Tregs.
- Induction of T-cell dysfunction. Sustained antigen presentation and stimulation with inflammatory cytokines induce T cell exhaustion, characterized by reduced proliferation and effector functions. However, cytotoxic functions may be rescued by immune checkpoint receptors blockade with monoclonal antibodies. It has been reported that exhausted T cells show increased expression of multiple checkpoint receptors, which may interfere with the response rate of patients to ICB monotherapies.
3.1. TIM-3
3.2. TIGIT
3.3. CD137
3.4. ICOS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Erauso, L.C.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Hernandez, C.; Fernandez, G.; Garcia-Granda, M.J.; Blanco, E.; Vera, R.; Kochan, G.; et al. Resistance to PD-L1/PD-1 Blockade Immunotherapy. A Tumor-Intrinsic or Tumor-Extrinsic Phenomenon? Front. Pharmacol. 2020, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernández-Rubio, L.; Bocanegra, A.; Echaide, M.; Garnica, M.; Ramos, P.; Fernández-Hinojal, G.; Vera, R.; et al. Clinical landscape of LAG-3-targeted therapy. Immunol.-Oncol. Technol. 2022, 14, 100079. [Google Scholar] [CrossRef] [PubMed]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernández-Rubio, L.; Morente, P.; Fernández-Hinojal, G.; Echaide, M.; Garnica, M.; et al. Understanding LAG-3 Signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar] [CrossRef] [PubMed]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar] [CrossRef] [Green Version]
- Huard, B.; Prigent, P.; Pagès, F.; Bruniquel, D.; Triebel, F. T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur. J. Immunol. 1996, 26, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Huard, B.; Tournier, M.; Hercend, T.; Triebel, F.; Faure, F. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur. J. Immunol. 1994, 24, 3216–3221. [Google Scholar] [CrossRef]
- Andrews, L.P.; Marciscano, A.E.; Drake, C.G.; Vignali, D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017, 276, 80–96. [Google Scholar] [CrossRef]
- Workman, C.J.; Cauley, L.S.; Kim, I.-J.; Blackman, M.A.; Woodland, D.L.; Vignali, D.A.A. Lymphocyte Activation Gene-3 (CD223) Regulates the Size of the Expanding T Cell Population Following Antigen Activation In Vivo. J. Immunol. 2004, 172, 5450–5455. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Dugger, K.J.; Vignali, D.A.A. Cutting Edge: Molecular Analysis of the Negative Regulatory Function of Lymphocyte Activation Gene-3. J. Immunol. 2002, 169, 5392–5395. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.B.; Horton, B.L.; Zheng, Y.; Duan, Y.; Powell, J.D.; Gajewski, T.F. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J. Exp. Med. 2017, 214, 381–400. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Eppolito, C.; Lele, S.; Shrikant, P.; Matsuzaki, J.; Odunsi, K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 2015, 6, 27359–27377. [Google Scholar] [CrossRef] [PubMed]
- Grosso, J.F.; Goldberg, M.V.; Getnet, D.; Bruno, T.C.; Yen, H.-R.; Pyle, K.J.; Hipkiss, E.; Vignali, D.A.A.; Pardoll, D.M.; Drake, C.G. Functionally Distinct LAG-3 and PD-1 Subsets on Activated and Chronically Stimulated CD8 T Cells. J. Immunol. 2009, 182, 6659–6669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, J.F.; Kelleher, C.C.; Harris, T.J.; Maris, C.H.; Hipkiss, E.L.; De Marzo, A.; Anders, R.; Netto, G.; Getnet, D.; Bruno, T.C.; et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Investig. 2007, 117, 3383–3392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chihara, N.; Madi, A.; Kondo, T.; Zhang, H.; Acharya, N.; Singer, M.; Nyman, J.; Marjanovic, N.D.; Kowalczyk, M.S.; Wang, C.; et al. Induction and transcriptional regulation of the co-inhibitory gene module in T cells. Nature 2018, 558, 454–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackburn, S.D.; Shin, H.; Haining, W.N.; Zou, T.; Workman, C.J.; Polley, A.; Betts, M.R.; Freeman, G.J.; A A Vignali, D.; Wherry, E.J. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2008, 10, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camisaschi, C.; Casati, C.; Rini, F.; Perego, M.; De Filippo, A.; Triebel, F.; Parmiani, G.; Belli, F.; Rivoltini, L.; Castelli, C. LAG-3 Expression Defines a Subset of CD4+CD25highFoxp3+ Regulatory T Cells That Are Expanded at Tumor Sites. J. Immunol. 2010, 184, 6545–6551. [Google Scholar] [CrossRef] [Green Version]
- Workman, C.J.; Vignali, D.A.A. Negative Regulation of T Cell Homeostasis by Lymphocyte Activation Gene-3 (CD223). J. Immunol. 2005, 174, 688–695. [Google Scholar] [CrossRef] [PubMed]
- White, A.M.; Wraith, D.C. Tr1-Like T Cells—An Enigmatic Regulatory T Cell Lineage. Front. Immunol. 2016, 7, 355. [Google Scholar] [CrossRef] [Green Version]
- Huard, B.; Tournier, M.; Triebel, F. LAG-3 does not define a specific mode of natural killing in human. Immunol. Lett. 1998, 61, 109–112. [Google Scholar] [CrossRef]
- Baixeras, E.; Huard, B.; Miossec, C.; Jitsukawa, S.; Martin, M.; Hercend, T.; Auffray, C.; Triebel, F.; Piatier-Tonneau, D. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J. Exp. Med. 1992, 176, 327–337. [Google Scholar] [CrossRef]
- Huang, C.-T.; Workman, C.J.; Flies, D.; Pan, X.; Marson, A.L.; Zhou, G.; Hipkiss, E.L.; Ravi, S.; Kowalski, J.; Levitsky, H.I.; et al. Role of LAG-3 in Regulatory T Cells. Immunity 2004, 21, 503–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuazo, M.; Arasanz, H.; Fernández-Hinojal, G.; García-Granda, M.J.; Gato, M.; Bocanegra, A.; Martínez, M.; Hernández, B.; Teijeira, L.; Morilla, I.; et al. Functional systemic CD 4 immunity is required for clinical responses to PD -L1/PD -1 blockade therapy. EMBO Mol. Med. 2019, 11, e10293. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, J.; Gnjatic, S.; Mhawech-Fauceglia, P.; Beck, A.; Miller, A.; Tsuji, T.; Eppolito, C.; Qian, F.; Lele, S.; Shrikant, P.; et al. Tumor-infiltrating NY-ESO-1–specific CD8 + T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 7875–7880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruniquel, D.; Borie, N.; Hannier, S.; Triebel, F. Regulation of expression of the human lymphocyte activation gene-3 (LAG-3) molecule, a ligand for MHC class II. Immunogenetics 1998, 48, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, F.; Manetti, R.; Cosmi, L.; Galli, G.; Heusser, C.H.; Romagnani, S.; Maggi, E. Opposite role for interleukin-4 and interferon-γ on CD30 and lymphocyte activation gene-3 (LAG-3) expression by activated naive T cells. Eur. J. Immunol. 1997, 27, 2239–2244. [Google Scholar] [CrossRef]
- Annunziato, F.; Manetti, R.; Tomasévic, I.; Giudizi, M.; Biagiotti, R.; Giannò, V.; Germano, P.; Mavilia, C.; Maggi, E.; Romagnani, S. Expression and release of LAG-3-encoded protein by human CD4 + T cells are associated with IFN-γ production. FASEB J. 1996, 10, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Lee, S.J.; Park, C.-G.; Lee, Y.S.; Chun, T. Trafficking of LAG-3 to the Surface on Activated T Cells via Its Cytoplasmic Domain and Protein Kinase C Signaling. J. Immunol. 2014, 193, 3101–3112. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Ou, M.T.; Karuppagounder, S.S.; Kam, T.-I.; Yin, X.; Xiong, Y.; Ge, P.; Umanah, G.E.; Brahmachari, S.; Shin, J.-H.; et al. Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science 2016, 353. [Google Scholar] [CrossRef] [Green Version]
- Angelopoulou, E.; Paudel, Y.N.; Villa, C.; Shaikh, M.F.; Piperi, C. Lymphocyte-Activation Gene 3 (LAG3) Protein as a Possible Therapeutic Target for Parkinson’s Disease: Molecular Mechanisms Connecting Neuroinflammation to α-Synuclein Spreading Pathology. Biology 2020, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhou, M.; Qiu, J.; Lin, Y.; Chen, X.; Huang, S.; Mo, M.; Liu, H.; Peng, G.; Zhu, X.; et al. Association of LAG3 genetic variation with an increased risk of PD in Chinese female population. J. NeuroInflamm. 2019, 16, 270. [Google Scholar] [CrossRef]
- Lino, A.C.; Dang, V.D.; Lampropoulou, V.; Welle, A.; Joedicke, J.; Pohar, J.; Simon, Q.; Thalmensi, J.; Baures, A.; Flühler, V.; et al. LAG-3 Inhibitory Receptor Expression Identifies Immunosuppressive Natural Regulatory Plasma Cells. Immunity 2018, 49, 120–133.e9. [Google Scholar] [CrossRef] [PubMed]
- Kisielow, M.; Kisielow, J.; Capoferri-Sollami, G.; Karjalainen, K. Expression of lymphocyte activation gene 3 (LAG-3) on B cells is induced by T cells. Eur. J. Immunol. 2005, 35, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Donia, M.; Andersen, R.; Kjeldsen, J.W.; Fagone, P.; Munir, S.; Nicoletti, F.; Andersen, M.H.; Straten, P.T.; Svane, I.M. Aberrant Expression of MHC Class II in Melanoma Attracts Inflammatory Tumor-Specific CD4+ T- Cells, Which Dampen CD8+ T-cell Antitumor Reactivity. Cancer Res. 2015, 75, 3747–3759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemon, P.; Jean-Louis, F.; Ramgolam, K.; Brignone, C.; Viguier, M.; Bachelez, H.; Triebel, F.; Charron, D.; Aoudjit, F.; Al-Daccak, R.; et al. MHC Class II Engagement by Its Ligand LAG-3 (CD223) Contributes to Melanoma Resistance to Apoptosis. J. Immunol. 2011, 186, 5173–5183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huard, B.; Prigent, P.; Tournier, M.; Bruniquel, D.; Triebel, F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol. 1995, 25, 2718–2721. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The promising immune checkpoint LAG-3: From tumor microenvironment to cancer immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol. Res. 2015, 3, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Feng, Y.; Fang, S. Overexpression of ezrin and galectin-3 as predictors of poor prognosis of cervical cancer. Braz. J. Med. Biol. Res. 2017, 50, e5356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Wang, J.; Yang, G.; Yu, N.; Huang, Z.; Xu, H.; Li, J.; Qiu, J.; Zeng, X.; Chen, S.; et al. Posttranscriptional regulation of Galectin-3 by miR-128 contributes to colorectal cancer progression. Oncotarget 2017, 8, 15242–15251. [Google Scholar] [CrossRef] [Green Version]
- Chung, L.-Y.; Tang, S.-J.; Wu, Y.-C.; Sun, G.-H.; Liu, H.-Y.; Sun, K.-H. Galectin-3 augments tumor initiating property and tumorigenicity of lung cancer through interaction with β-catenin. Oncotarget 2014, 6, 4936–4952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, Q.; Celias, D.P.; Wu, C.; Cole, A.R.; Singh, S.; Mason, C.; Dong, S.; Tran, T.H.; Amarasinghe, G.K.; Ruffell, B.; et al. LAG3 ectodomain structure reveals functional interfaces for ligand and antibody recognition. Nat. Immunol. 2022, 23, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Huard, B.; Mastrangeli, R.; Prigent, P.; Bruniquel, D.; Donini, S.; El-Tayar, N.; Maigret, B.; Dréano, M.; Triebel, F. Characterization of the major histocompatibility complex class II binding site on LAG-3 protein. Proc. Natl. Acad. Sci. USA 1997, 94, 5744–5749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Liu, J.; Liu, D.; Liu, B.; Wang, M.; Hu, Z.; Du, X.; Tang, L.; He, F. LSECtin Expressed on Melanoma Cells Promotes Tumor Progression by Inhibiting Antitumor T-cell Responses. Cancer Res. 2014, 74, 3418–3428. [Google Scholar] [CrossRef] [Green Version]
- Zuazo, M.; Arasanz, H.; Bocanegra, A.; Chocarro, L.; Vera, R.; Escors, D.; Kagamu, H.; Kochan, G. Systemic CD4 immunity: A powerful clinical biomarker for PD-L1/PD-1 immunotherapy. EMBO Mol. Med. 2020, 12, e12706. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Arasanz, H.; Chocarro, L.; Bocanegra, A.; Zuazo, M.; Fernandez-Hinojal, G.; Blanco, E.; Vera, R.; Escors, D.; Kochan, G. Systemic Blood Immune Cell Populations as Biomarkers for the Outcome of Immune Checkpoint Inhibitor Therapies. Int. J. Mol. Sci. 2020, 21, 2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernandez, G.; Chocarro, L.; Vera, R.; Kochan, G.; Escors, D. Systemic CD4 Immunity as a Key Contributor to PD-L1/PD-1 Blockade Immunotherapy Efficacy. Front. Immunol. 2020, 11, 586907. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Shi, X.; Jia, X.; Yang, Y. Identification and validation of an immune-related gene signature predictive of overall survival in colon cancer. Aging 2020, 12, 26095–26120. [Google Scholar] [CrossRef] [PubMed]
- Saka, D.; Gökalp, M.; Piyade, B.; Cevik, N.C.; Arik Sever, E.; Unutmaz, D.; Ceyhan, G.O.; Demir, I.E.; Asimgil, H. Mechanisms of T-Cell Exhaustion in Pancreatic Cancer. Cancers 2020, 12, 2274. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.-W.; Mao, L.; Yu, G.-T.; Bu, L.-L.; Ma, S.-R.; Liu, B.; Gutkind, J.S.; Kulkarni, A.B.; Zhang, W.-F.; Sun, Z.-J. LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. OncoImmunology 2016, 5, e1239005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datar, I.; Sanmamed, M.F.; Wang, J.; Henick, B.S.; Choi, J.; Badri, T.; Dong, W.; Mani, N.; Toki, M.; Mejías, L.D.; et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non–Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin. Cancer Res. 2019, 25, 4663–4673. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, T.; Xuan, Q.; Zhao, H.; Qin, L.; Zhang, Q. Lymphocyte-Activation Gene-3 Expression and Prognostic Value in Neoadjuvant-Treated Triple-Negative Breast Cancer. J. Breast Cancer 2018, 21, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ye, J.; Ma, Y.; Hua, P.; Huang, Y.; Fu, X.; Li, D.; Yuan, M.; Xia, Z. Function of T regulatory type 1 cells is down-regulated and is associated with the clinical presentation of coronary artery disease. Hum. Immunol. 2018, 79, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Haudebourg, T.; Dugast, A.-S.; Coulon, F.; Usal, C.; Triebel, F.; Vanhove, B. Depletion of LAG-3 Positive Cells in Cardiac Allograft Reveals Their Role in Rejection and Tolerance. Transplantation 2007, 84, 1500–1506. [Google Scholar] [CrossRef]
- Rodriguez, A. High HDL-Cholesterol Paradox: SCARB1-LAG3-HDL Axis. Curr. Atheroscler. Rep. 2021, 23, 5. [Google Scholar] [CrossRef]
- Golden, D.; Kolmakova, A.; Sura, S.; Vella, A.T.; Manichaikul, A.; Wang, X.-Q.; Bielinski, S.J.; Taylor, K.D.; Chen, Y.-D.I.; Rich, S.S.; et al. Lymphocyte activation gene 3 and coronary artery disease. JCI Insight 2016, 1, e88628. [Google Scholar] [CrossRef] [Green Version]
- Slevin, S.M.; Garner, L.C.; Lahiff, C.; Tan, M.; Wang, L.M.; Ferry, H.; Greenaway, B.; Lynch, K.; Geremia, A.; Hughes, S.; et al. Lymphocyte Activation Gene (LAG)-3 Is Associated With Mucosal Inflammation and Disease Activity in Ulcerative Colitis. J. Crohn’s Colitis 2020, 14, 1446–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, J.-S.; Visperas, A.; Sanogo, Y.O.; Bechtel, J.J.; Dvorina, N.; Kim, S.; Jang, E.; Stohlman, S.A.; Shen, B.; Fairchild, R.L.; et al. An IL-27/Lag3 axis enhances Foxp3+ regulatory T cell–suppressive function and therapeutic efficacy. Mucosal Immunol. 2015, 9, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Duvefelt, K.; Svensson, F.; Masterman, T.; Jonasdottir, G.; Salter, H.; Emahazion, T.; Hellgren, D.; Falk, G.; Olsson, T.; et al. Two genes encoding immune-regulatory molecules (LAG3 and IL7R) confer susceptibility to multiple sclerosis. Genes Immun. 2005, 6, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Delmastro, M.M.; Styche, A.J.; Trucco, M.M.; Workman, C.J.; Vignali, D.A.; Piganelli, J.D. Modulation of Redox Balance Leaves Murine Diabetogenic TH1 T Cells “LAG-3-ing” Behind. Diabetes 2012, 61, 1760–1768. [Google Scholar] [CrossRef] [Green Version]
- Bettini, M.; Szymczak-Workman, A.L.; Forbes, K.; Castellaw, A.H.; Selby, M.; Pan, X.; Drake, C.G.; Korman, A.J.; Vignali, D.A.A. Cutting Edge: Accelerated Autoimmune Diabetes in the Absence of LAG-3. J. Immunol. 2011, 187, 3493–3498. [Google Scholar] [CrossRef]
- Doe, H.T.; Kimura, D.; Miyakoda, M.; Kimura, K.; Akbari, M.; Yui, K. Expression of PD-1/LAG-3 and cytokine production by CD4+T cells during infection withPlasmodiumparasites. Microbiol Immunol. 2016, 60, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.L.; Mehra, S.; Ahsan, M.H.; Selman, M.; Khader, S.; Kaushal, D. LAG3 Expression in Active Mycobacterium tuberculosis Infections. Am. J. Pathol. 2014, 185, 820–833. [Google Scholar] [CrossRef] [Green Version]
- Graydon, C.G.; Balasko, A.L.; Fowke, K.R. Roles, function and relevance of LAG3 in HIV infection. PLOS Pathog. 2019, 15, e1007429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jochems, S.P.; Jacquelin, B.; Tchitchek, N.; Busato, F.; Pichon, F.; Huot, N.; Liu, Y.; Ploquin, M.J.; Roché, E.; Cheynier, R.; et al. DNA methylation changes in metabolic and immune-regulatory pathways in blood and lymph node CD4 + T cells in response to SIV infections. Clin. Epigenetics 2020, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- Wuerdemann, N.; Pütz, K.; Eckel, H.; Jain, R.; Wittekindt, C.; Huebbers, C.U.; Sharma, S.J.; Langer, C.; Gattenlöhner, S.; Büttner, R.; et al. LAG-3, TIM-3 and VISTA Expression on Tumor-Infiltrating Lymphocytes in Oropharyngeal Squamous Cell Carcinoma—Potential Biomarkers for Targeted Therapy Concepts. Int. J. Mol. Sci. 2020, 22, 379. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-J.; Zhang, Y.; Jin, G.-X.; Yao, L.; Wu, D.-Q. Expression of LAG-3 is coincident with the impaired effector function of HBV-specific CD8+ T cell in HCC patients. Immunol. Lett. 2012, 150, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Coulon, P.-G.; Srivastava, R.; Vahed, H.; Kim, G.J.; Walia, S.S.; Yamada, T.; Fouladi, M.A.; Ly, V.T.; Benmohamed, L. Blockade of LAG-3 Immune Checkpoint Combined With Therapeutic Vaccination Restore the Function of Tissue-Resident Anti-viral CD8+ T Cells and Protect Against Recurrent Ocular Herpes Simplex Infection and Disease. Front. Immunol. 2018, 9, 2922. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Coulon, P.-G.; Prakash, S.; Srivastava, R.; Geertsema, R.; Dhanushkodi, N.; Lam, C.; Nguyen, V.; Gorospe, E.; Nguyen, A.M.; et al. Blockade of PD-1 and LAG-3 Immune Checkpoints Combined with Vaccination Restores the Function of Antiviral Tissue-Resident CD8 + T RM Cells and Reduces Ocular Herpes Simplex Infection and Disease in HLA Transgenic Rabbits. J. Virol. 2019, 93, e00827-19. [Google Scholar] [CrossRef] [Green Version]
- Richter, K.; Agnellini, P.; Oxenius, A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. Int. Immunol. 2009, 22, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [Green Version]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion during Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019, 37, 457–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernandez-Rubio, L.; Echaide, M.; Garnica, M.; Ramos, P.; Piñeiro, S.; Kochan, G.; Escors, D. LAG-3 Role in Infection. In Proceedings of the 1st International Electronic Conference on Molecular Sciences: Druggable Targets of Emerging Infectious Disease, 31 August 2021; Volume 2021. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernandez-Rubio, L.; Echaide, M.; Garnica, M.; Ramos, P.; Piñeiro, S.; Kochan, G.; Escors, D. LAG-3 Role in Cardiovascular Diseases. In Proceedings of the MOL2NET’22, Conference on Molecular, Biomedical & Computational Sciences and Engineering, Basel, Switzerland, 23 March 2022. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernandez-Rubio, L.; Echaide, M.; Garnica, M.; Ramos, P.; Piñeiro, S.; Kochan, G.; Escors, D. LAG-3 Role in Inflammatory Diseases. In Proceedings of the MOL2NET’22, Conference on Molecular, Biomedical & Computational Sciences and Engineering, Basel, Switzerland, 23 March 2022. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernandez-Rubio, L.; Echaide, M.; Garnica, M.; Ramos, P.; Piñeiro, S.; Kochan, G.; Escors, D. LAG-3 Role in Neurological Diseases. In Proceedings of the MOL2NET’22, Conference on Molecular, Biomedical & Computational Sciences and Engineering, Basel, Switzerland, 23 March 2022. [Google Scholar] [CrossRef]
- Chocarro, L.; Blanco, E.; Arasanz, H.; Fernandez-Rubio, L.; Bocanegra, A.; Echaide, M.; Garnica, M.; Ramos, P.; Piñeiro, S.; Kochan, G.; et al. Role of the next-generation immune checkpoint LAG-3 in response and resistance to cancer immunotherapy. In Proceedings of the MOL2NET’22, Conference on Molecular, Biomedical & Computational Sciences and Engineering, Basel, Switzerland, 23 March 2022. [Google Scholar] [CrossRef]
- RELATLIMAB. Available online: https://drugs.ncats.io/substance/AF75XOF6W3 (accessed on 1 July 2022).
- Ascierto, P.A.; Melero, I.; Bhatia, S.; Bono, P.; Sanborn, R.E.; Lipson, E.J.; Callahan, M.K.; Gajewski, T.; Gomez-Roca, C.A.; Hodi, F.S.; et al. Initial efficacy of anti-lymphocyte activation gene-3 (anti–LAG-3; BMS-986016) in combination with nivolumab (nivo) in pts with melanoma (MEL) previously treated with anti–PD-1/PD-L1 therapy. J. Clin. Oncol. 2017, 35, 9520. [Google Scholar] [CrossRef]
- Lipson, E.; Long, G.; Tawbi, H.; Schadendorf, D.; Atkinson, V.; Maurer, M.; Simonsen, K.; Harbison, C.; Hodi, F. CA224-047: A randomized, double-blind, phase II/III study of relatlimab (anti–LAG-3) in combination with nivolumab (anti–PD-1) versus nivolumab alone in previously untreated metastatic or unresectable melanoma. Ann. Oncol. 2018, 29, viii464–viii465. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Bono, P.; Bhatia, S.; Melero, I.; Nyakas, M.S.; Svane, I.-M.; Larkin, J.; Gomez-Roca, C.; Schadendorf, D.; Dummer, R.; et al. Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti–PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarker-enriched populations. Ann. Oncol. 2017, 28, v611–v612. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; González-Rodríguez, A.P.; Payer, Á.R.; González-García, E.; López-Soto, A.; Gonzalez, S. LAG-3 Blockade with Relatlimab (BMS-986016) Restores Anti-Leukemic Responses in Chronic Lymphocytic Leukemia. Cancers 2021, 13, 2112. [Google Scholar] [CrossRef]
- Clinical Trials Register—Search for 2018-003278-28. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2018-003278-28 (accessed on 1 July 2022).
- Ellis, J.; Marks, D.J.; Srinivasan, N.; Barrett, C.; Hopkins, T.G.; Richards, A.; Fuhr, R.; Albayaty, M.; Coenen, M.; Liefaard, L.; et al. Depletion of LAG-3 + T Cells Translated to Pharmacology and Improvement in Psoriasis Disease Activity: A Phase I Randomized Study of mAb GSK2831781. Clin. Pharmacol. Ther. 2020, 109, 1293–1303. [Google Scholar] [CrossRef]
- Savitsky, D.; Ward, R.; Riordan, C.; Mundt, C.; Jennings, S.; Connolly, J.; Findeis, M.; Sanicola, M.; Underwood, D.; Nastri, H.; et al. Abstract 3819: INCAGN02385 is an antagonist antibody targeting the co-inhibitory receptor LAG-3 for the treatment of human malignancies. Cancer Res. 2018, 78, 3819. [Google Scholar] [CrossRef]
- IERAMILIMAB. Available online: https://drugs.ncats.io/substance/OI8P0SFD4R (accessed on 1 July 2022).
- Lin, C.-C.; Garralda, E.; Schöffski, P.; Hong, D.; Siu, L.; Martin, M.; Maur, M.; Hui, R.; Soo, R.; Chiu, J.; et al. 387 A Phase II, multicenter study of the safety and efficacy of LAG525 in combination with spartalizumab in patients with advanced malignancies. J. Immunother. Cancer 2020, 8, A412. [Google Scholar] [CrossRef]
- Uboha, N.V.; Milhem, M.M.; Kovacs, C.; Amin, A.; Magley, A.; Das Purkayastha, D.; Piha-Paul, S.A. Phase II study of spartalizumab (PDR001) and LAG525 in advanced solid tumors and hematologic malignancies. J. Clin. Oncol. 2019, 37, 2553. [Google Scholar] [CrossRef]
- Hong, D.S.; Schoffski, P.; Calvo, A.; Sarantopoulos, J.; De Olza, M.O.; Carvajal, R.D.; Prawira, A.; Kyi, C.; Esaki, T.; Akerley, W.L.; et al. Phase I/II study of LAG525 ± spartalizumab (PDR001) in patients (pts) with advanced malignancies. J. Clin. Oncol. 2018, 36, 3012. [Google Scholar] [CrossRef]
- Garralda, E.; Sukari, A.; Lakhani, N.J.; Patnaik, A.; Lou, Y.; Im, S.-A.; Golan, T.; Geva, R.; Wermke, M.; De Miguel, M.; et al. A phase 1 first-in-human study of the anti-LAG-3 antibody MK4280 (favezelimab) plus pembrolizumab in previously treated, advanced microsatellite stable colorectal cancer. J. Clin. Oncol. 2021, 39, 3584. [Google Scholar] [CrossRef]
- Gregory, G.P.; Zinzani, P.L.; Palcza, J.; Healy, J.A.; Orlowski, R.J.; Nahar, A.; Armand, P. Abstract CT106: Anti-LAG-3 antibody MK-4280 in combination with pembrolizumab for the treatment of hematologic malignancies: A Phase I/II study. Cancer Res. 2019, 79, CT106. [Google Scholar] [CrossRef]
- FAVEZELIMAB. Available online: https://drugs.ncats.io/substance/H1396W7D1H (accessed on 1 July 2022).
- Burova, E.; Hermann, A.; Dai, J.; Ullman, E.; Halasz, G.; Potocky, T.; Hong, S.; Liu, M.; Allbritton, O.; Woodruff, A.; et al. Preclinical Development of the Anti-LAG-3 Antibody REGN3767: Characterization and Activity in Combination with the Anti-PD-1 Antibody Cemiplimab in Human PD-1xLAG-3–Knockin Mice. Mol. Cancer Ther. 2019, 18, 2051–2062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FIANLIMAB. Available online: https://drugs.ncats.io/substance/OX5LRQ5H6K (accessed on 1 July 2022).
- Hamid, O.; Wang, D.; Kim, T.M.; Kim, S.-W.; Lakhani, N.J.; Johnson, M.L.; Groisberg, R.; Papadopoulos, K.P.; Kaczmar, J.M.; Middleton, M.R.; et al. Clinical activity of fianlimab (REGN3767), a human anti-LAG-3 monoclonal antibody, combined with cemiplimab (anti-PD-1) in patients (pts) with advanced melanoma. J. Clin. Oncol. 2021, 39, 9515. [Google Scholar] [CrossRef]
- Nanda, R.; Liu, M.C.; Yau, C.; Shatsky, R.; Pusztai, L.; Wallace, A.; Chien, A.J.; Forero-Torres, A.; Ellis, E.; Han, H.; et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020, 6, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Lakhani, N.J.; Johnson, M.L.; Park, H.; Wang, D.; Yap, T.; Dowlati, A.; Maki, R.G.; Lynce, F.; Ulahannan, S.V.; et al. First-in-human study of REGN3767 (R3767), a human LAG-3 monoclonal antibody (mAb), ± cemiplimab in patients (pts) with advanced malignancies. J. Clin. Oncol. 2019, 37, 2508. [Google Scholar] [CrossRef]
- Spreafico, A.; Janku, F.; Rodon, J.A.; Tolcher, A.W.; Chandana, S.R.; Oliva, M.; Musalli, S.; Knauss, L.; Kragh, L.; Alifrangis, L.; et al. A phase I study of Sym021, an anti-PD-1 antibody (Ab), alone and in combination with Sym022 (anti-LAG-3) or Sym023 (anti-TIM-3). Ann. Oncol. 2019, 30. [Google Scholar] [CrossRef]
- Lakhani, N.; Spreafico, A.; Tolcher, A.; Rodon, J.; Janku, F.; Chandana, S.; Oliva, M.; Sharma, M.; Abdul-Karim, R.; Hansen, U.; et al. 1019O Phase I studies of Sym021, an anti-PD-1 antibody, alone and in combination with Sym022 (anti-LAG-3) or Sym023 (anti-TIM-3). Ann. Oncol. 2020, 31, S704. [Google Scholar] [CrossRef]
- Ghosh, S.; Sharma, G.; Travers, J.; Kumar, S.; Choi, J.; Jun, H.T.; Kehry, M.; Ramaswamy, S.; Jenkins, D. TSR-033, a novel therapeutic antibody targeting LAG-3, enhances T-cell function and the activity of PD-1 blockade in vitro and in vivo. Mol. Cancer Ther. 2019, 18, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Kim, H.; Sung, E.; Jung, U.; Hong, Y.; Lee, H.; Ko, M.; Park, Y.; Park, C.K.; Kim, S.J.; et al. Abstract 1633: ABL501, PD-L1 x LAG-3, a bispecific antibody promotes enhanced human T cell activation through targeting simultaneously two immune checkpoint inhibitors, LAG-3 and PD-L1. Cancer Res. 2021, 81, 1633. [Google Scholar] [CrossRef]
- Sung, E.; Ko, M.; Won, J.-Y.; Jo, Y.; Park, E.; Kim, H.; Choi, E.; Jung, U.-J.; Jeon, J.; Kim, Y.; et al. LAG-3xPD-L1 bispecific antibody potentiates antitumor responses of T cells through dendritic cell activation. Mol. Ther. 2022, 30, S1525. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ni, H.; Zhang, P.; Guo, X.; Wu, M.; Shen, H.; Wang, J.; Wu, W.; Wu, Z.; Ding, J.; et al. PD-L1/LAG-3 bispecific antibody enhances tumor-specific immunity. OncoImmunology 2021, 10, 1943180. [Google Scholar] [CrossRef] [PubMed]
- Powderly, J.D.; Hurwitz, H.; Ryan, D.P.; Laheru, D.A.; Pandya, N.B.; Lohr, J.; Moore, P.A.; Bonvini, E.; Wigginton, J.M.; Crocenzi, T.S. A phase 1, first-in-human, open label, dose escalation study of MGD007, a humanized gpA33 × CD3 DART molecule, in patients with relapsed/refractory metastatic colorectal carcinoma. J. Clin. Oncol. 2016, 34, TPS3628. [Google Scholar] [CrossRef]
- Hedvat, M.; Bonzon, C.; Bernett, M.J.; Moore, G.L.; Avery, K.; Rashid, R.; Nisthal, A.; Schubert, S.; Varma, R.; Lee, S.-H.; et al. Abstract 2784: Simultaneous checkpoint-checkpoint or checkpoint-costimulatory receptor targeting with bispecific antibodies promotes enhanced human T cell activation. Cancer Res. 2018, 78, 2784. [Google Scholar] [CrossRef]
- Everett, K.L.; Kraman, M.; Wollerton, F.P.; Zimarino, C.; Kmiecik, K.; Gaspar, M.; Pechouckova, S.; Allen, N.L.; Doody, J.F.; Tuna, M. Generation of Fcabs targeting human and murine LAG-3 as building blocks for novel bispecific antibody therapeutics. Methods 2018, 154, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Kraman, M.; Faroudi, M.; Allen, N.L.; Kmiecik, K.; Gliddon, D.; Seal, C.; Koers, A.; Wydro, M.M.; Batey, S.; Winnewisser, J.; et al. FS118, a Bispecific Antibody Targeting LAG-3 and PD-L1, Enhances T-Cell Activation Resulting in Potent Antitumor Activity. Clin. Cancer Res. 2020, 26, 3333–3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, T.; Wong, D.; Hu-Lieskovan, S.; Papadopoulos, K.; Morrow, M.; Grabowska, U.; Gliddon, D.; Holz, J.-B.; LoRusso, P. 395 A first-in-human study of FS118, a tetravalent bispecific antibody targeting LAG-3 and PD-L1, in patients with advanced cancer and resistance to PD-(L)1 therapy. J. ImmunoTherapy Cancer 2020, 8, A420. [Google Scholar] [CrossRef]
- Yap, T.; Papadopoulos, K.P.; Lorusso, P.; Wong, D.J.; Hu-Lieskovan, S.; Holz, J.-B. A first-in-human phase I study of FS118, an anti-LAG-3/PD-L1 bispecific antibody in patients with solid tumors that have progressed on prior PD-1/PD-L1 therapy. J. Clin. Oncol. 2019, 37, TPS2652. [Google Scholar] [CrossRef]
- Crescendo Biologics and Cancer Research UK Sign Clinical Development Partnership to Develop CB213, a Novel Bispecific Humabody® Therapeutic | Business Wire. Available online: https://www.businesswire.com/news/home/20200505005080/en/Crescendo-Biologics-and-Cancer-Research-UK-sign-Clinical-Development-Partnership-to-develop-CB213-a-novel-bispecific-Humabody%C2%AE-therapeutic (accessed on 1 July 2022).
- Edwards, C.J.; Sette, A.; Cox, C.; Di Fiore, B.; Wyre, C.; Sydoruk, D.; Yadin, D.; Hayes, P.; Stelter, S.; Bartlett, P.D.; et al. The multi-specific VH-based Humabody CB213 co-targets PD1 and LAG3 on T cells to promote anti-tumour activity. Br. J. Cancer 2021, 126, 1168–1177. [Google Scholar] [CrossRef]
- Haftcheshmeh, S.M.; Zamani, P.; Mashreghi, M.; Nikpoor, A.R.; Tavakkol-Afshari, J.; Jaafari, M.R. Immunoliposomes bearing lymphocyte activation gene 3 fusion protein and P5 peptide: A novel vaccine for breast cancer. Biotechnol. Prog. 2020, 37, e3095. [Google Scholar] [CrossRef] [PubMed]
- Cappello, P.; Triebel, F.; Iezzi, M.; Caorsi, C.; Quaglino, E.; Lollini, P.L.; Amici, A.; Di Carlo, E.; Musiani, P.; Giovarelli, M.; et al. LAG-3 Enables DNA Vaccination to Persistently Prevent Mammary Carcinogenesis in HER-2/neu Transgenic BALB/c. Cancer Res. 2003, 63, 2518–2525. [Google Scholar] [PubMed]
- Fougeray, S.; Brignone, C.; Triebel, F. A soluble LAG-3 protein as an immunopotentiator for therapeutic vaccines: Preclinical evaluation of IMP321. Vaccine 2006, 24, 5426–5433. [Google Scholar] [CrossRef] [PubMed]
- Andreae, S.; Piras, F.; Burdin, N.; Triebel, F. Maturation and Activation of Dendritic Cells Induced by Lymphocyte Activation Gene-3 (CD223). J. Immunol. 2002, 168, 3874–3880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brignone, C.; Grygar, C.; Marcu, M.; Schäkel, K.; Triebel, F. A soluble form of lymphocyte activation gene-3 (IMP321) induces activation of a large range of human effector cytotoxic cells. J. Immunol. 2007, 179, 4202–4211. [Google Scholar] [CrossRef] [Green Version]
- El Mir, S.; Triebel, F. A Soluble Lymphocyte Activation Gene-3 Molecule Used as a Vaccine Adjuvant Elicits Greater Humoral and Cellular Immune Responses to Both Particulate and Soluble Antigens. J. Immunol. 2000, 164, 5583–5589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brignone, C.; Grygar, C.; Marcu, M.; Perrin, G.; Triebel, F. IMP321 (sLAG-3), an immunopotentiator for T cell responses against a HBsAg antigen in healthy adults: A single blind randomised controlled phase I study. J. Immune Based Ther. Vaccines 2007, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Brignone, C.; Grygar, C.; Marcu, M.; Perrin, G.; Triebel, F. IMP321 (sLAG-3) safety and T cell response potentiation using an influenza vaccine as a model antigen: A single-blind phase I study. Vaccine 2007, 25, 4641–4650. [Google Scholar] [CrossRef]
- Casati, C.; Camisaschi, C.; Rini, F.; Arienti, F.; Rivoltini, L.; Triebel, F.; Parmiani, G.; Castelli, C. Soluble Human LAG-3 Molecule Amplifies the In vitro Generation of Type 1 Tumor-Specific Immunity. Cancer Res. 2006, 66, 4450–4460. [Google Scholar] [CrossRef] [Green Version]
- Advanced Melanoma | OpdualagTM (Nivolumab and Relatlimab-Rmbw). Available online: https://www.opdualag.com/ (accessed on 1 July 2022).
- FDA Approves Opdualag for Unresectable or Metastatic Melanoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-opdualag-unresectable-or-metastatic-melanoma (accessed on 1 July 2022).
- Official Title of Study: A Randomized, Double-Blind Phase 2/3 Study of Relatlimab Combined with Nivolumab Versus Nivolumab in Participants with Previously Untreated Metastatic or Unresectable Melanoma Protocol(S) CA224-047. Available online: https://adisinsight.springer.com/trials/700294223 (accessed on 1 July 2022).
- Fda and Cder, “Highlights of Prescribing Information”. Available online: www.fda.gov/medwatch (accessed on 1 July 2022).
- Lipson, E.J.; Tawbi, H.A.; Schadendorf, D.; Ascierto, P.A.; Matamala, L.; Castillo Gutierrez, E.; Rutkowski, P.; Gogas, H.; Lao, C.D.; Janoski de Menezes, J. Relatlimab (RELA) plus nivolumab (NIVO) versus NIVO in first-line advanced melanoma: Primary phase III results from RELATIVITY-047 (CA224-047). J. Clin. Oncol. 2021, 39, 9503. [Google Scholar] [CrossRef]
- Long, G.V.; Hodi, F.S.; Lipson, E.J.; Schadendorf, D.; Ascierto, P.A.; Matamala, L.; Salman, P.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.; et al. Relatlimab and nivolumab versus nivolumab in previously untreated metastatic or unresectable melanoma: Overall survival and response rates from RELATIVITY-047 (CA224-047). J. Clin. Oncol. 2022, 40, 360385. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [Green Version]
- Rotte, A.; Jin, J.Y.; Lemaire, V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann. Oncol. 2018, 29, 71–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 2021, 14, 45. [Google Scholar] [CrossRef] [PubMed]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhu, Y.; Li, G.; Huang, H.; Zhang, G.; Wang, F.; Sun, J.; Yang, Q.; Zhang, X.; Lu, B. TIM-3 Expression Characterizes Regulatory T Cells in Tumor Tissues and Is Associated with Lung Cancer Progression. PLoS ONE 2012, 7, e30676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, A.C.; Anderson, D.E.; Bregoli, L.; Hastings, W.D.; Kassam, N.; Lei, C.; Chandwaskar, R.; Karman, J.; Su, E.W.; Hirashima, M.; et al. Promotion of Tissue Inflammation by the Immune Receptor Tim-3 Expressed on Innate Immune Cells. Science 2007, 318, 1141–1143. [Google Scholar] [CrossRef]
- Ndhlovu, L.C.; Lopez-Vergès, S.; Barbour, J.D.; Jones, R.B.; Jha, A.R.; Long, B.R.; Schoeffler, E.C.; Fujita, T.; Nixon, D.F.; Lanier, L.L. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 2012, 119, 3734–3743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Zhu, C.; Kondo, Y.; Anderson, A.C.; Gandhi, A.; Russell, A.F.; Dougan, S.K.; Petersen, B.-S.; Melum, E.; Pertel, T.; et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015, 517, 386–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freeman, G.J.; Casasnovas, J.M.; Umetsu, D.T.; DeKruyff, R.H. TIMgenes: A family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol. Rev. 2010, 235, 172–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka-Akita, H.; Fujioka, Y.; Ohba, Y.; Gorman, J.V.; Colgan, J.D.; et al. Tumor-infiltrating DCs suppress nucleic acid–mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 2012, 13, 832–842. [Google Scholar] [CrossRef]
- Qin, S.; Dong, B.; Yi, M.; Chu, Q.; Wu, K. Prognostic Values of TIM-3 Expression in Patients With Solid Tumors: A Meta-Analysis and Database Evaluation. Front. Oncol. 2020, 10, 1288. [Google Scholar] [CrossRef] [PubMed]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef]
- Liu, J.-F.; Wu, L.; Yang, L.-L.; Deng, W.-W.; Mao, L.; Wu, H.; Zhang, W.-F.; Sun, Z.-J. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J. Exp. Clin. Cancer Res. 2018, 37, 44. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Munger, M.; Veenstra, R.G.; Weigel, B.J.; Hirashima, M.; Munn, D.; Murphy, W.J.; Azuma, M.; Anderson, A.C.; Kuchroo, V.K.; et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011, 117, 4501–4510. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, S.; Hu, Y.; Yang, Z.; Li, J.; Liu, X.; Deng, L.; Wang, Y.; Zhang, X.; Jiang, T.; et al. Targeting PD-1 and Tim-3 Pathways to Reverse CD8 T-Cell Exhaustion and Enhance Ex Vivo T-Cell Responses to Autologous Dendritic/Tumor Vaccines. J. Immunother. 2016, 39, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Sun, Z.; Benallaoua, M.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Kuchroo, V.; Zarour, H.M. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen–specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 2010, 207, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, P.; Penninger, J.M.; Timms, E.; Wakeham, A.; Shahinian, A.; Lee, K.P.; Thompson, C.B.; Griesser, H.; Mak, T.W. Lymphoproliferative Disorders with Early Lethality in Mice Deficient in Ctla-4. Science 1995, 270, 985–988. [Google Scholar] [CrossRef]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune Dilated Cardiomyopathy in PD-1 Receptor-Deficient Mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Murtaza, A.; Laken, H.; Correia, J.D.S.; McNeeley, P.; Altobell, L.; Zhang, J.; Vancutsem, P.; Wilcoxen, K.; Jenkins, D. Discovery of TSR-022, a novel, potent anti-human TIM-3 therapeutic antibody. Eur. J. Cancer 2016, 69, S102. [Google Scholar] [CrossRef]
- Falchook, G.S.; Ribas, A.; Davar, D.; Eroglu, Z.; Wang, J.S.; Luke, J.J.; Hamilton, E.P.; Di Pace, B.; Wang, T.; Ghosh, S.; et al. Phase 1 trial of TIM-3 inhibitor cobolimab monotherapy and in combination with PD-1 inhibitors nivolumab or dostarlimab (AMBER). J. Clin. Oncol. 2022, 40, 2504. [Google Scholar] [CrossRef]
- Lepletier, A.; Madore, J.; O’Donnell, J.S.; Johnston, R.L.; Li, X.-Y.; McDonald, E.; Ahern, E.; Kuchel, A.; Eastgate, M.; Pearson, S.-A.; et al. Tumor CD155 Expression Is Associated with Resistance to Anti-PD1 Immunotherapy in Metastatic Melanoma. Clin. Cancer Res. 2020, 26, 3671–3681. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Madore, J.; Li, X.-Y.; Smyth, M.J. Tumor intrinsic and extrinsic immune functions of CD155. Semin. Cancer Biol. 2019, 65, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The Immunoreceptor TIGIT Regulates Antitumor and Antiviral CD8 + T Cell Effector Function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; A Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2008, 10, 48–57. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Xiao, Y.; Yang, Q.-C.; Yang, S.-C.; Yang, L.-L.; Sun, Z.-J. TIGIT/CD155 blockade enhances anti-PD-L1 therapy in head and neck squamous cell carcinoma by targeting myeloid-derived suppressor cells. Oral Oncol. 2021, 121, 105472. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, D.; Cichocki, F.; Zhang, B.; Yingst, A.; Spellman, S.R.; Cooley, S.; Verneris, M.R.; Blazar, B.R.; Miller, J.S. Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor. Cells Cancer Res. 2016, 76, 5696–5706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtulus, S.; Sakuishi, K.; Ngiow, S.-F.; Joller, N.; Tan, D.J.; Teng, M.; Smyth, M.; Kuchroo, V.K.; Anderson, A.C. TIGIT predominantly regulates the immune response via regulatory T cells. J. Clin. Investig. 2015, 125, 4053–4062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Liu, J.; Cui, J.; Ma, B.; Zhou, Q.; Yang, X.; Lu, Z.; Du, Y.; Su, C. Expression of TIGIT/CD155 and correlations with clinical pathological features in human hepatocellular carcinoma. Mol. Med. Rep. 2019, 20, 3773–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Zhao, E.; Zhu, C.; Zhao, W.; Wang, C.; Zhang, Z.; Zhao, G. TIGIT and PD-1 may serve as potential prognostic biomarkers for gastric cancer. Immunobiology 2020, 225, 151915. [Google Scholar] [CrossRef] [PubMed]
- Stålhammar, G.; Seregard, S.; Grossniklaus, H.E. Expression of immune checkpoint receptors Indoleamine 2,3-dioxygenase and T cell Ig and ITIM domain in metastatic versus nonmetastatic choroidal melanoma. Cancer Med. 2019, 8, 2784–2792. [Google Scholar] [CrossRef]
- Lee, W.J.; Lee, Y.J.; Choi, M.E.; Yun, K.A.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E. Expression of lymphocyte-activating gene 3 and T-cell immunoreceptor with immunoglobulin and ITIM domains in cutaneous melanoma and their correlation with programmed cell death 1 expression in tumor-infiltrating lymphocytes. J. Am. Acad. Dermatol. 2019, 81, 219–227. [Google Scholar] [CrossRef]
- Guillerey, C.; Harjunpää, H.; Carrié, N.; Kassem, S.; Teo, T.; Miles, K.; Krumeich, S.; Weulersse, M.; Cuisinier, M.; Stannard, K.; et al. TIGIT immune checkpoint blockade restores CD8+ T-cell immunity against multiple myeloma. Blood 2018, 132, 1689–1694. [Google Scholar] [CrossRef] [Green Version]
- Hung, A.L.; Maxwell, R.; Theodros, D.; Belcaid, Z.; Mathios, D.; Luksik, A.S.; Kim, E.; Wu, A.; Xia, Y.; Garzon-Muvdi, T.; et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. OncoImmunology 2018, 7, e1466769. [Google Scholar] [CrossRef] [PubMed]
- Banta, K.L.; Xu, X.; Chitre, A.S.; Au-Yeung, A.; Takahashi, C.; O’Gorman, W.E.; Wu, T.D.; Mittman, S.; Cubas, R.; Comps-Agrar, L.; et al. Mechanistic convergence of the TIGIT and PD-1 inhibitory pathways necessitates co-blockade to optimize anti-tumor CD8+ T cell responses. Immunity 2022, 55, 512–526.e9. [Google Scholar] [CrossRef]
- Chen, X.; Xue, L.; Ding, X.; Zhang, J.; Jiang, L.; Liu, S.; Hou, H.; Jiang, B.; Cheng, L.; Zhu, Q.; et al. An Fc-Competent Anti-Human TIGIT Blocking Antibody Ociperlimab (BGB-A1217) Elicits Strong Immune Responses and Potent Anti-Tumor Efficacy in Pre-Clinical Models. Front. Immunol. 2022, 13, 828319. [Google Scholar] [CrossRef]
- Frentzas, S.; Meniawy, T.; Kao, S.C.-H.; Wang, R.; Zuo, Y.; Zheng, H.; Tan, W. AdvanTIG-105: Phase 1 dose-escalation study of anti-TIGIT monoclonal antibody ociperlimab (BGB-A1217) in combination with tislelizumab in patients with advanced solid tumors. J. Clin. Oncol. 2021, 39, 2583. [Google Scholar] [CrossRef]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef]
- Genentech: Press Releases. 2021. Available online: https://www.gene.com/media/press-releases/14892/2021-01-04/genentechs-novel-anti-tigit-tiragolumab- (accessed on 1 July 2022).
- Rudin, C.M.; Liu, S.V.; Lu, S.; Soo, R.A.; Hong, M.H.; Lee, J.-S.; Bryl, M.; Dumoulin, D.W.; Rittmeyer, A.; Chiu, C.-H.; et al. SKYSCRAPER-02: Primary results of a phase III, randomized, double-blind, placebo-controlled study of atezolizumab (atezo) + carboplatin + etoposide (CE) with or without tiragolumab (tira) in patients (pts) with untreated extensive-stage small cell lung cancer (ES-SCLC). J. Clin. Oncol. 2022, 40, LBA8507. [Google Scholar] [CrossRef]
- Niu, J.; Maurice-Dror, C.; Lee, D.; Kim, D.-W.; Nagrial, A.; Voskoboynik, M.; Chung, H.; Mileham, K.; Vaishampayan, U.; Rasco, D.; et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer. Ann. Oncol. 2021, 33, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.; Cho, B.; Juergens, R.; Cheng, Y.; De Castro, G.; Erman, M.; Bauman, J.; Takahashi, T.; Schwarzenberger, P.; Zhang, P.; et al. P14.01 Phase 3 Study of First-Line Pembrolizumab ± Vibostolimab (anti-TIGIT) in Patients With PD-L1—Positive Metastatic NSCLC. J. Thorac. Oncol. 2021, 16, S1010–S1011. [Google Scholar] [CrossRef]
- Garni-Wagner, B.A.; Lee, Z.H.; Kim, Y.-J.; Wilde, C.; Kang, C.-Y.; Kwon, B.S. 4-1BB Is Expressed on CD45RAhiROhiTransitional T Cell in Humans. Cell. Immunol. 1996, 169, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wortzman, M.E.; Clouthier, D.L.; McPherson, A.J.; Lin, G.H.Y.; Watts, T.H. The contextual role of TNFR family members in CD8+T-cell control of viral infections. Immunol. Rev. 2013, 255, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.; Carvajal, R.D.; Marabelle, A.; Patel, S.P.; Lorusso, P.M.; Rasmussen, E.; Juan, G.; Upreti, V.V.; Beers, C.; Ngarmchamnanrith, G.; et al. Dose escalation results from a first-in-human, phase 1 study of glucocorticoid-induced TNF receptor–related protein agonist AMG 228 in patients with advanced solid tumors. J. Immunother. Cancer 2018, 6, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmanoukian, A.S.; Infante, J.R.; Aljumaily, R.; Naing, A.; Chintakuntlawar, A.V.; Rizvi, N.A.; Ross, H.J.; Gordon, M.; Mallinder, P.R.; Elgeioushi, N.; et al. Safety and Clinical Activity of MEDI1873, a Novel GITR Agonist, in Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 6196–6203. [Google Scholar] [CrossRef]
- Heinhuis, K.M.; Carlino, M.; Joerger, M.; Di Nicola, M.; Meniawy, T.; Rottey, S.; Moreno, V.; Gazzah, A.; Delord, J.-P.; Paz-Ares, L.; et al. Safety, Tolerability, and Potential Clinical Activity of a Glucocorticoid-Induced TNF Receptor–Related Protein Agonist Alone or in Combination With Nivolumab for Patients With Advanced Solid Tumors: A Phase 1/2a Dose-Escalation and Cohort-Expansion Clinical Trial. JAMA Oncol. 2020, 6, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Geva, R.; Voskoboynik, M.; Dobrenkov, K.; Mayawala, K.; Gwo, J.; Wnek, R.; Chartash, E.; Long, G. First-in-human phase 1 study of MK-1248, an anti–glucocorticoid-induced tumor necrosis factor receptor agonist monoclonal antibody, as monotherapy or with pembrolizumab in patients with advanced solid tumors. Cancer 2020, 126, 4926–4935. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.P.; Autio, K.A.; Golan, T.; Dobrenkov, K.; Chartash, E.; Chen, Q.; Wnek, R.; Long, G.V. Phase I Study of MK-4166, an Anti-human Glucocorticoid-Induced TNF Receptor Antibody, Alone or with Pembrolizumab in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 1904–1911. [Google Scholar] [CrossRef]
- Gutierrez, M.; Moreno, V.; Heinhuis, K.M.; Olszanski, A.J.; Spreafico, A.; Ong, M.; Chu, Q.S.; Carvajal, R.D.; Trigo, J.; Ochoa de Olza, M.; et al. OX40 Agonist BMS-986178 Alone or in Combination With Nivolumab and/or Ipilimumab in Patients With Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Moiseyenko, A.; Muggia, F.; Condamine, T.; Pulini, J.; Janik, J.E.; Cho, D.C. Sequential therapy with INCAGN01949 followed by ipilimumab and nivolumab in two patients with advanced ovarian carcinoma. Gynecol. Oncol. Rep. 2020, 34, 100655. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Burris, H.A.; Luken, M.J.D.M.; Pishvaian, M.J.; Bang, Y.-J.; Gordon, M.; Awada, A.; Camidge, D.R.; Hodi, F.S.; McArthur, G.A.; et al. First-In-Human Phase I Study of the OX40 Agonist MOXR0916 in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Postel-Vinay, S.; Lam, V.K.; Ros, W.; Bauer, T.M.; Hansen, A.R.; Cho, D.C.; Hodi, F.S.; Schellens, J.H.; Litton, J.K.; Aspeslagh, S.; et al. Abstract CT150: A first-in-human phase I study of the OX40 agonist GSK3174998 (GSK998) +/- pembrolizumab in patients (Pts) with selected advanced solid tumors (ENGAGE-1). Cancer Res. 2020, 80, CT150. [Google Scholar] [CrossRef]
- Duhen, R.; Ballesteros-Merino, C.; Frye, A.K.; Tran, E.; Rajamanickam, V.; Chang, S.-C.; Koguchi, Y.; Bifulco, C.B.; Bernard, B.; Leidner, R.S.; et al. Neoadjuvant anti-OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells. Nat. Commun. 2021, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Glisson, B.S.; Leidner, R.S.; Ferris, R.L.; Powderly, J.; Rizvi, N.A.; Keam, B.; Schneider, R.; Goel, S.; Ohr, J.P.; Burton, J.; et al. Safety and Clinical Activity of MEDI0562, a Humanized OX40 Agonist Monoclonal Antibody, in Adult Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020, 26, 5358–5367. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Spano, J.-P.; Angevin, E.; Doi, T.; Bullock, A.J.; Harris, W.P.; Hamid, O.; Gougis, P.; Forgie, A.; Yang, W.; et al. Analysis of OX40 agonist antibody (PF-04518600) in patients with hepatocellular carcinoma. J. Clin. Oncol. 2020, 38, 523. [Google Scholar] [CrossRef]
- Shapira-Frommer, R.; van Dongen, M.G.; Dobrenkov, K.; Chartash, E.; Liu, F.; Li, C.; Wnek, R.; Patel, M. O83 Phase 1 study of an anti-CD27 agonist as monotherapy and in combination with pembrolizumab in patients with advanced solid tumors. J. ImmunoTherapy Cancer 2020, 8, A2. [Google Scholar] [CrossRef]
- Ansell, S.M.; Flinn, I.; Taylor, M.H.; Sikic, B.I.; Brody, J.; Nemunaitis, J.; Feldman, A.; Hawthorne, T.R.; Rawls, T.; Keler, T.; et al. Safety and activity of varlilumab, a novel and first-in-class agonist anti-CD27 antibody, for hematologic malignancies. Blood Adv. 2020, 4, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Sznol, M.; Hodi, F.S.; Margolin, K.; McDermott, D.F.; Ernstoff, M.S.; Kirkwood, J.M.; Wojtaszek, C.; Feltquate, D.; Logan, T. Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). J. Clin. Oncol. 2008, 26, 3007. [Google Scholar] [CrossRef]
- Timmerman, J.; Herbaux, C.; Ribrag, V.; Zelenetz, A.D.; Houot, R.; Neelapu, S.S.; Logan, T.; Lossos, I.S.; Urba, W.; Salles, G.; et al. Urelumab alone or in combination with rituximab in patients with relapsed or refractory B-cell lymphoma. Am. J. Hematol. 2020, 95, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.H.; Logan, T.F.; Hodi, F.S.; McDermott, D.; Melero, I.; Hamid, O.; Schmidt, H.; Robert, C.; Chiarion-Sileni, V.; Ascierto, P.A.; et al. Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody. Clin. Cancer Res. 2017, 23, 1929–1936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, N.H.; He, A.R.; Doi, T.; Levy, R.; Bhatia, S.; Pishvaian, M.J.; Cesari, R.; Chen, Y.; Davis, C.B.; Huang, B.; et al. Phase I Study of Single-Agent Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Patients with Advanced Cancer. Clin. Cancer Res. 2018, 24, 1816–1823. [Google Scholar] [CrossRef] [Green Version]
- Tolcher, A.W.; Sznol, M.; Hu-Lieskovan, S.; Papadopoulos, K.P.; Patnaik, A.; Rasco, D.W.; Di Gravio, D.; Huang, B.; Gambhire, D.; Chen, Y.; et al. Phase Ib Study of Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Combination with Pembrolizumab (MK-3475) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 5349–5357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, E.E.W.; Pishvaian, M.J.; Shepard, D.R.; Wang, D.; Weiss, J.; Johnson, M.L.; Chung, C.H.; Chen, Y.; Huang, B.; Davis, C.B.; et al. A phase Ib study of utomilumab (PF-05082566) in combination with mogamulizumab in patients with advanced solid tumors. J. Immunother. Cancer 2019, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Chiappori, A.; Thompson, J.; Eskens, F.; Spano, J.-P.; Doi, T.; Hamid, O.; Diab, A.; Rizvi, N.; Hu-Lieskovan, S.; Ros, W.; et al. P860 Results from a combination of OX40 (PF-04518600) and 4–1BB (utomilumab) agonistic antibodies in melanoma and non-small cell lung cancer in a phase 1 dose expansion cohort. J. Immunother. Cancer 2020, 8, A9–A10. [Google Scholar] [CrossRef]
- Martin-Orozco, N.; Li, Y.; Wang, Y.; Liu, S.; Hwu, P.; Liu, Y.-J.; Dong, C.; Radvanyi, L. Melanoma Cells Express ICOS Ligand to Promote the Activation and Expansion of T-Regulatory Cells. Cancer Res. 2010, 70, 9581–9590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khayyamian, S.; Hutloff, A.; Büchner, K.; Gräfe, M.; Henn, V.; Kroczek, R.A.; Mages, H.W. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4 + T cells. Proc. Natl. Acad. Sci. USA 2002, 99, 6198–6203. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Dong, Y.; Yang, Q.; Xu, W.; Jiang, S.; Yu, Z.; Yu, K.; Zhang, S. Acute Myeloid Leukemia Cells Express ICOS Ligand to Promote the Expansion of Regulatory T Cells. Front. Immunol. 2018, 9, 2227. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-J.; Kim, S.-N.; Jeon, M.-S.; Yi, T.; Song, S.U. ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells. Sci. Rep. 2017, 7, 44486. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, O.; Nabissi, M.; Morelli, M.B.; Torquati, L.; Amantini, C.; Santoni, G. ICOS-L as a Potential Therapeutic Target for Cancer Immunotherapy. Curr. Protein Pept. Sci. 2018, 19, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Gigoux, M.; Shang, J.; Pak, Y.; Xu, M.; Choe, J.; Mak, T.W.; Suh, W.-K. Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. USA 2009, 106, 20371–20376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrad, C.; Gregorio, J.; Wang, Y.-H.; Ito, T.; Meller, S.; Hanabuchi, S.; Anderson, S.; Atkinson, N.; Ramirez, P.T.; Liu, Y.-J.; et al. Plasmacytoid Dendritic Cells Promote Immunosuppression in Ovarian Cancer via ICOS Costimulation of Foxp3+ T-Regulatory Cells. Cancer Res. 2012, 72, 5240–5249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Yang, M.; Wang, Y.-H.; Lande, R.; Gregorio, J.; Perng, O.A.; Qin, X.-F.; Liu, Y.-J.; Gilliet, M. Plasmacytoid dendritic cells prime IL-10–producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 2007, 204, 105–115. [Google Scholar] [CrossRef]
- Strauss, L.; Bergmann, C.; Szczepanski, M.J.; Lang, S.; Kirkwood, J.M.; Whiteside, T.L. Expression of ICOS on Human Melanoma-Infiltrating CD4+CD25highFoxp3+ T Regulatory Cells: Implications and Impact on Tumor-Mediated Immune Suppression. J. Immunol. 2008, 180, 2967–2980. [Google Scholar] [CrossRef] [Green Version]
- Faget, J.; Bendriss-Vermare, N.; Gobert, M.; Durand, I.; Olive, D.; Biota, C.; Bachelot, T.; Treilleux, I.; Goddard-Leon, S.; Lavergne, E.; et al. ICOS-Ligand Expression on Plasmacytoid Dendritic Cells Supports Breast Cancer Progression by Promoting the Accumulation of Immunosuppressive CD4+ T Cells. Cancer Res. 2012, 72, 6130–6141. [Google Scholar] [CrossRef] [Green Version]
- Chavez, J.C.; Foss, F.M.; William, M.B.M.; E Brammer, J.; Smith, S.M.; Prica, A.; Zain, J.M.; Tuscano, J.M.; Glenn, M.; Mehta-Shah, N.; et al. A Phase I Study of Anti-ICOS Antibody MEDI-570 for Relapsed/Refractory (R/R) Peripheral T-Cell Lymphoma (PTCL) and Angioimmunoblastic T-Cell Lymphoma (AITL) (NCI-9930). Blood 2020, 136, 5–6. [Google Scholar] [CrossRef]
- Patel, M.R.; Naing, A.; Iii, H.A.B.; Lin, C.-C.; Curigliano, G.; Thistlethwaite, F.; Minchom, A.R.; Ascierto, P.A.; De Braud, F.G.; Cecchini, M.; et al. A phase 1/2 open-label study of KY1044, an anti-ICOS antibody with dual mechanism of action, as single agent and in combination with atezolizumab, in adult patients with advanced malignancies. J. Clin. Oncol. 2021, 39, 2624. [Google Scholar] [CrossRef]
- Sainson, R.C.; Thotakura, A.K.; Kosmac, M.; Borhis, G.; Parveen, N.; Kimber, R.; Carvalho, J.; Henderson, S.J.; Pryke, K.L.; Okell, T.; et al. An Antibody Targeting ICOS Increases Intratumoral Cytotoxic to Regulatory T-cell Ratio and Induces Tumor Regression. Cancer Immunol. Res. 2020, 8, 1568–1582. [Google Scholar] [CrossRef]
- Hansen, A.; Bauer, T.; Moreno, V.; Maio, M.; Groenland, S.; Martin-Liberal, J.; Gan, H.; Rischin, D.; Millward, M.; Olszanski, A.; et al. First in human study with GSK3359609 [GSK609], inducible T cell co-stimulator (ICOS) receptor agonist in patients [Pts] with advanced, solid tumors: Preliminary results from INDUCE-1. Ann. Oncol. 2018, 29, viii404. [Google Scholar] [CrossRef]
- Fan, X.; Quezada, S.; Sepulveda, M.A.; Sharma, P.; Allison, J.P. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J. Exp. Med. 2014, 211, 715–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burris, H.A.; Callahan, M.K.; Tolcher, A.W.; Kummar, S.; Falchook, G.S.; Pachynski, R.K.; Tykodi, S.S.; Gibney, G.T.; Seiwert, T.Y.; Gainor, J.F.; et al. Phase 1 safety of ICOS agonist antibody JTX-2011 alone and with nivolumab (nivo) in advanced solid tumors; predicted vs observed pharmacokinetics (PK) in ICONIC. J. Clin. Oncol. 2017, 35, 3033. [Google Scholar] [CrossRef]
- Yap, T.A.; Burris, H.A.; Kummar, S.; Falchook, G.S.; Pachynski, R.K.; Lorusso, P.; Tykodi, S.S.; Gibney, G.T.; Gainor, J.F.; Rahma, O.E.; et al. ICONIC: Biologic and clinical activity of first in class ICOS agonist antibody JTX-2011 +/- nivolumab (nivo) in patients (pts) with advanced cancers. J. Clin. Oncol. 2018, 36, 3000. [Google Scholar] [CrossRef]
- Kobziev, O.; Bulat, I.; Ostapenko, Y.; Zvirbule, Z.; Ursol, G.; Boyko, V.; Paramonov, V.; Hashambhoy-Ramsay, Y.; Hart, C.; Harvey, C.; et al. Phase 2 study of PD-1 inhibitor JTX-4014 alone and in combination with vopratelimab, an ICOS agonist, in biomarker-selected subjects with metastatic NSCLC after one prior platinum-containing regimen (SELECT). J. Clin. Oncol. 2021, 39, TPS9137. [Google Scholar] [CrossRef]


| Opdualag (n = 355) | Nivolumab (n = 359) | ||
|---|---|---|---|
| AEs | Musculoskeletal pain | 54.6% | 33.1% |
| Fatigue | 29.3% | 20.6% | |
| Asthenia | 13.5% | 9.2% | |
| Pyrexia | 12.4% | 9.2% | |
| Headache | 18.0% | 13.1% | |
| Cough | 14.1% | 10.6% | |
| Rash | 17.4% | 13.6% | |
| Pruritus | 24.8% | 17.3% | |
| Diarrhea | 23.1% | 17.3% | |
| Nausea | 17.7% | 16.4% | |
| Constipation | 11.0% | 7.0% | |
| Decreased appetite | 15.5% | 7.5% | |
| Anaemia | 14.1% | 10.3% | |
| Increased AST | 9.9% | 4.7% | |
| Increased ALT | 10.1% | 5.85% | |
| Hypothyroidism | 16.3% | 13.1% | |
| SAEs | Anaemia | 1.4% | 1.1% |
| Acute myocardial infarction | 1.1% | 0.6% | |
| Myocarditis | 1.1% | 0.3% | |
| Adrenal insufficiency | 1.4% | 0.0% | |
| Colitis | 1.4% | 0.3% | |
| Diarrhea | 1.1% | 0.8% | |
| General health deterioration | 0.6% | 1.7% | |
| Pyrexia | 0.9% | 1.4 % | |
| Pneumonia | 1.4% | 0.8% | |
| Urinary tract infection | 0.9% | 1.7% | |
| Back pain | 1.1% | 0.6% | |
| Malignant neoplasm progression | 11.0% | 13.1% | |
| Metastases to central nervous system | 1.1% | 0.8% | |
| Pneumonitis | 1.1% | 0.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chocarro, L.; Bocanegra, A.; Blanco, E.; Fernández-Rubio, L.; Arasanz, H.; Echaide, M.; Garnica, M.; Ramos, P.; Piñeiro-Hermida, S.; Vera, R.; et al. Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor. Cells 2022, 11, 2351. https://doi.org/10.3390/cells11152351
Chocarro L, Bocanegra A, Blanco E, Fernández-Rubio L, Arasanz H, Echaide M, Garnica M, Ramos P, Piñeiro-Hermida S, Vera R, et al. Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor. Cells. 2022; 11(15):2351. https://doi.org/10.3390/cells11152351
Chicago/Turabian StyleChocarro, Luisa, Ana Bocanegra, Ester Blanco, Leticia Fernández-Rubio, Hugo Arasanz, Miriam Echaide, Maider Garnica, Pablo Ramos, Sergio Piñeiro-Hermida, Ruth Vera, and et al. 2022. "Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor" Cells 11, no. 15: 2351. https://doi.org/10.3390/cells11152351
APA StyleChocarro, L., Bocanegra, A., Blanco, E., Fernández-Rubio, L., Arasanz, H., Echaide, M., Garnica, M., Ramos, P., Piñeiro-Hermida, S., Vera, R., Escors, D., & Kochan, G. (2022). Cutting-Edge: Preclinical and Clinical Development of the First Approved Lag-3 Inhibitor. Cells, 11(15), 2351. https://doi.org/10.3390/cells11152351