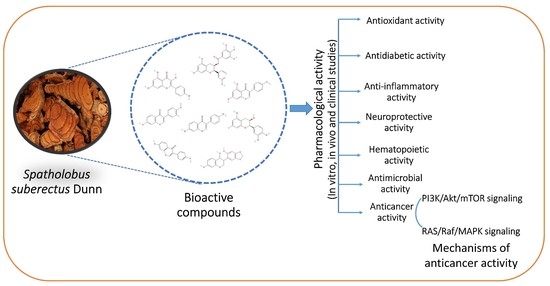
A Review of the Pharmacological Potential of Spatholobus suberectus Dunn on Cancer
Abstract
:1. Introduction
2. Phytochemistry
3. Pharmacological Activity of SSD
3.1. Antioxidant Activity
3.2. Antidiabetic Activity
3.3. Anti-Inflammatory Activity
3.4. Neuroprotective Activity
3.5. Hematopoietic Activity
3.6. Antimicrobial Activity
3.7. Other Activities
4. Anticancer Activity of SSD
5. Mechanism of Anticancer Activity of SSD
5.1. RAS/Raf/MAPK Signaling
5.2. PI3K/Akt/mTOR Signaling
6. Clinical Studies
7. Toxicity Studies
8. Future Perspectives
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sukalingam, K.; Ganesan, K.; Xu, B. Trianthema portulacastrum L. (giant pigweed): Phytochemistry and pharmacological properties. Phytochem. Rev. 2017, 16, 461–478. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2017, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Gani, S.; Murugesan, A.G.; Pandian, M.R. Preliminary Toxicity and Phytochemical Studies of Aqueous Bark Extract of Helicteres isora L. Int. J. Pharmacol. 2007, 3, 96–100. [Google Scholar] [CrossRef]
- Tadesse, S.; Ganesan, K.; Nair, S.; Letha, N.; Gani, S. Preliminary phytochemical investigation of Different Solvent Extracts of Centella asiatica L. (Family: Apiaceae)—An Ethiopian weed. Int. J. Pharma. Chem. Biol. Sci. 2016, 6, 97–102. [Google Scholar]
- Sinaga, M.; Ganesan, K.; Kumar Nair, S.K.P.; Gani, S.B. Preliminary Phytochemical Analysis and In Vitro Antibacterial Activity of Bark and Seeds of Ethiopian Neem (Azadirachta indica A. Juss). World J. Pharm. Pharm. Sci. 2016, 5, 1714–1723. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Lentils and Their Health Promoting Effects. Int. J. Mol. Sci. 2017, 18, 2390. [Google Scholar] [CrossRef]
- Islam, T.; Ganesan, K.; Xu, B. New Insight into Mycochemical Profiles and Antioxidant Potential of Edible and Medicinal Mushrooms: A Review. Int. J. Med. Mushrooms 2019, 21, 237–251. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N. Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef]
- Xu, B.; Ganesan, K.; Mickymaray, S.; Alfaiz, F.A.; Thatchinamoorthi, R.; Aboody, M.S.A. Immunomodulatory and antineoplastic efficacy of common spices and their connection with phenolic antioxidants. Bioact. Compd. Health Dis. 2020, 3, 15. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Zhang, J.; Gong, Y.; Liu, G.-Z.; Gao, L.-L.; Zhang, P.; Cai, C.-T. Morphological and physiological responses of Spatholobus suberectus Dunn to nitrogen and water availability. Photosynthetica 2019, 57, 1130–1141. [Google Scholar] [CrossRef]
- Pang, J.; Guo, J.P.; Jin, M.; Chen, Z.Q.; Wang, X.W.; Li, J.W. Antiviral effects of aqueous extract from Spatholobus suberectus Dunn. against coxsackievirus B3 in mice. Chin. J. Integr. Med. 2011, 17, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Wu, L.; Wei, K.; Liang, Y.; Song, Z.; Zhou, X.; Wang, S.; Li, M.; Wu, Q.; Zhang, K.; et al. A draft genome for Spatholobus suberectus. Sci. Data 2019, 6, 113. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Q.; Ganesan, K.; Kewu, Z.; Shen, J.; Gang, F.; Luo, X.; Chen, J. The Antitriple Negative Breast cancer Efficacy of Spatholobus suberectus Dunn on ROS-Induced Noncanonical Inflammasome Pyroptotic Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5187569. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Lin, C.S.; Lai, G.H. Phytochemical characteristics, free radical scavenging activities, and neuroprotection of five medicinal plant extracts. Evid. Based Complement. Altern. Med. 2012, 2012, 984295. [Google Scholar] [CrossRef]
- Wang, K.H.; Lin, R.D.; Hsu, F.L.; Huang, Y.H.; Chang, H.C.; Huang, C.Y.; Lee, M.H. Cosmetic applications of selected traditional Chinese herbal medicines. J. Ethnopharmacol. 2006, 106, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ravipati, A.S.; Zhang, L.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; Shanmugam, K.; Munch, G.; Wu, M.J.; et al. Antioxidant and anti-inflammatory activities of selected Chinese medicinal plants and their relation with antioxidant content. BMC Complement. Altern. Med. 2012, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.N.; Qu, X.B.; Guan, S.H.; Xu, P.P.; Shi, Y.Y.; Guo, D.A. Chemical constituents of Spatholobus suberectus. Chin. J. Nat. Med. 2012, 10, 32–35. [Google Scholar] [CrossRef]
- Cui, Y.J.; Liu, P.; Chen, R.Y. Studies on the chemical constituents of Spatholobus suberectus Dunn. Yao Xue Xue Bao 2002, 37, 784–787. [Google Scholar]
- Cheng, J.; Liang, H.; Wang, Y.; Zhao, Y.Y. Studies on the constituents from the stems of Spatholobus suberectus. Zhongguo Zhong Yao Za Zhi 2003, 28, 1153–1155. [Google Scholar]
- Cui, Y.J.; Liu, P.; Chen, R.Y. Studies on the active constituents in vine stem of Spatholobus suberectus. Zhongguo Zhong Yao Za Zhi 2005, 30, 121–123. [Google Scholar]
- Wang, H.; Liu, Y.; Zeng, Z.; He, W. Study on HPLC chromatographic fingerprint of anti-tumor active site SSCE of Caulis spatholobi. Zhongguo Zhong Yao Za Zhi 2011, 36, 2525–2529. [Google Scholar] [PubMed]
- Zhang, M.; Chen, H.; Li, J.; Pei, Y.; Liang, Y. Antioxidant properties of tartary buckwheat extracts as affected by different thermal processing methods. LWT Food Sci. Technol. 2010, 43, 181–185. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wang, Y.M.; Xu, M.; Wu, D.M.; Chen, J.H.; Yan, X.P. Antioxidant Activity, Phenol and Flavonoid Contents of Fourteen Mulberry Varieties Leaves. Adv. Mater. Res. 2013, 781–784, 1454–1459. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Chen, J.; Zhu, D.; Zhou, H.; Wang, X. A study on anticancer activity of Caulis spatholobi extract on human osteosarcoma Saos-2 cells. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Fu, Y.; Wang, Z.; Yang, D.; Chen, J.; Wang, D. Determination on the Contents of Condensed Tannins in Spatholobus suberectus Dunn. Extracts and Primary Study on their Anti-tumor Activities. Acta Sci. Nat. Univ. Sunyatseni 2011, 50, 75–80. [Google Scholar]
- Zhang, Y.; Guo, L.; Duan, L.; Dong, X.; Zhou, P.; Liu, E.H.; Li, P. Simultaneous determination of 16 phenolic constituents in Spatholobi caulis by high performance liquid chromatography/electrospray ionization triple quadrupole mass spectrometry. J. Pharm. Biomed. Anal. 2015, 102, 110–118. [Google Scholar] [CrossRef]
- Sun, J.Q.; Zhang, G.L.; Zhang, Y.; Nan, N.; Sun, X.; Yu, M.W.; Wang, H.; Li, J.P.; Wang, X.M. Spatholobus suberectus Column Extract Inhibits Estrogen Receptor Positive Breast Cancer via Suppressing ER MAPK PI3K/AKT Pathway. Evid. Based Complement. Altern. Med. 2016, 2016, 2934340. [Google Scholar] [CrossRef]
- Mei, Y.; Wei, L.; Chai, C.; Zou, L.; Liu, X.; Chen, J.; Tan, M.; Wang, C.; Cai, Z.; Zhang, F.; et al. A Method to Study the Distribution Patterns for Metabolites in Xylem and Phloem of Spatholobi caulis. Molecules 2019, 25, 167. [Google Scholar] [CrossRef]
- Peng, F.; Meng, C.W.; Zhou, Q.M.; Chen, J.P.; Xiong, L. Cytotoxic Evaluation against Breast Cancer Cells of Isoliquiritigenin Analogues from Spatholobus suberectus and Their Synthetic Derivatives. J. Nat. Prod. 2016, 79, 248–251. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, L.; Yang, X.W.; Zhang, Y.B.; Xu, W.; Zhang, P.; Zhao, W.; Peng, K.F.; Gong, Y.; Liu, N.F. Simultaneous detection and quantification of 57 compounds in Spatholobi caulis applying ultra-fast liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2020, 43, 4247–4262. [Google Scholar] [CrossRef]
- Suganya, N.; Bhakkiyalakshmi, E.; Sarada, D.V.; Ramkumar., K.M. Reversibility of endothelial dysfunction in diabetes: Role of polyphenols. Br. J. Nutr. 2016, 116, 223–246. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, T.; Jin, X. UPLC-MS/MS assay for simultaneous determination of four compounds in rat plasma: Application to pharmacokinetic study after oral administration of Caulis spatholobi extract. Biomed. Chromatogr. 2016, 30, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Latha, M.; Ramkumar, K.M.; Pari, L.; Damodaran, P.N.; Rajeshkannan, V.; Suresh, T. Phytochemical and antimicrobial study of an antidiabetic plant: Scoparia dulcis L. J. Med. Food. 2006, 9, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.L.; Wan, J.Y.; Li, P.; Qi, L.W. Ultrasonic/microwave assisted extraction and diagnostic ion filtering strategy by liquid chromatography-quadrupole time-of-flight mass spectrometry for rapid characterization of flavonoids in Spatholobus suberectus. J. Chromatogr. A 2011, 1218, 5774–5786. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Chung, B.; Kim, C.K.; Oh, D.C.; Oh, K.B.; Shin, J. Spatholobus suberectus Dunn. constituents inhibit sortase A and Staphylococcus aureus cell clumping to fibrinogen. Arch. Pharm. Res. 2017, 40, 518–523. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Meng, X.; Bao, Y.; Wang, S.; Li, T. 3D microfluidic in vitro model and bioinformatics integration to study the effects of Spatholobi caulis tannin in cervical cancer. Sci. Rep. 2018, 8, 12285. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Guan, R.; Chen, J.; Yang, D.; Zhao, Z.; Wang, D. Chemical characterization of procyanidins from Spatholobus suberectus and their antioxidative and anticancer activities. J. Funct. Foods 2015, 12, 468–477. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, L.; Feng, L.; Guo, F.; Li, Y. Characterization of total phenolic constituents from the stems of Spatholobus suberectus using LC-DAD-MS(n) and their inhibitory effect on human neutrophil elastase activity. Molecules 2013, 18, 7549–7556. [Google Scholar] [CrossRef]
- Liu, C.; Ma, L.; Chen, R.Y.; Liu, P. Determination of catechin and its analogues in Spatholobus suberectus by RP-HPLC. Zhongguo Zhong Yao Za Zhi 2005, 30, 1433–1435. [Google Scholar]
- Peng, F.; Zhu, H.; Meng, C.W.; Ren, Y.R.; Dai, O.; Xiong, L. New Isoflavanes from Spatholobus suberectus and Their Cytotoxicity against Human Breast Cancer Cell Lines. Molecules 2019, 24, 3218. [Google Scholar] [CrossRef]
- Wang, L.X.; Zheng, H.R.; Ren, F.C.; Chen, T.G.; Li, X.M.; Jiang, X.J.; Wang, F. Polysubstituted Isoflavonoids from Spatholobus suberectus, Flemingia macrophylla, and Cudrania cochinchinensis. Nat. Prod. Bioprospecting 2017, 7, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Zhang, Y.B.; Yang, X.W.; Yang, Y.F.; Xu, W.; Zhao, W.; Peng, K.F.; Gong, Y.; Liu, N.F.; Zhang, P. Anti-Inflammatory Activity of Some Characteristic Constituents from the Vine Stems of Spatholobus suberectus. Molecules 2019, 24, 154. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Sung, S.H.; Park, J.H.; Kim, Y.C. Flavonoids from Spatholobus suberectus. Arch. Pharm. Res. 2004, 27, 589–592. [Google Scholar] [CrossRef]
- Liu, X.Y.; Xu, W.; Yang, X.W.; Zhang, P.; Zhao, W.; Gong, Y.; Liu, N.F. Study on non-flavonoids chemical constituents from Spatholobi caulis. Zhongguo Zhong Yao Za Zhi 2020, 45, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Xu, W.; Yang, X.W.; Zhang, P.; Zhao, W.; Gong, Y.; Liu, N.F. Isolation and identification of flavonoids from Spatholobi caulis. Zhongguo Zhong Yao Za Zhi 2020, 45, 1384–1392. [Google Scholar] [CrossRef]
- Peng, F.; Xiong, L.; Peng, C. (-)-Sativan Inhibits Tumor Development and Regulates miR-200c/PD-L1 in Triple Negative Breast Cancer Cells. Front. Pharm. 2020, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Huan, L.Y.; Zhao, W.R.; Tang, N.; Jin, Y.; Tang, J.Y. Spatholobi Caulis extracts promote angiogenesis in HUVECs in vitro and in zebrafish embryos in vivo via up-regulation of VEGFRs. J. Ethnopharmacol. 2017, 200, 74–83. [Google Scholar] [CrossRef]
- Lu, D.; He, H.; Wu, B.; Yao, S. Cytotoxic effect on cancer cells and structural identification of phenols from Spatholobi caulis by HPLC-ESI-MS(n). Nat. Prod. Commun. 2009, 4, 809–812. [Google Scholar]
- Liu, Q.; Kwan, K.Y.; Cao, T.; Yan, B.; Ganesan, K.; Jia, L.; Zhang, F.; Lim, C.; Wu, Y.; Feng, Y.; et al. Broad-spectrum antiviral activity of Spatholobus suberectus Dunn against SARS-CoV-2, SARS-CoV-1, H5N1, and other enveloped viruses. Phytother. Res. 2022, 36, 3232–3247. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Wada-Takahashi, S.; Takamichi, M.; Watanabe, K.; Yoshida, A.; Yoshino, F.; Miyamoto, C.; Maehata, Y.; Sugiyama, S.; Takahashi, S.S.; et al. Reactive oxygen species scavenging activity of Jixueteng evaluated by electron spin resonance (ESR) and photon emission. Nat. Prod. Commun. 2014, 9, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Banbury, L.K.; Leach, D.N. Antioxidant activity of 45 Chinese herbs and the relationship with their TCM characteristics. Evid. Based Complement. Altern. Med. 2008, 5, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.F.; Jiang, L.H.; Zhao, W.D.; Xi-Nan, M.; Huang, S.Q.; Yang, J.; Hu, T.J.; Chen, H.L. Immunomodulatory and antioxidant effects of total flavonoids of Spatholobus suberectus Dunn on PCV2 infected mice. Sci. Rep. 2017, 7, 8676. [Google Scholar] [CrossRef]
- Chen, H.L.; Yang, J.; Fu, Y.F.; Meng, X.N.; Zhao, W.D.; Hu, T.J. Effect of total flavonoids of Spatholobus suberectus Dunn on PCV2 induced oxidative stress in RAW264.7 cells. BMC Complement. Altern. Med. 2017, 17, 244. [Google Scholar] [CrossRef]
- Kim, H.; Yi, S.S.; Lee, H.K.; Heo, T.H.; Park, S.K.; Jun, H.S.; Song, K.D.; Kim, S.J. Antiproliferative Effect of Vine Stem Extract from Spatholobus suberectus Dunn on Rat C6 Glioma Cells Through Regulation of ROS, Mitochondrial Depolarization, and P21 Protein Expression. Nutr. Cancer 2018, 70, 605–619. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, C.; Liu, X.; Guo, Y. Protective effect of Spatholobus suberectus on brain tissues in cerebral ischemia. Am. J. Transl. Res. 2016, 8, 3963–3969. [Google Scholar]
- Tan, X.; Dong, X.Z.; Guo, D.H.; Wang, S.; Li, M.H.; Zhao, R.Q.; Liu, P. Anti-radiation effect and mechanism studies of ethanol extracts from Spatholobus suberectus and its active component catechin. Zhongguo Zhong Yao Za Zhi 2016, 41, 1718–1724. [Google Scholar] [CrossRef]
- Dong, X.Z.; Wang, Y.N.; Tan, X.; Liu, P.; Guo, D.H.; Yan, C. Protective Effect of JXT Ethanol Extract on Radiation-Induced Hematopoietic Alteration and Oxidative Stress in the Liver. Oxid. Med. Cell. Longev. 2018, 2018, 9017835. [Google Scholar] [CrossRef]
- Islam, T.; Ganesan, K.; Xu, B. Insights into health-promoting effects of Jew’s ear (Auricularia auricula-judae). Trends Food Sci. Technol. 2021, 114, 552–569. [Google Scholar] [CrossRef]
- Ganesan, K.; Quiles, J.L.; Daglia, M.; Xiao, J.; Xu, B. Dietary phytochemicals modulate intestinal epithelial barrier dysfunction and autoimmune diseases. Food Front. 2021, 2, 357–382. [Google Scholar] [CrossRef]
- Ganesan, K.; Ramkumar, K.M.; Xu, B. Vitexin restores pancreatic β-cell function and insulin signaling through Nrf2 and NF-κB signaling pathways. Eur. J. Pharmacol. 2020, 888, 173606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Alam, M.B.; Lee, S.H.; Kim, Y.J.; Lee, S.; An, H.; Choi, H.J.; Son, H.U.; Park, C.H.; Kim, H.H.; et al. Spatholobus suberectus Exhibits Antidiabetic Activity In Vitro and In Vivo through Activation of AKT-AMPK Pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 6091923. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Hur, J.; Choi, J.; Kim, Y.; Park, H.Y.; Ha, S.K. Spatholobus suberectus Ameliorates Diabetes-Induced Renal Damage by Suppressing Advanced Glycation End Products in db/db Mice. Int. J. Mol. Sci. 2018, 19, 2774. [Google Scholar] [CrossRef]
- Ganesan, K.; Du, B.; Chen, J. Effects and mechanisms of dietary bioactive compounds on breast cancer prevention. Pharmacol. Res. 2022, 178, 105974. [Google Scholar] [CrossRef]
- Jayasuriya, R.; Dhamodharan, U.; Ali, D.; Ganesan, K.; Xu, B.; Ramkumar, K.M. Targeting Nrf2/Keap1 signaling pathway by bioactive natural agents: Possible therapeutic strategy to combat liver disease. Phytomedicine 2021, 92, 153755. [Google Scholar] [CrossRef]
- Ganesan, K.; Jayachandran, M.; Xu, B. Diet-Derived Phytochemicals Targeting Colon Cancer Stem Cells and Microbiota in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 3976. [Google Scholar] [CrossRef]
- Ganesan, K.; Guo, S.; Fayyaz, S.; Zhang, G.; Xu, B. Targeting Programmed Fusobacterium nucleatum Fap2 for Colorectal Cancer Therapy. Cancers 2019, 11, 1592. [Google Scholar] [CrossRef] [Green Version]
- Shim, S.H. 20S proteasome inhibitory activity of flavonoids isolated from Spatholobus suberectus. Phytother. Res. 2011, 25, 615–618. [Google Scholar] [CrossRef]
- Li, R.W.; David Lin, G.; Myers, S.P.; Leach, D.N. Anti-inflammatory activity of Chinese medicinal vine plants. J. Ethnopharmacol. 2003, 85, 61–67. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, K.; Liu, W.; Fu, F.; Xu, J. Combined Autodock and comparative molecular field analysis study on predicting 5-lipoxygenase inhibitory activity of flavonoids isolated from Spatholobus suberectus Dunn. Z. Fur Naturforschung. C J. Biosci. 2015, 70, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, E.N.; Jeong, G.S. Oral Administration of Liquiritigenin Confers Protection from Atopic Dermatitis through the Inhibition of T Cell Activation. Biomolecules 2020, 10, 786. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Zhang, Y.B.; Yang, X.W.; Xu, W.; Liu, L.; Zhang, P.; Gong, Y.; Liu, N.F.; Peng, K.F. Simultaneous determination of twenty-five compounds with anti-inflammatory activity in Spatholobi caulis by using an optimized UFLC-MS/MS method: An application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2021, 204, 114267. [Google Scholar] [CrossRef] [PubMed]
- Park, H.R.; Lee, H.; Lee, J.J.; Yim, N.H.; Gu, M.J.; Ma, J.Y. Protective Effects of Spatholobi caulis Extract on Neuronal Damage and Focal Ischemic Stroke/Reperfusion Injury. Mol. Neurobiol. 2018, 55, 4650–4666. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, D.X.; Chen, R.Y.; Chen, M.L.; Yin, J.F.; Chen, G.Y. Effect of catechin on bone marrow cell cycle and gene expression of hematopoietic growth factors. Yao Xue Xue Bao 2004, 39, 424–428. [Google Scholar] [PubMed]
- Chang, J.; Sun, W.; Zeng, J.; Xue, Y.; Zhang, Y.; Pan, X.; Zhou, Y.; Lai, M.; Bian, G.; Zhou, Q.; et al. Establishment of an in vitro system based on AGM-S3 co-culture for screening traditional herbal medicines that stimulate hematopoiesis. J. Ethnopharmacol. 2019, 240, 111938. [Google Scholar] [CrossRef]
- Su, E.Y.; Chen, H.S. Clinical observation on aplastic anemia treated by Spatholobus suberectus Composita. Zhongguo Zhong Xi Yi Jie He Za Zhi 1997, 17, 213–215. [Google Scholar] [PubMed]
- Anandhakumar, S.; Krishnamoorthy, G.; Ramkumar, K.M.; Raichur, A.M. Preparation of collagen peptide functionalized chitosan nanoparticles by ionic gelation method: An effective carrier system for encapsulation and release of doxorubicin for cancer drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 378–385. [Google Scholar] [CrossRef]
- Guan, Y.; An, P.; Zhang, Z.; Zhang, F.; Yu, Y.; Wu, Q.; Shi, Y.; Guo, X.; Tao, Y.; Wang, F. Screening identifies the Chinese medicinal plant Caulis spatholobi as an effective HAMP expression inhibitor. J. Nutr. 2013, 143, 1061–1066. [Google Scholar] [CrossRef]
- Wang, D.X.; Chen, M.L.; Yin, J.F.; Liu, P. Effect of SS8, the active part of Spatholobus suberectus Dunn, on proliferation of hematopoietic progenitor cells in mice with bone marrow depression. Zhongguo Zhong Yao Za Zhi 2003, 28, 152–155. [Google Scholar]
- Wang, D.X.; Liu, P.; Chen, Y.H.; Chen, R.Y.; Guo, D.H.; Ren, H.Y.; Chen, M.L. Stimulating effect of catechin, an active component of Spatholobus suberectus Dunn, on bioactivity of hematopoietic growth factor. Chin. Med. J. 2008, 121, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.L.; Lam, M.L.; Au, T.K.; Ip, D.T.; Ng, T.B.; Fong, W.P.; Wan, D.C. A comparison of human immunodeficiency virus type-1 protease inhibition activities by the aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2000, 67, 2889–2896. [Google Scholar] [CrossRef]
- Guo, J.P.; Pang, J.; Wang, X.W.; Shen, Z.Q.; Jin, M.; Li, J.W. In vitro screening of traditionally used medicinal plants in China against enteroviruses. World J. Gastroenterol. 2006, 12, 4078–4081. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Gan, R.Y.; Zhang, D.; Farha, A.K.; Habimana, O.; Mavumengwana, V.; Li, H.B.; Wang, X.H.; Corke, H. Large-Scale Screening of 239 Traditional Chinese Medicinal Plant Extracts for Their Antibacterial Activities against Multidrug-Resistant Staphylococcus aureus and Cytotoxic Activities. Pathogens 2020, 9, 185. [Google Scholar] [CrossRef]
- Chen, S.R.; Wang, A.Q.; Lin, L.G.; Qiu, H.C.; Wang, Y.T.; Wang, Y. In Vitro Study on Anti-Hepatitis C Virus Activity of Spatholobus suberectus Dunn. Molecules 2016, 21, 1367. [Google Scholar] [CrossRef]
- Park, W.; Ahn, C.H.; Cho, H.; Kim, C.K.; Shin, J.; Oh, K.B. Inhibitory Effects of Flavonoids from Spatholobus suberectus on Sortase A and Sortase A-Mediated Aggregation of Streptococcus mutans. J. Microbiol. Biotechnol. 2017, 27, 1457–1460. [Google Scholar] [CrossRef]
- Ashour, M.L.; Youssef, F.S.; Gad, H.A.; Wink, M. Inhibition of Cytochrome P450 (CYP3A4) Activity by Extracts from 57 Plants Used in Traditional Chinese Medicine (TCM). Pharmacogn. Mag. 2017, 13, 300–308. [Google Scholar] [CrossRef]
- Pao, L.H.; Hu, O.Y.; Fan, H.Y.; Lin, C.C.; Liu, L.C.; Huang, P.W. Herb-drug interaction of 50 Chinese herbal medicines on CYP3A4 activity in vitro and in vivo. Am. J. Chin. Med. 2012, 40, 57–73. [Google Scholar] [CrossRef]
- Lee, B.J.; Jo, I.Y.; Bu, Y.; Park, J.W.; Maeng, S.; Kang, H.; Jang, W.; Hwang, D.S.; Lee, W.; Min, K.; et al. Antiplatelet effects of Spatholobus suberectus via inhibition of the glycoprotein IIb/IIIa receptor. J. Ethnopharmacol. 2011, 134, 460–467. [Google Scholar] [CrossRef]
- Han, A.R.; Park, H.J.; Chen, D.; Jang, D.S.; Kim, H.J.; Lee, S.K.; Seo, E.K. Topoisomerase-II-inhibitory principles from the stems of Spatholobus suberectus. Chem. Biodivers. 2007, 4, 1487–1491. [Google Scholar] [CrossRef]
- Ha, H.; Shim, K.S.; An, H.; Kim, T.; Ma, J.Y. Water extract of Spatholobus suberectus inhibits osteoclast differentiation and bone resorption. BMC Complement. Altern. Med. 2013, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Im, N.K.; Lee, S.G.; Lee, D.S.; Park, P.H.; Lee, I.S.; Jeong, G.S. Spatholobus suberectus inhibits osteoclastogenesis and stimulates chondrogenesis. Am. J. Chin. Med. 2014, 42, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Park, M.N.; Kim, C.; Kang, B.; Song, H.S.; Lee, H.; Kim, S.H.; Shim, B.S.; Kim, B. MiR-657/ATF2 Signaling Pathway Has a Critical Role in Spatholobus suberectus Dunn Extract-Induced Apoptosis in U266 and U937 Cells. Cancers 2019, 11, 150. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, D.M.; Loo, T.Y.; Cheng, Y.; Chen, L.L.; Shen, J.G.; Yang, D.P.; Chow, L.W.; Guan, X.Y.; Chen, J.P. Spatholobus suberectus inhibits cancer cell growth by inducing apoptosis and arresting cell cycle at G2/M checkpoint. J. Ethnopharmacol. 2011, 133, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, T.; Chang, T.T.; Chiang, J.H.; Sun, M.F.; Yen, H.R. Improved Survival With Integration of Chinese Herbal Medicine Therapy in Patients with Acute Myeloid Leukemia: A Nationwide Population-Based Cohort Study. Integr. Cancer Ther. 2017, 16, 156–164. [Google Scholar] [CrossRef]
- Bajracharya, P.; Lee, E.J.; Lee, D.M.; Shim, S.H.; Kim, K.J.; Lee, S.H.; Bae, J.J.; Chun, S.S.; Lee, T.K.; Kwon, S.H.; et al. Effect of different ingredients in traditional Korean medicine for human uterine leiomyoma on normal myometrial and leiomyomal smooth muscle cell proliferation. Arch. Pharm. Res. 2009, 32, 1555–1563. [Google Scholar] [CrossRef]
- Xie, F.; Wang, M.; Su, Y.; Xiao, K.; Chu, X.; Long, S.; Li, L.; Zhang, X.; Xue, P.; Zhu, S. Unveiling Potential Mechanisms of Spatholobi caulis against Lung Metastasis of Malignant Tumor by Network Pharmacology and Molecular Docking. Evid. Based Complement. Altern. Med. 2022, 2022, 1620539. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Jin, C.; Cheng, Y.Y.; Zhang, Q.X.; Li, X.X.; Zhang, L. Molecular mechanism of Spatholobi caulis in treatment of lung cancer based on network pharmacology and molecular docking. Zhongguo Zhong Yao Za Zhi 2021, 46, 837–844. [Google Scholar] [CrossRef]
- Zhu, S.C.; Cai, J.; Wu, C.Y.; Cheng, C.S. Molecular mechanism of Spatholobi caulis in treatment of ovarian cancer based on network pharmacology and experimental verification. Zhongguo Zhong Yao Za Zhi 2022, 47, 786–795. [Google Scholar] [CrossRef]
- Cho, C.H.; Kwon, S.H.; Cha, S.D.; Kwon, K.Y. Spatholobus suberectus Dunn inhibits the growth of cervical cancer cell. Int. J. Gynecol. Cancer 2004, 14, 233. [Google Scholar] [CrossRef]
- Gulati, R.; Nandi, D.; Sarkar, K.; Venkataraman, P.; Ramkumar, K.M.; Ranjan, P.; Janardhanan, R. Exosomes as Theranostic Targets: Implications for the Clinical Prognosis of Aggressive Cancers. Front Mol. Biosci. 2022, 9, 890768. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, J.; Meng, X.; Li, T.; Wang, S.; Bao, Y. The Pharmacological Effects of Spatholobi caulis Tannin in Cervical Cancer and Its Precise Therapeutic Effect on Related circRNA. Mol. Ther. Oncolytics 2019, 14, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fu, Q.; He, W.; Sun, Y.K.; Wang, Y.Z.; Wang, X.M. Non-apoptotic programmed cell death induced by extract of Spatholobus suberctus in human lung cancer A549 cells. Zhongguo Zhong Yao Za Zhi 2008, 33, 2040–2044. [Google Scholar] [PubMed]
- Ha, E.S.; Lee, E.O.; Yoon, T.J.; Kim, J.H.; Park, J.O.; Lim, N.C.; Jung, S.K.; Yoon, B.S.; Kim, S.H. Methylene chloride fraction of Spatholobi caulis induces apoptosis via caspase dependent pathway in U937 cells. Biol. Pharm. Bull. 2004, 27, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tang, Q.; Luo, L.; Gao, Z.; Zhang, S. A preliminary study on the anticancer efficacy of Caulis spatholobi compound 1802. Pak. J. Pharm. Sci. 2018, 31, 1145–1150. [Google Scholar]
- Baek, S.J.; Kim, J.S.; Jackson, F.R.; Eling, T.E.; McEntee, M.F.; Lee, S.H. Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis 2004, 25, 2425–2432. [Google Scholar] [CrossRef]
- Lim, Y.C.; Lee, S.H.; Song, M.H.; Yamaguchi, K.; Yoon, J.H.; Choi, E.C.; Baek, S.J. Growth inhibition and apoptosis by (-)-epicatechin gallate are mediated by cyclin D1 suppression in head and neck squamous carcinoma cells. Eur. J. Cancer 2006, 42, 3260–3266. [Google Scholar] [CrossRef]
- Chen, H.M.; Wu, Y.C.; Chia, Y.C.; Chang, F.R.; Hsu, H.K.; Hsieh, Y.C.; Chen, C.C.; Yuan, S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009, 286, 161–171. [Google Scholar] [CrossRef]
- Phung, H.M.; Lee, H.; Lee, S.; Jang, D.; Kim, C.-E.; Kang, K.S.; Seo, C.-S.; Choi, Y.-K. Analysis and Anticancer Effects of Active Compounds from Spatholobi Caulis in Human Breast Cancer Cells. Processes 2020, 8, 1193. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Liu, P.; Chen, Q.; Situ, H.; Xie, T.; Zhang, J.; Peng, C.; Lin, Y.; Chen, J. MicroRNA-25 regulates chemoresistance-associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014, 5, 7013–7026. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; Du, Q.; Peng, C.; Wang, N.; Tang, H.; Xie, X.; Shen, J.; Chen, J. A Review: The Pharmacology of Isoliquiritigenin. Phytother. Res. 2015, 29, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Tang, H.; Du, J.; Chen, J.; Peng, C. Isoliquiritigenin Suppresses EMT-Induced Metastasis in Triple-Negative Breast Cancer through miR-200c/C-JUN/Catenin. Am. J. Chin. Med. 2021, 49, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Tang, H.; Liu, P.; Shen, J.; Guan, X.; Xie, X.; Gao, J.; Xiong, L.; Jia, L.; Chen, J.; et al. Isoliquiritigenin modulates miR-374a/PTEN/Akt axis to suppress breast cancer tumorigenesis and metastasis. Sci. Rep. 2017, 7, 9022. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Z.; Peng, C.; You, J.; Shen, J.; Han, S.; Chen, J. Dietary compound isoliquiritigenin targets GRP78 to chemosensitize breast cancer stem cells via β-catenin/ABCG2 signaling. Carcinogenesis 2014, 35, 2544–2554. [Google Scholar] [CrossRef]
- Tang, H.; Peng, F.; Huang, X.; Xie, X.; Chen, B.; Shen, J.; Gao, F.; You, J.; Xie, X.; Chen, J. Neoisoliquiritigenin Inhibits Tumor Progression by Targeting GRP78-β-catenin Signaling in Breast Cancer. Curr. Cancer Drug Targets 2018, 18, 390–399. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Z.; Wang, Y.; Xie, X.; Shen, J.; Peng, C.; You, J.; Peng, F.; Tang, H.; Guan, X.; et al. Dietary compound isoliquiritigenin prevents mammary carcinogenesis by inhibiting breast cancer stem cells through WIF1 demethylation. Oncotarget 2015, 6, 9854–9876. [Google Scholar] [CrossRef]
- Gao, F.; Zhang, J.; Fu, C.; Xie, X.; Peng, F.; You, J.; Tang, H.; Wang, Z.; Li, P.; Chen, J. iRGD-modified lipid-polymer hybrid nanoparticles loaded with isoliquiritigenin to enhance anti-breast cancer effect and tumor-targeting ability. Int. J. Nanomed. 2017, 12, 4147–4162. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Han, S.; Wang, N.; Mo, F.; Loo, T.Y.; Shen, J.; Huang, H.; Chen, J. Bioactivity-guided identification and cell signaling technology to delineate the lactate dehydrogenase a inhibition effects of Spatholobus suberectus on breast cancer. PLoS ONE 2013, 8, e56631. [Google Scholar] [CrossRef]
- Padmavathi, G.; Ramkumar, K.M. MicroRNA mediated regulation of the major redox homeostasis switch, Nrf2, and its impact on oxidative stress-induced ischemic/reperfusion injury. Arch. Biochem. Biophys. 2021, 698, 108725. [Google Scholar] [CrossRef]
- Xie, H.; Valera, V.A.; Merino, M.J.; Amato, A.M.; Signoretti, S.; Linehan, W.M.; Sukhatme, V.P.; Seth, P. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol. Cancer Ther. 2009, 8, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.Y.; Loo, T.Y.; Shen, J.G.; Wang, N.; Wang, D.M.; Yang, D.P.; Mo, S.L.; Guan, X.Y.; Chen, J.P. LDH-A silencing suppresses breast cancer tumorigenicity through induction of oxidative stress mediated mitochondrial pathway apoptosis. Breast Cancer Res. Treat. 2012, 131, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Suganya, N.; Bhakkiyalakshmi, E.; Subin, T.S.; Krishnamurthi, K.; Devi, S.S.; Lau, K.; Sekar, T.V.; Paulmurugan, R.; Ramkumar, K.M. Proteomic identification of pterostilbene-mediated anticancer activities in HepG2 cells. Chem. Res. Toxicol. 2014, 27, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, Q.; Guo, Y.; Yang, Q.; Yin, J.; Ran, Q.; Liu, L.; Zhao, Z.; Wang, Y.; Li, Y.; et al. Extract of Caulis spatholobi, a novel platelet inhibitor, efficiently suppresses metastasis of colorectal cancer by targeting tumor cell-induced platelet aggregation. Biomed. Pharm. 2020, 123, 109718. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.C.; Kim, S.A.; Song, G.Y.; Baek, N.I.; Park, Y.D.; Ryu, S.Y.; Saiki, I.; Kim, S.H. Effects of the ethyl acetate fraction of Spatholobi caulis on tumour cell aggregation and migration. Phytother. Res. 2003, 17, 163–167. [Google Scholar] [CrossRef]
- Chen, X.; Li, Q.; Kan, X.X.; Wang, Y.J.; Li, Y.J.; Yang, Q.; Xiao, H.B.; Chen, Y.; Weng, X.G.; Cai, W.Y.; et al. Extract of Caulis spatholobi, a novel blocker targeting tumor cell-induced platelet aggregation, inhibits breast cancer metastasis. Oncol. Rep. 2016, 36, 3215–3224. [Google Scholar] [CrossRef]
- Inami, K.; Asada, Y.; Harada, T.; Okayama, Y.; Usui, N.; Mochizuki, M. Antimutagenic components in Spatholobus suberectus Dunn against N-methyl-N-nitrosourea. Genes Environ. 2019, 41, 22. [Google Scholar] [CrossRef]
- Da, J.; Xu, M.; Wang, Y.; Li, W.; Lu, M.; Wang, Z. Kaempferol Promotes Apoptosis While Inhibiting Cell Proliferation via Androgen-Dependent Pathway and Suppressing Vasculogenic Mimicry and Invasion in Prostate Cancer. Anal. Cell. Pathol. 2019, 2019, 1907698. [Google Scholar] [CrossRef]
- Forbes, A.M.; Lin, H.; Meadows, G.G.; Meier, G.P. Synthesis and anticancer activity of new flavonoid analogs and inconsistencies in assays related to proliferation and viability measurements. Int. J. Oncol. 2014, 45, 831–842. [Google Scholar] [CrossRef]
- Pham, H.N.T.; Sakoff, J.A.; Vuong, Q.V.; Bowyer, M.C.; Scarlett, C.J. Comparative cytotoxic activity between kaempferol and gallic acid against various cancer cell lines. Data Brief 2018, 21, 1033–1036. [Google Scholar] [CrossRef]
- Qiu, W.; Lin, J.; Zhu, Y.; Zhang, J.; Zeng, L.; Su, M.; Tian, Y. Kaempferol Modulates DNA Methylation and Downregulates DNMT3B in Bladder Cancer. Cell. Physiol. Biochem. 2017, 41, 1325–1335. [Google Scholar] [CrossRef]
- Wu, P.; Meng, X.; Zheng, H.; Zeng, Q.; Chen, T.; Wang, W.; Zhang, X.; Su, J. Kaempferol Attenuates ROS-Induced Hemolysis and the Molecular Mechanism of Its Induction of Apoptosis on Bladder Cancer. Molecules 2018, 23, 2592. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, P.; Jiang, Z.; Chen, G.; Rivera, A.; Phasakda, A.; Zhang, Q.; Zheng, S.; Wang, G.; Chen, Q.H. Nitrogen-containing derivatives of O-tetramethylquercetin: Synthesis and biological profiles in prostate cancer cell models. Bioorg. Chem. 2019, 87, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.Q.; Venkateswaran, V.; Viswanathan, L.; Teahan, S.J.; Fleshner, N.E.; Klotz, L.H. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006, 9, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ma, X.; Li, Q.; Yang, Y.; Xu, X.; Sun, J.; Yu, M.; Cao, K.; Yang, L.; Yang, G.; et al. Anti-cancer effects of fisetin on mammary carcinoma cells via regulation of the PI3K/Akt/mTOR pathway: In vitro and in vivo studies. Int. J. Mol. Med. 2018, 42, 811–820. [Google Scholar] [CrossRef]
- Zhang, X.J.; Jia, S.S. Fisetin inhibits laryngeal carcinoma through regulation of AKT/NF-κB/mTOR and ERK1/2 signaling pathways. Biomed. Pharm. 2016, 83, 1164–1174. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, C.; Huang, H.; Yang, B.; Xiao, G.; Kong, D.; Tian, Q.; Song, Q.; Song, Y.; Tan, H.; et al. The Natural Compound Myricetin Effectively Represses the Malignant Progression of Prostate Cancer by Inhibiting PIM1 and Disrupting the PIM1/CXCR4 Interaction. Cell. Physiol. Biochem. 2018, 48, 1230–1244. [Google Scholar] [CrossRef]
- Li, J.; Qu, W.; Cheng, Y.; Sun, Y.; Jiang, Y.; Zou, T.; Wang, Z.; Xu, Y.; Zhao, H. The inhibitory effect of intravesical fisetin against bladder cancer by induction of p53 and down-regulation of NF-kappa B pathways in a rat bladder carcinogenesis model. Basic Clin. Pharmacol. Toxicol. 2014, 115, 321–329. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Ye, X.; Xue, S.; Shi, J.; Pan, J.; Chen, Q. Inhibition effects and induction of apoptosis of flavonoids on the prostate cancer cell line PC-3 in vitro. Food Chem. 2013, 138, 48–53. [Google Scholar] [CrossRef]
- Sun, F.; Zheng, X.Y.; Ye, J.; Wu, T.T.; Wang, J.; Chen, W. Potential anticancer activity of myricetin in human T24 bladder cancer cells both in vitro and in vivo. Nutr. Cancer 2012, 64, 599–606. [Google Scholar] [CrossRef]
- Dang, Q.; Song, W.; Xu, D.; Ma, Y.; Li, F.; Zeng, J.; Zhu, G.; Wang, X.; Chang, L.S.; He, D.; et al. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol. Carcinog. 2015, 54, 831–840. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Siddiqui, I.A.; Verma, A.K.; Mukhtar, H. Fisetin Enhances Chemotherapeutic Effect of Cabazitaxel against Human Prostate Cancer Cells. Mol. Cancer Ther. 2016, 15, 2863–2874. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.K.; Syed, D.N.; Khan, M.I.; Adhami, V.M.; Gong, Y.; Lucey, J.A.; Mukhtar, H. Dietary flavonoid fisetin increases abundance of high-molecular-mass hyaluronan conferring resistance to prostate oncogenesis. Carcinogenesis 2016, 37, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Lippman, S.M.; El-Naggar, A.K. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Saini, K.S.; Loi, S.; de Azambuja, E.; Metzger-Filho, O.; Saini, M.L.; Ignatiadis, M.; Dancey, J.E.; Piccart-Gebhart, M.J. Targeting the PI3K/AKT/mTOR and Raf/MEK/ERK pathways in the treatment of breast cancer. Cancer Treat. Rev. 2013, 39, 935–946. [Google Scholar] [CrossRef]
- Lu, H.; Guo, Y.; Gupta, G.; Tian, X. Mitogen-Activated Protein Kinase (MAPK): New Insights in Breast Cancer. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 51–59. [Google Scholar] [CrossRef]
- Chen, J.; Hou, R.; Zhang, X.; Ye, Y.; Wang, Y.; Tian, J. Calycosin suppresses breast cancer cell growth via ERβ-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. PLoS ONE 2014, 9, e91245. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Han, S.; Wang, D.; Mo, S.; Yu, L.; Huang, H.; Tsui, K.; Shen, J.; Chen, J. Dietary compound isoliquiritigenin inhibits breast cancer neoangiogenesis via VEGF/VEGFR-2 signaling pathway. PLoS ONE 2013, 8, e68566. [Google Scholar] [CrossRef]
- Ozbay, T.; Nahta, R. Delphinidin Inhibits HER2 and Erk1/2 Signaling and Suppresses Growth of HER2-Overexpressing and Triple Negative Breast Cancer Cell Lines. Breast Cancer 2011, 5, 143–154. [Google Scholar] [CrossRef]
- Farabegoli, F.; Govoni, M.; Spisni, E.; Papi, A. EGFR inhibition by (-)-epigallocatechin-3-gallate and IIF treatments reduces breast cancer cell invasion. Biosci. Rep. 2017, 37, BSR20170168. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, J.; Du, Y.; Ding, J.; Liu, J.Y. The green tea polyphenol EGCG potentiates the antiproliferative activity of sunitinib in human cancer cells. Tumour Biol. 2016, 37, 8555–8566. [Google Scholar] [CrossRef]
- Xin, M.; Wang, Y.; Ren, Q.; Guo, Y. Formononetin and metformin act synergistically to inhibit growth of MCF-7 breast cancer cells in vitro. Biomed. Pharmacother. 2019, 109, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhao, X.; Ye, Y.; Wang, Y.; Tian, J. Estrogen receptor beta-mediated proliferative inhibition and apoptosis in human breast cancer by calycosin and formononetin. Cell. Physiol. Biochem. 2013, 32, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Mo, B.; Hu, C.; Liu, H.; Qi, H.; Wang, X.; Xu, J. Genistein induces cell apoptosis in MDA-MB-231 breast cancer cells via the mitogen-activated protein kinase pathway. Toxicol. In Vitro 2008, 22, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, M.; Ono, M.; Higuchi, T.; Chen, C.; Hara, T.; Nakano, S. Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci. 2014, 105, 252–257. [Google Scholar] [CrossRef]
- Ponnusamy, L.; Kothandan, G.; Manoharan, R. Berberine and Emodin abrogates breast cancer growth and facilitates apoptosis through inactivation of SIK3-induced mTOR and Akt signaling pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165897. [Google Scholar] [CrossRef]
- Braal, C.L.; Hussaarts, K.; Seuren, L.; Oomen-de Hoop, E.; de Bruijn, P.; Buck, S.A.J.; Bos, M.; Thijs-Visser, M.F.; Zuetenhorst, H.J.M.; Mathijssen-van Stein, D.; et al. Influence of green tea consumption on endoxifen steady-state concentration in breast cancer patients treated with tamoxifen. Breast Cancer Res. Treat. 2020, 184, 107–113. [Google Scholar] [CrossRef]
- Shan, Y.; Cheng, Y.; Zhang, Y.; Guan, F.Q.; Sun, H.; Ren, X.C.; Chen, Y.; Feng, X.; Yang, J.M. Triticuside A, a dietary flavonoid, inhibits proliferation of human breast cancer cells via inducing apoptosis. Nutr. Cancer 2013, 65, 891–899. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; Prakasam, G.; Chattopadhyay, S.; Rehman, A.U.; Padder, R.A.; Ansari, M.A.; Irshad, R.; Mangalhara, K.; Bamezai, R.N.K.; Husain, M.; et al. Curcumin decreases Warburg effect in cancer cells by down-regulating pyruvate kinase M2 via mTOR-HIF1α inhibition. Sci. Rep. 2018, 8, 8323. [Google Scholar] [CrossRef]
- Ke, J.Y.; Banh, T.; Hsiao, Y.H.; Cole, R.M.; Straka, S.R.; Yee, L.D.; Belury, M.A. Citrus flavonoid naringenin reduces mammary tumor cell viability, adipose mass, and adipose inflammation in obese ovariectomized mice. Mol. Nutr. Food Res. 2017, 61, 1600934. [Google Scholar] [CrossRef]
- Serrano, M.L.; Sánchez-Gómez, M.; Bravo, M.M.; Yakar, S.; LeRoith, D. Differential expression of IGF-I and insulin receptor isoforms in HPV positive and negative human cervical cancer cell lines. Horm. Metab. Res. 2008, 40, 661–667. [Google Scholar] [CrossRef]
- López-Knowles, E.; O’Toole, S.A.; McNeil, C.M.; Millar, E.K.A.; Qiu, M.R.; Crea, P.; Daly, R.J.; Musgrove, E.A.; Sutherland, R.L. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int. J. Cancer 2010, 126, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Xu, L.; Ye, M.; Liao, M.; Du, H.; Chen, H. Formononetin inhibits migration and invasion of MDA-MB-231 and 4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through PI3K/AKT signaling pathways. Horm. Metab. Res. 2014, 46, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Hsu, L.S.; Lin, C.L.; Hong, H.M.; Pan, M.H.; Way, T.D.; Chen, W.J. 3,5,4′-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol. Appl. Pharmacol. 2013, 272, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ye, H.; Wang, Q.Q.; Li, W.T.; Yu, B.B.; Bai, Y.M.; Xu, G.H. Chinese Herbal Medicine for Chemotherapy-Induced Leukopenia: A Systematic Review and Meta-Analysis of High-Quality Randomized Controlled Trials. Front. Pharm. 2021, 12, 573500. [Google Scholar] [CrossRef]
- Wang, J.-L. Jiawei sancai fengsui tang treatment after chemotherapy leukopenia disease. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 248–250. [Google Scholar]
- Zou, J. Clinical study of sanhuang sanxian decoction on the treatment of advanced lung cancer patients with chemotherapy leukopenia. China J. Chin. Med. 2015, 30, 325–327. [Google Scholar]
- Li, J.; Tuo, T.; Zhang, C.; Wang, J.; Xu, W. Clinical efficacy and mechanism of bazhen decoction on leukocyte reduction induced by chemotherapy in lung cancer. World Chin. Med. 2020, 15, 94–98. [Google Scholar]
- Li, Z.-X.; Liu, X.-P. Chinese Medicinal Herb Decoction for Treating Thrombocytopenia after Radio-Chemotherapy. CN102335294B, 31 July 2013. [Google Scholar]
- Fan, Y.; Liu, J.; Miao, J.; Zhang, X.; Yan, Y.; Bai, L.; Chang, J.; Wang, Y.; Wang, L.; Bian, Y.; et al. Anti-inflammatory activity of the Tongmai Yangxin pill in the treatment of coronary heart disease is associated with estrogen receptor and NF-κB signaling pathway. J. Ethnopharmacol. 2021, 276, 114106. [Google Scholar] [CrossRef]
- Su, E.Y.; Fang, Y.H.; Chen, H.S. Clinical observation of treating 62 patients with severe aplastic anemia failing in immunosuppressive therapy by integrative medicine. Zhongguo Zhongxiyi Jiehe Zazhi Chin. J. Integr. Tradit. West. Med. 2012, 32, 1616–1620. [Google Scholar]
- Fleischer, T.; Chang, T.T.; Chiang, J.H.; Chang, C.M.; Hsieh, C.Y.; Yen, H.R. Adjunctive Chinese herbal medicine therapy improves survival of patients with chronic myeloid leukemia: A nationwide population-based cohort study. Cancer Med. 2016, 5, 640–648. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, J.; Li, B.; Yu, R.; Hu, Y.; Li, X.; Peng, G.; Zhang, M.; Zhu, M. Tongmai Yangxin intervening in myocardial remodeling after PCI for coronary heart disease: Study protocol for a double-blind, randomized controlled trial. Trials 2020, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-H.; Zhu, M.; Wang, D.-G.; Yang, Y.-S.; Tan, T.; Zhu, H.; He, J.-F. Yangxin Tongmai Formula ameliorates impaired glucose tolerance in children with Graves’ disease through upregulation of the insulin receptor levels. Acta Pharmacol. Sin. 2018, 39, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiaojin, H.; Jun, S.; Lin, Z.; Yiqun, Z. Effect of gubi Recipe in treating knee osteoarthritis with symptom of kidney deficiency and collateral obstruction. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 207–211. [Google Scholar]
- Ma, H.; Huang, K. Effects of Bushen Huoxue Recipe on Knee Osteoarthritis Patients with Kidney Deficiency and Blood Stasis Syndrome and Its Influence on Serum Cytokines. World Chin. Med. 2019, 14, 696–699. [Google Scholar]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharm. 2013, 38, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Li, Y.; Sarkar, F.H. The bounty of nature for changing the cancer landscape. Mol. Nutr. Food Res. 2016, 60, 1251–1263. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, S.B.; Wang, Y.C.; Li, X.Q.; Zheng, L.H.; Zhao, M.G. Neuroprotective effects of formononetin against NMDA-induced apoptosis in cortical neurons. Phytother. Res. 2013, 27, 1770–1775. [Google Scholar] [CrossRef]
- Kwon, K.R.; Alam, M.B.; Park, J.H.; Kim, T.H.; Lee, S.H. Attenuation of UVB-Induced Photo-Aging by Polyphenolic-Rich Spatholobus suberectus Stem Extract Via Modulation of MAPK/AP-1/MMPs Signaling in Human Keratinocytes. Nutrients 2019, 11, 1341. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, S.; Guo, W.; Xie, Z.; Zheng, Y.; Cao, Z.; Zhou, Y. Transcriptome analysis provides insights into the immune responsive pathways and genes in the head kidney of tiger grouper (Epinephelus fuscoguttatus) fed with Spatholobus suberectus, Phellodendron amurense, or Eclipta prostrata. Fish Shellfish Immunol. 2018, 73, 100–111. [Google Scholar] [CrossRef]
- Chen, D.H.; Luo, X.; Yu, M.Y.; Zhao, Y.Q.; Cheng, Y.F.; Yang, Z.R. Effect of Spatholobus suberectus on the bone marrow cells and related cytokines of mice. Zhongguo Zhong Yao Za Zhi 2004, 29, 352–355. [Google Scholar]
- Shao, Z. Spatholobus suberectus Stem Extract Improves the Protective Effect of Heparin on Cerulein-Induced Pancreatitis. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Class | Sub-Class | Chemical Name | Structure | References |
|---|---|---|---|---|
| Carotenoids | Apocarotenoids | Blumenol A |  | [33] |
| Coumestans | Coumestans | Medicagol |  | [34] |
| Flavanoids | Flavonols | Rutin |  | [28] |
| Flavanols | Epigallocatechin |  | [26,28,34] | |
| Procyanidin B2 |  | [26,28,35] | ||
| Epicatechin gallate |  | [34,36,37,38] | ||
| Methyl gallate |  | [34,36,37,38] | ||
| Ethyl gallate |  | [34,36,37,38] | ||
| 3,7-dihydroxy-6-methoxyflavonol |  | [34,36,37,38] | ||
| (-)-Catechin |  | [30] | ||
| (-)-Epiafzelechin |  | [30] | ||
| (-)-Epicatechin |  | [20,26,27,28,30,34,35,39] | ||
| (-)-Gallocatechin |  | [20,26,28,30,34,39] | ||
| (+)-Catechin |  | [35] | ||
| (+)-Gallocatechin |  | [35] | ||
| Flavanone | Suberectin |  | [18] | |
| Hesperetin |  | [34] | ||
| Naringenin |  | [26,28,33,34,35] | ||
| Flavonols | Kaempferol |  | [28] | |
| Isoflavonoids | Biochanin A |  | [34,36,37,38] | |
| 2′-Hydroxygenistein |  | [30] | ||
| 3′-Methoxydaidzein |  | [30] | ||
| 5,7-Dihydroxy-4′-Methoxyisoflavone |  | [26,28,30,34,35,40] | ||
| 7,2′,4′-Trihydroxy-8,3′-Dimethoxyisoflavan |  | [41] | ||
| 7,2′-Dihydroxy-4′,5′-Methylenedioxyisoflavan-4-Ol |  | [35] | ||
| 7,4′-Dihydroxy-8,2′,3′-Trimethoxyisoflavan |  | [41] | ||
| 7,4′-Dihydroxy-8-Methoxy-Isoflavone |  | [33] | ||
| Afrormosin |  | [34,35] | ||
| Butesuperin A |  | [30] | ||
| Calycosin |  | [18,26,27,28,30,34] | ||
| Daidzein |  | [18,26,27,28,30,34,35,42] | ||
| Daidzin |  | [28,30] | ||
| Dulcisflavan |  | [33] | ||
| Formononetin |  | [18,26,27,28,30,34,35,42] | ||
| Genistein |  | [26,27,28,30,34,35,40,42,43] | ||
| Genistin |  | [28,30,42] | ||
| Glycyroside |  | [33] | ||
| Isoliquiritigenin |  | [26,28,30,34,35,42] | ||
| Liquiritigenin |  | [26,28,30,33,34,35,42] | ||
| Maackiain |  | [30,40,43] | ||
| Medicarpin |  | [28,43] | ||
| Ononin |  | [20,26,28,30,34] | ||
| Prunetin |  | [26,27,28,34] | ||
| Pseudobaptigenin |  | [34,43] | ||
| Puerarin |  | [27] | ||
| Sativan |  | [30,35,43] | ||
| Chalcones | Butein |  | [34] | |
| Dihydroflavonols | Dihydroquercetin |  | [26,28] | |
| Flavanone | (2R,3R)-3,7-dihydroxyflavanone |  | [33] | |
| (2S)-7-hydroxy-6-methoxy-flavanone |  | [43] | ||
| (2S,3R)-3,7-dihydroxy-6-methoxy-flavanone |  | [35] | ||
| 7-hydroxyflavanone |  | [35] | ||
| Lignans | Lignans | (+)-Epipinoresinol |  | [30] |
| (+)-Medioresinol |  | [30,33] | ||
| (+)-Pinoresinol |  | [30] | ||
| (+)-Syringaresinol |  | [30] | ||
| Isolariciresinol |  | [30] | ||
| Prestegane B |  | [33] | ||
| Non-flavonoids | Pterocarpans | (6aR,11aR)-maackiain |  | [30] |
| (6aR,11aR)-medicarpin |  | [30] | ||
| Terpenoids | Lupeol |  | [33] | |
| Lupeone |  | [33] | ||
| Betulinic Acid |  | [19] | ||
| Glycyrrhizin |  | [27] | ||
| Steroids | β-sitosterol |  | [20] | |
| β-sitosterone |  | [19] | ||
| Daucosterol |  | [30] | ||
| Anthraquinones | Aloe-emodin |  | [30] | |
| Physcion (emodin-3-methyl ether) |  | [33] | ||
| Chrysophanol |  | [33] | ||
| Emodin |  | [20] | ||
| Rhein |  | [19] | ||
| Lactones | Angelicin |  | [30] | |
| n-butyl-O-β-D-fructopyranoside |  | [30] | ||
| Glycosides | 2-methoxy-4-(2′-ethoxyl)-phenol-1-O-β-D-glucopyranosi de |  | [33] | |
| 5-O-(β-apiosyl-(1→2)-O-β-xylopyranosyl)gentisic acid |  | [33] | ||
| 15-O-(α-rhamnopyranosyl)-aloe-emodin |  | [20] | ||
| 1-O-(β-apiosyl-(1→6)-O-β-glucopyranosyl)-3-O-methyl phloroglucinol |  | [19] | ||
| Other polyphenols | Furanocoumarins | 5,7-Dihydroxycoumarin |  | [30] |
| glyoxylic acid | Allantoin |  | [30] | |
| Phenethyl alcohol | Benzene ethanol |  | [33] | |
| Phenolic acids | Hydroxybenzoic acids | 3,4-dihydroxybenzoic acid |  | [34,36,37,38] |
| 3,4-dihydroxybenzaldehyde |  | [34,36,37,38] | ||
| β-glucogallin |  | [34,36,37,38] | ||
| p-hydroxy benzoic acid |  | [27,30] | ||
| Protocatechuic acid |  | [30] | ||
| Protocatechuic acid ethyl ester |  | [33] | ||
| Protocatechuic acid methyl ester |  | [30] | ||
| Syringic acid |  | [20] | ||
| Vanillic acid |  | [20] | ||
| Saponins | Phytosterol | Daucosterol |  | [20] |
| Active Constituents of SSD | Dose | Positive Control | Mechanism of Action | Activity | References |
|---|---|---|---|---|---|
| (3S)-7-hydroxy-8,2′,4′-trimethoxyisoflavane, (3S)-7-hydroxy-8,2′-dimethoxy-4′,5′-methylenedioxyisoflavane, (S)-sativan and maackiain. | 25.1–93.6 μM (IC50) | - | - | Anticancer activity | [40] |
| 7,2′,4′-trihydroxy-8,3′-dimethoxyisoflavan | >40 μM (IC50) | Paclitaxel, cisplatin | - | [41] | |
| 7,4′-dihydroxy-8,2′,3′-trimethoxyisoflavan | >19.11 μM (IC50) | Paclitaxel, cisplatin | - | [41] | |
| 7-Hydroxyflavanone | 5.21 μM | Epoximicin (IC50 = 65 nM) | 20S proteasome inhibition | [69] | |
| Aqueous extract | 1000 μg/mL | - | - | [96] | |
| 50–300 μg/mL | Docetaxel | G2/M checkpoint; Apoptosis induction; ROS induction | [94] | ||
| 50–300 μg/mL | - | BC cell apoptosis; G2/M phase arrest; ROS accumulation and inhibition of LDH-A | [118] | ||
| 0.07 μg/mL | - | - | [122] | ||
| 5–20 mg/plate | - | - | [126] | ||
| CHCl3-soluble and EtOAc-soluble fractions | 100 µg/mL | Epoximicin (IC50 = 65 nM) | 20S proteasome inhibition | [69] | |
| EGC | 60–100 μM | - | LDH-A, HIF-1α | [118] | |
| Ethanol (60% (v/v) in water) extracts | 10.89–52.58 μg/mL (IC50) | Docetaxel | ROS induced pyroptosis | [119] | |
| Ethanol extracts | 10–20 μg/mL | - | ROS induced apoptosis | [93] | |
| Ethyl acetate fraction of methanol extract | 7.5–15 mg/plate | - | - | [126] | |
| Formononetin | >1000 μg/plate | - | - | [126] | |
| Gallocatechin, catechin, epicatechin | - | - | - | [118] | |
| Genistein | 9.26 μM | Epoximicin (IC50 = 65 nM) | 20S proteasome inhibition | [69] | |
| Genistein and EGC | 12.5–100 μg/mL | - | ROS induced apoptosis | [93] | |
| Isoliquiritigenin | 4.88 μM | Epoximicin (IC50 = 65 nM) | 20S proteasome inhibition | [69] | |
| Isoliquiritigenin analogues | 0.71–7.9 μM (IC50) | Paclitaxel | - | [29] | |
| Liquiritigenin, daidzein, medicarpin, and formononetin | >100 μM | Epoximicin (IC50 = 65 nM) | 20S proteasome inhibition | [69] | |
| Medicarpin, isoliquiritigenin, genistein and naringenin | 100–500 μg/plate | - | Cell cycle inhibition; Antioxidant | [126] | |
| Methanol extract | 5–20 mg/plate | - | - | [126] | |
| Sativan | 10–100 μM | - | miR-200c/PD-L1 regulation; apoptosis induction | [46] | |
| Sub-column extracts from ethanol (80% (v/v) in water) extracts | 40–320 μg/mL | - | Reduced activity of ER and downregulation of PI3K/AKT and MAPK pathway | [27] | |
| Kaempferol | 28.8 ± 1.5 μM (with 1 nM dihydrotestosterone) | - | Inhibits the activation of androgen receptors | [127] | |
| 58.3 ± 3.5 μM (with 1 nM dihydrotestosterone) | - | Apoptosis induction | |||
| 38.35 ± 1.94 μM | Cell cycle inhibition; Antioxidant | [128] | |||
| 50 μM | Antioxidant, antimicrobial and cytotoxic activities | [129] | |||
| 78.4 μM (24 h), 38.1 μM (48 h) | Inhibition of DNA methylation | [130] | |||
| 54.7 μM | Promoted antioxidant enzymes; inhibited ROS generation and lipid peroxidation; inhibiting the function of phosphorylated AKT (p-AKT), CyclinD1, CDK4, Bid, Mcl-1, and Bcl-xL; promoted p-BRCA1, p-ATM, p53, p21, p38, Bax and Bid expression | [131] | |||
| Fisetin | 34.1 ± 7.7 μM | Docetaxel | ROS induced apoptosis | [132] | |
| 32.50 μM | - | Cell cycle arrest; antiproliferative effect | [133] | ||
| 20, 40 and 80 µM | - | Suppressed cell proliferation metastasis and invasiveness; induced the apoptosis | [134] | ||
| 10–100 μM | - | Suppressed cell proliferation by regulating PI3K/AKT/NF-κB | [135] | ||
| Myricetin | 94.48 μM | - | Inhibiting PIM1 and disrupting the PIM1/CXCR4 interaction | [136] | |
| 47.6 μM | - | Cell cycle inhibition; Antioxidant | [137] | ||
| 37.5–300 μM | - | Inhibition of cell proliferation | [138] | ||
| 10–100 μM | - | Promoted cell cycle arrest at G2/M, induced apoptosis, modulated Bcl-2 family proteins and activated caspase-3 | [139] |
| Active Constituents of SSD | Model | Doses and Route of Administration | Positive Control | Mechanism of Action | Activity | References |
|---|---|---|---|---|---|---|
| Aqueous extract | BALB/c Nude mice | 1 g/kg b.w., p.o. | Docetaxel | G2/M checkpoint; Apoptosis induction; ROS induction | Anti-cancer activity | [94] |
| BALB/c Nude mice | 1 g/kg b.w., p.o. | - | BC cell apoptosis; G2/M phase arrest; ROS accumulation and inhibition of LDH-A | [118] | ||
| EGC | BALB/c Nude mice | 20, 40 mg/kg b.w, i.p. | - | LDH-A, HIF-1α | [118] | |
| Ethanol (60% (v/v) in water) extracts | BALB/c Nude mice | 0.8 g/kg b.w, p.o. | Docetaxel | ROS-induced pyroptosis | [119] | |
| Sativan | BALB/c Nude mice | 25, 50 mg/kg b.w, i.p. daily for 4 weeks | - | miR-200c/PD-L1 regulation; Apoptosis induction | [46] | |
| Kaempferol | BALB/c nude mice | 50, 100, 150 mg/kg b.w. p.o daily for 4 weeks | - | Suppression of tumor growth and metastasis; modulating DNA methylation by inhibiting DNMT3B | [130,140] | |
| Fisetin | Athymic nude male mice; transgenic TRAMP mouse | 20, 40 mg/kg; 3 times/week for 7 weeks | - | Tumor growth Inhibition by decreasing proliferation; inducing apoptosis; metastasis inhibition; synthesis and degradation inhibition of hyaluronan, an enzyme involved in cancer progression; overall survival increase | [141,142] | |
| Myricetin | Wistar rats | 25 mg/kg b.w. every 2 days for 40 days | - | Tumor growth inhibition; upregulation of p53 and downregulation of NF-κB pathway | [137] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Ganesan, K.; Liu, Q.; Chen, J. A Review of the Pharmacological Potential of Spatholobus suberectus Dunn on Cancer. Cells 2022, 11, 2885. https://doi.org/10.3390/cells11182885
Zhang F, Ganesan K, Liu Q, Chen J. A Review of the Pharmacological Potential of Spatholobus suberectus Dunn on Cancer. Cells. 2022; 11(18):2885. https://doi.org/10.3390/cells11182885
Chicago/Turabian StyleZhang, Feng, Kumar Ganesan, Qingqing Liu, and Jianping Chen. 2022. "A Review of the Pharmacological Potential of Spatholobus suberectus Dunn on Cancer" Cells 11, no. 18: 2885. https://doi.org/10.3390/cells11182885
APA StyleZhang, F., Ganesan, K., Liu, Q., & Chen, J. (2022). A Review of the Pharmacological Potential of Spatholobus suberectus Dunn on Cancer. Cells, 11(18), 2885. https://doi.org/10.3390/cells11182885