Comparison of Two Software Packages for Perfusion Imaging: Ischemic Core and Penumbra Estimation and Patient Triage in Acute Ischemic Stroke
Abstract
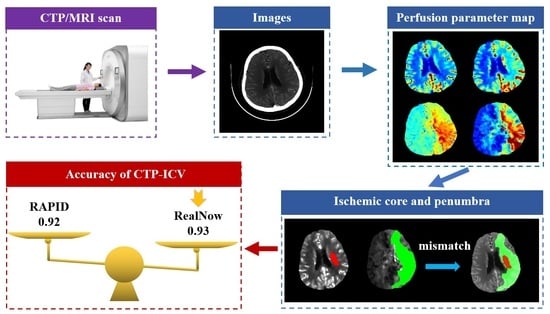
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Image Acquisition and Postprocessing
2.3. Data Analysis
3. Results
3.1. Patient Characteristics
3.2. Comparison of Measurements between Packages
3.3. Comparison of Patient Triage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mokli, Y.; Pfaff, J.; dos Santos, D.P.; Herweh, C.; Nagel, S. Computer-Aided Imaging Analysis in Acute Ischemic Stroke—Background and Clinical Applications. Neurol. Res. Pract. 2019, 1, 23. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, 211. [Google Scholar] [CrossRef]
- Christensen, S.; Lansberg, M.G. CT Perfusion in Acute Stroke: Practical Guidance for Implementation in Clinical Practice. J. Cereb. Blood Flow Metab. 2019, 39, 1664–1668. [Google Scholar] [CrossRef]
- Copen, W.A.; Schaefer, P.W.; Wu, O. MR Perfusion Imaging in Acute Ischemic Stroke. Neuroimaging Clin. N. Am. 2011, 21, 259–283. [Google Scholar] [CrossRef]
- Demeestere, J.; Wouters, A.; Christensen, S.; Lemmens, R.; Lansberg, M.G. Review of Perfusion Imaging in Acute Ischemic Stroke: From Time to Tissue. Stroke 2020, 51, 1017–1024. [Google Scholar] [CrossRef]
- Liu, Y.; Karonen, J.O.; Vanninen, R.L.; Østergaard, L.; Roivainen, R.; Nuutinen, J.; Perkio, J.; Könönen, M.; Hämäläinen, A.; Vanninen, E.J.; et al. Cerebral Hemodynamics in Human Acute Ischemic Stroke: A Study with Diffusion- and Perfusion-Weighted Magnetic Resonance Imaging and SPECT. J. Cereb. Blood Flow Metab. 2000, 20, 910–920. [Google Scholar] [CrossRef]
- Lindsay, E. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. J. Emerg. Med. 2018, 54, 583–584. [Google Scholar] [CrossRef]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W. Use of Imaging to Select Patients for Late Window Endovascular Therapy. Stroke 2018, 49, 2256–2260. [Google Scholar] [CrossRef]
- Koopman, M.S.; Berkhemer, O.A.; Geuskens, R.R.E.G.; Emmer, B.J.; van Walderveen, M.A.A.; Jenniskens, S.F.M.; van Zwam, W.H.; van Oostenbrugge, R.J.; van der Lugt, A.; Dippel, D.W.J.; et al. Comparison of Three Commonly Used CT Perfusion Software Packages in Patients with Acute Ischemic Stroke. J. NeuroIntervent. Surg. 2019, 11, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Austein, F.; Riedel, C.; Kerby, T.; Meyne, J.; Binder, A.; Lindner, T.; Huhndorf, M.; Wodarg, F.; Jansen, O. Comparison of Perfusion CT Software to Predict the Final Infarct Volume after Thrombectomy. Stroke 2016, 47, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- Harston, G.W.J.; Rane, N.; Shaya, G.; Thandeswaran, S.; Cellerini, M.; Sheerin, F.; Kennedy, J. Imaging Biomarkers in Acute Ischemic Stroke Trials: A Systematic Review. AJNR Am. J. Neuroradiol. 2015, 36, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Bivard, A.; Kleinig, T.; Miteff, F.; Butcher, K.; Lin, L.; Levi, C.; Parsons, M. Ischemic core thresholds change with time to reperfusion: A case control study. Ann. Neurol. 2017, 82, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Rava, R.A.; Snyder, K.V.; Mokin, M.; Waqas, M.; Allman, A.B.; Senko, J.L.; Podgorsak, A.R.; Shiraz Bhurwani, M.M.; Hoi, Y.; Siddiqui, A.H.; et al. Assessment of a Bayesian Vitrea CT Perfusion Analysis to Predict Final Infarct and Penumbra Volumes in Patients with Acute Ischemic Stroke: A Comparison with RAPID. AJNR Am. J. Neuroradiol. 2020, 41, 206–212. [Google Scholar] [CrossRef]
- Ramos-Cabrer, P.; Campos, F.; Sobrino, T.; Castillo, J. Targeting the Ischemic Penumbra. Stroke 2011, 42, S7–S11. [Google Scholar] [CrossRef]
- Liu, S.; Levine, S.R.; Winn, H.R. Targeting Ischemic Penumbra: Part I—From Pathophysiology to Therapeutic Strategy. J. Exp. Stroke Transl. Med. 2010, 3, 47–55. [Google Scholar] [CrossRef]
- Shi, Z.; Li, J.; Zhao, M.; Zhang, M.; Wang, T.; Chen, L.; Liu, Q.; Wang, H.; Lu, J.; Zhao, X. Baseline Cerebral Ischemic Core Quantified by Different Automatic Software and Its Predictive Value for Clinical Outcome. Front. Neurosci. 2021, 15, 608799. [Google Scholar] [CrossRef]
- Kudo, K.; Sasaki, M.; Yamada, K.; Momoshima, S.; Utsunomiya, H.; Shirato, H.; Ogasawara, K. Differences in CT Perfusion Maps Generated by Different Commercial Software: Quantitative Analysis by Using Identical Source Data of Acute Stroke Patients. Radiology 2010, 254, 200–209. [Google Scholar] [CrossRef]
- Bathla, G.; Limaye, K.; Policeni, B.; Klotz, E.; Juergens, M.; Derdeyn, C. Achieving Comparable Perfusion Results across Vendors. The next Step in Standardizing Stroke Care: A Technical Report. J. Neurointervent. Surg. 2019, 11, 1257–1260. [Google Scholar] [CrossRef]
- Fahmi, F.; Marquering, H.A.; Streekstra, G.J.; Beenen, L.F.M.; Velthuis, B.K.; VanBavel, E.; Majoie, C.B. Differences in CT Perfusion Summary Maps for Patients with Acute Ischemic Stroke Generated by 2 Software Packages. AJNR Am. J. Neuroradiol. 2012, 33, 2074–2080. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Lev, M.H.; Goldmakher, G.V.; Kamalian, S.; Schaefer, P.W.; Furie, K.L.; Gonzalez, R.G.; Sanelli, P.C. Arterial Input Function Placement for Accurate CT Perfusion Map Construction in Acute Stroke. AJR Am. J. Roentgenol. 2010, 194, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Peerlings, D.; Bennink, E.; Dankbaar, J.W.; Velthuis, B.K.; de Jong, H.W.A.M.; Majoie, C.B.; Roos, Y.B.; Duijm, L.E.; Keizer, K.; van der Lugt, A.; et al. Variation in Arterial Input Function in a Large Multicenter Computed Tomography Perfusion Study. Eur. Radiol. 2021, 31, 8317–8325. [Google Scholar] [CrossRef] [PubMed]
- Bathla, G.; Ortega-Gutierrez, S.; Klotz, E.; Juergens, M.; Zevallos, C.B.; Ansari, S.; Ward, C.E.; Policeni, B.; Samaniego, E.; Derdeyn, C. Comparing the Outcomes of Two Independent Computed Tomography Perfusion Softwares and Their Impact on Therapeutic Decisions in Acute Ischemic Stroke. J. Neurointervent. Surg. 2020, 12, 1028–1032. [Google Scholar] [CrossRef]
- Bulwa, Z.; Dasenbrock, H.; Osteraas, N.; Cherian, L.; Crowley, R.W.; Chen, M. Incidence of Unreliable Automated Computed Tomography Perfusion Maps. J. Stroke Cerebrovasc. Dis. 2019, 28, 104471. [Google Scholar] [CrossRef]
- Kauw, F.; Heit, J.J.; Martin, B.W.; van Ommen, F.; Kappelle, L.J.; Velthuis, B.K.; de Jong, H.W.A.M.; Dankbaar, J.W.; Wintermark, M. Computed Tomography Perfusion Data for Acute Ischemic Stroke Evaluation Using Rapid Software: Pitfalls of Automated Postprocessing. J. Comput. Assist. Tomogr. 2020, 44, 75–77. [Google Scholar] [CrossRef]
- Markus, H.S.; Lythgoe, D.J.; Ostegaard, L.; O’Sullivan, M.; Williams, S.C.R. Reduced Cerebral Blood Flow in White Matter in Ischaemic Leukoaraiosis Demonstrated Using Quantitative Exogenous Contrast Based Perfusion MRI. J. Neurol. Neurosurg. Psychiatry 2000, 69, 48–53. [Google Scholar] [CrossRef]
- Kranz, P.G.; Eastwood, J.D. Does diffusion-weighted imaging represent the ischemic core? An evidence-based systematic review. AJNR. Am. J. Neuroradiol. 2009, 30, 1206–1212. [Google Scholar] [CrossRef]
- Asdaghi, N.; Campbell, B.C.; Butcher, K.S.; Coulter, J.I.; Modi, J.; Qazi, A.; Goyal, M.; Demchuk, A.M.; Coutts, S.B. DWI reversal is associated with small infarct volume in patients with TIA and minor stroke. AJNR. Am. J. Neuroradiol. 2014, 35, 660–666. [Google Scholar] [CrossRef]
- Ermine, C.M.; Bivard, A.; Parsons, M.W.; Baron, J.-C. The Ischemic Penumbra: From Concept to Reality. Int. J. Stroke 2021, 16, 497–509. [Google Scholar] [CrossRef]
- Kaithoju, S. Ischemic Stroke: Risk Stratification, Warfarin Teatment and Outcome Measure. J. Atr. Fibrillation 2015, 8, 1144. [Google Scholar] [CrossRef]
- Boldsen, J.K.; Engedal, T.S.; Pedraza, S.; Cho, T.-H.; Thomalla, G.; Nighoghossian, N.; Baron, J.-C.; Fiehler, J.; Østergaard, L.; Mouridsen, K. Better Diffusion Segmentation in Acute Ischemic Stroke through Automatic Tree Learning Anomaly Segmentation. Front. Neuroinformatics 2018, 12, 21. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Inoue, M.; Yamagami, H.; Fujita, K.; Tanaka, K.; Ando, D.; Sonoda, K.; Kamogawa, N.; Koga, M.; Ihara, M.; et al. Use of Diffusion-Weighted Imaging-Alberta Stroke Program Early Computed Tomography Score (DWI-ASPECTS) and Ischemic Core Volume to Determine the Malignant Profile in Acute Stroke. J. Am. Heart Assoc. 2019, 8, e012558. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Delman, B.N.; Fifi, J.T.; Tuhrim, S.; Wheelwright, D.; Doshi, A.H.; Mocco, J.; Nael, K. Estimation of Ischemic Core Volume Using Computed Tomographic Perfusion: Bayesian Versus Singular Value Deconvolution Postprocessing. Stroke 2018, 49, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Wetterling, F.; Chatzikonstantinou, E.; Tritschler, L.; Meairs, S.; Fatar, M.; Schad, L.R.; Ansar, S. Investigating Potentially Salvageable Penumbra Tissue in an in Vivo Model of Transient Ischemic Stroke Using Sodium, Diffusion, and Perfusion Magnetic Resonance Imaging. BMC Neurosci. 2016, 17, 82. [Google Scholar] [CrossRef]
- Cereda, C.W.; Christensen, S.; Campbell, B.C.; Mishra, N.K.; Mlynash, M.; Levi, C.; Straka, M.; Wintermark, M.; Bammer, R.; Albers, G.W.; et al. A Benchmarking Tool to Evaluate Computer Tomography Perfusion Infarct Core Predictions against a DWI Standard. J. Cereb. Blood Flow Metab. 2016, 36, 1780–1789. [Google Scholar] [CrossRef]
- Mokin, M.; Levy, E.I.; Saver, J.L.; Siddiqui, A.H.; Goyal, M.; Bonafé, A.; Cognard, C.; Jahan, R.; Albers, G.W. Predictive Value of RAPID Assessed Perfusion Thresholds on Final Infarct Volume in SWIFT PRIME (Solitaire with the Intention for Thrombectomy as Primary Endovascular Treatment). Stroke 2017, 48, 932–938. [Google Scholar] [CrossRef]
- Hoving, J.W.; Marquering, H.A.; Majoie, C.B.L.M.; Yassi, N.; Sharma, G.; Liebeskind, D.S.; van der Lugt, A.; Roos, Y.B.; van Zwam, W.; van Oostenbrugge, R.J.; et al. Volumetric and Spatial Accuracy of Computed Tomography Perfusion Estimated Ischemic Core Volume in Patients with Acute Ischemic Stroke. Stroke 2018, 49, 2368–2375. [Google Scholar] [CrossRef]
- Albers, G.W.; Goyal, M.; Jahan, R.; Bonafe, A.; Diener, H.-C.; Levy, E.I.; Pereira, V.M.; Cognard, C.; Cohen, D.J.; Hacke, W.; et al. Ischemic Core and Hypoperfusion Volumes Predict Infarct Size in SWIFT PRIME: CT Perfusion Volumes. Ann. Neurol. 2016, 79, 76–89. [Google Scholar] [CrossRef]
- Pisana, F.; Henzler, T.; Schönberg, S.; Klotz, E.; Schmidt, B.; Kachelrieß, M. Noise reduction and functional maps image quality improvement in dynamic CT perfusion using a new k-means clustering guided bilateral filter (KMGB). Med. Phys. 2017, 44, 3464–3482. [Google Scholar] [CrossRef]
- Najm, M.A.; Kuang, H.; Fedorico, A.; Jogiat, U.; Goyal, M.; Hill, M.D.; Demchuk, A.; Menon, B.K.; Qiu, W. Automated brain extraction from head CT and CTA images using convex optimization with shape propagation. Comput. Methods Programs Biomed. 2019, 176, 1–8. [Google Scholar] [CrossRef]
- Mouridsen, K.; Christensen, S.; Gyldensted, L.; Ostergaard, L. Automatic selection of arterial input function using cluster analysis. Magn. Reson. Med. 2010, 55, 524–531. [Google Scholar] [CrossRef] [PubMed]










| Groups | CTP Group (n = 174) | MRI Group (n = 420) |
|---|---|---|
| Age in years (SD) | 65 (20.6) | 64 (17.1) |
| Gender(M/F) | 135/39 | 286/134 |
| Occlusion sites, n (%) | ||
| Middle cerebral artery | 101 (58.0) | 218 (51.9) |
| Posterior cerebral artery | 17 (9.8) | 58(13.8) |
| Anterior cerebral artery | 11 (6.3) | 32 (7.6) |
| Internal carotid artery | 33 (18.9) | 74 (17.6) |
| Basilar artery | 12 (6.8) | 38(9.0) |
| Package A | Package B | |||
|---|---|---|---|---|
| CTP group | ICV, mL | Mean (SD) | 14.9 (36.0) | 14.0 (28.3) |
| median [IQR] | 0.0 [0.0–6.4] | 2.7 [0.0–13.1] | ||
| PV, mL | Mean (SD) | 81.1 (95.7) | 83.2 (91.4) | |
| median [IQR] | 39.2 [12.0–129.1] | 43.7 [14.6–128.3] | ||
| MRI group | ICV, mL | Mean (SD) | 52.4 (69.5) | 48.9 (69.1) |
| median [IQR] | 24.0 [12.0–64.0] | 21 [0.0–59.5] | ||
| PV, mL | Mean (SD) | 68.4 (77.3) | 61.6 (72.6) | |
| median [IQR] | 44.0 [4.0–108.0] | 38.3 [0.0–99.4] | ||
| CTP Group | MRI Group | |||
|---|---|---|---|---|
| ICV | PV | ICV | PV | |
| Mean difference (SD), mL | 0.89 (12.7) | −2.0 (13.0) | 3.5 (4.1) | 6.8 (46.9) |
| 95% Lower LoA (95% CI) | −24.1 (−27.4 to −20.9) | −27.6 (−30.9 to −24.3) | −4.5 (−5.1 to −3.8) | −81.2 (−89.6 to −72.8) |
| 95% Upperer LoA (95% CI) | 25.9 (22.6 to 29.2) | 23.4 (20.1 to 26.8) | 11.5 (10.8 to 12.2) | 102.9 (94.6 to 111.3) |
| ICC (95% CI) | 0.95 (0.94 to 0.97) | 0.99 (0.98 to 0.99) | 0.99 (0.98 to 0.99) | 0.87 (0.84 to 0.89) |
| Wilcoxon test (p value) | 0.264 | 0.354 | 0.463 | 0.178 |
| RAPID | RealNow | |
|---|---|---|
| Mean difference (SD), mL | −4.65 (16.3) | −3.65 (16.3) |
| 95% Lower LoA (95% CI) | −8.14 (−11.2 to −5.05) | −7.06 (−9.64 to −4.47) |
| 95% Upperer LoA (95% CI) | 17.4 (14.4 to 20.5) | 14.3 (11.8 to 16.9) |
| ICC (95% CI) | 0.92 (0.86 to 0.95) | 0.94 (0.90 to 0.97) |
| Wilcoxon test (p value) | 0.144 | 0.253 |
| ICC | Subgroup | Package A | Package B | |||
|---|---|---|---|---|---|---|
| Eligible | Not Eligible | Eligible | Not Eligible | |||
| CTP group | 0.90 | ICV > 70 mL | 16 | 3 | 19 | 0 |
| ICV < 70 mL | 145 | 10 | 153 | 2 | ||
| MRI group | 0.93 | ICV > 70 mL | 125 | 5 | 130 | 0 |
| ICV < 70 mL | 285 | 5 | 280 | 10 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Nan, Y.; Ju, J.; Zhou, J.; Xiao, H.; Wang, S. Comparison of Two Software Packages for Perfusion Imaging: Ischemic Core and Penumbra Estimation and Patient Triage in Acute Ischemic Stroke. Cells 2022, 11, 2547. https://doi.org/10.3390/cells11162547
Zhou X, Nan Y, Ju J, Zhou J, Xiao H, Wang S. Comparison of Two Software Packages for Perfusion Imaging: Ischemic Core and Penumbra Estimation and Patient Triage in Acute Ischemic Stroke. Cells. 2022; 11(16):2547. https://doi.org/10.3390/cells11162547
Chicago/Turabian StyleZhou, Xiang, Yashi Nan, Jieyang Ju, Jingyu Zhou, Huanhui Xiao, and Silun Wang. 2022. "Comparison of Two Software Packages for Perfusion Imaging: Ischemic Core and Penumbra Estimation and Patient Triage in Acute Ischemic Stroke" Cells 11, no. 16: 2547. https://doi.org/10.3390/cells11162547
APA StyleZhou, X., Nan, Y., Ju, J., Zhou, J., Xiao, H., & Wang, S. (2022). Comparison of Two Software Packages for Perfusion Imaging: Ischemic Core and Penumbra Estimation and Patient Triage in Acute Ischemic Stroke. Cells, 11(16), 2547. https://doi.org/10.3390/cells11162547