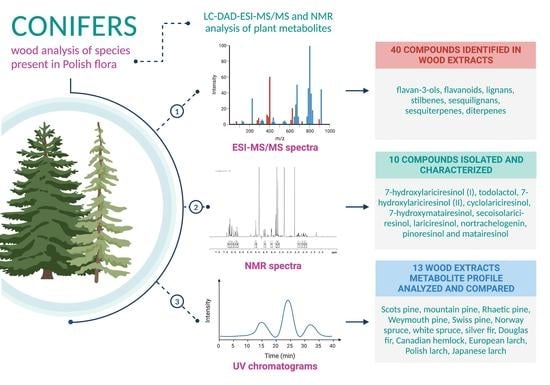
LC-DAD–ESI-MS/MS and NMR Analysis of Conifer Wood Specialized Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and General Experimental Procedures
2.2. Plant Material and Extract Preparation
2.3. Isolation of Lignans
2.4. Preparative Chromatography
2.5. General NMR Procedures
2.6. Phytochemical Characterization by LC-DAD–ESI-MS/MS Method
3. Results
3.1. Flavan-3-ols
3.2. Flavonoids
3.3. Lignans
3.4. Sesquilignans
3.5. Stilbenes
3.6. Sesquiterpenoids and Diterpenoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farjon, A. A Handbook of the World’s Conifers; Brill: Leiden, The Netherlands, 2010. [Google Scholar]
- Zajączkowski, G.J.M.; Jabłoński, T.; Szmidla, H.; Kowalska, A.; Małachowska, J.; Piwnicki, J. Raport o stanie lasów w Polsce 2020; Państwowe Gospodarstwo Leśne Lasy Państwowe: Warsaw, Poland, 2021. [Google Scholar]
- Farjon, A.; Filer, D. An Atlas of the World’s Conifers: An Analysis of their Distribution, Biogeography, Diversity and Conservation Status; Brill: Leiden, The Netherlands, 2013. [Google Scholar]
- Rosik-Dulewska, C. Podstawy Gospodarki Odpadami; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2015. [Google Scholar]
- Mantau, U. Wood flow analysis: Quantification of resource potentials, cascades and carbon effects. Biomass Bioenergy 2015, 79, 28–38. [Google Scholar] [CrossRef]
- Hassan, M.K.; Villa, A.; Kuittinen, S.; Jänis, J.; Pappinen, A. An assessment of side-stream generation from Finnish forest industry. J. Mater. Cycles Waste Manag. 2019, 21, 265–280. [Google Scholar] [CrossRef]
- Kumar, R.; Tsvetkov, D.E.; Varshney, V.K.; Nifantiev, N.E. Chemical constituents from temperate and subtropical trees with reference to knotwood. Ind. Crops Prod. 2020, 145, 112077. [Google Scholar] [CrossRef]
- Lunder, M.; Roskar, I.; Hosek, J.; Strukelj, B. Silver Fir (Abies alba) Extracts Inhibit Enzymes Involved in Blood Glucose Management and Protect against Oxidative Stress in High Glucose Environment. Plant. Foods Hum. Nutr 2019, 74, 47–53. [Google Scholar] [CrossRef]
- Huang, X.X.; Bai, M.; Zhou, L.; Lou, L.L.; Liu, Q.B.; Zhang, Y.; Li, L.Z.; Song, S.J. Food Byproducts as a New and Cheap Source of Bioactive Compounds: Lignans with Antioxidant and Anti-inflammatory Properties from Crataegus pinnatifida Seeds. J. Agric. Food Chem. 2015, 63, 7252–7260. [Google Scholar] [CrossRef]
- Sammartino, A.; Tommaselli, G.A.; Gargano, V.; di Carlo, C.; Attianese, W.; Nappi, C. Short-term effects of a combination of isoflavones, lignans and Cimicifuga racemosa on climacteric-related symptoms in postmenopausal women: A double-blind, randomized, placebo-controlled trial. Gynecol. Endocrinol. 2006, 22, 646–650. [Google Scholar] [CrossRef]
- Vo, Q.V.; Nam, P.C.; Bay, M.V.; Thong, N.M.; Cuong, N.D.; Mechler, A. Density functional theory study of the role of benzylic hydrogen atoms in the antioxidant properties of lignans. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Willför, S.; Reunanen, M.; Eklund, P.; Sjöholm, R.; Kronberg, L.; Fardim, P.; Pietarinen, S.; Holmbom, B. Oligolignans in Norway spruce and Scots pine knots and Norway spruce stemwood. Wood Res. Technol. Holzforschung 2004, 58, 345–354. [Google Scholar] [CrossRef]
- Cosentino, M.; Marino, F.; Maio, R.C.; Delle Canne, M.G.; Luzzani, M.; Paracchini, S.; Lecchini, S. Immunomodulatory activity of the lignan 7-hydroxymatairesinol potassium acetate (HMR/lignan) extracted from the heartwood of Norway spruce (Picea abies). Int. Immunopharmacol. 2010, 10, 339–343. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef]
- Biasiotto, G.; Zanella, I.; Predolini, F.; Archetti, I.; Cadei, M.; Monti, E.; Luzzani, M.; Pacchetti, B.; Mozzoni, P.; Andreoli, R.; et al. 7-Hydroxymatairesinol improves body weight, fat and sugar metabolism in C57BJ/6 mice on a high-fat diet. Br. J. Nutr. 2018, 120, 751–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nistor Baldea, L.A.; Martineau, L.C.; Benhaddou-Andaloussi, A.; Arnason, J.T.; Levy, E.; Haddad, P.S. Inhibition of intestinal glucose absorption by anti-diabetic medicinal plants derived from the James Bay Cree traditional pharmacopeia. J. Ethnopharmacol. 2010, 132, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Spoor, D.C.; Martineau, L.C.; Leduc, C.; Benhaddou-Andaloussi, A.; Meddah, B.; Harris, C.; Burt, A.; Fraser, M.H.; Coonishish, J.; Joly, E.; et al. Selected plant species from the Cree pharmacopoeia of northern Quebec possess anti-diabetic potential. Can. J. Physiol. Pharmacol. 2006, 84, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Harbilas, D.; Vallerand, D.; Brault, A.; Saleem, A.; Arnason, J.T.; Musallam, L.; Haddad, P.S. Larix laricina, an Antidiabetic Alternative Treatment from the Cree of Northern Quebec Pharmacopoeia, Decreases Glycemia and Improves Insulin Sensitivity In Vivo. Evid Based Complement. Alternat. Med. 2012, 2012, 296432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, N.; Guerrero-Analco, J.A.; Musallam, L.; Saleem, A.; Muhammad, A.; Walshe-Roussel, B.; Cuerrier, A.; Arnason, J.T.; Haddad, P.S. Adipogenic constituents from the bark of Larix laricina du Roi (K. Koch; Pinaceae), an important medicinal plant used traditionally by the Cree of Eeyou Istchee (Quebec, Canada) for the treatment of type 2 diabetes symptoms. J. Ethnopharmacol. 2012, 141, 1051–1057. [Google Scholar] [CrossRef]
- D’Andrea, G. Pycnogenol: A blend of procyanidins with multifaceted therapeutic applications? Fitoterapia 2010, 81, 724–736. [Google Scholar] [CrossRef]
- Shimada, T.; Tokuhara, D.; Tsubata, M.; Kamiya, T.; Kamiya-Sameshima, M.; Nagamine, R.; Takagaki, K.; Sai, Y.; Miyamoto, K.; Aburada, M. Flavangenol (pine bark extract) and its major component procyanidin B1 enhance fatty acid oxidation in fat-loaded models. Eur. J. Pharmacol. 2012, 677, 147–153. [Google Scholar] [CrossRef]
- Poussard, S.; Pires-Alves, A.; Diallo, R.; Dupuy, J.W.; Dargelos, E. A natural antioxidant pine bark extract, Oligopin®, regulates the stress chaperone HSPB1 in human skeletal muscle cells: A proteomics approach. Phytother. Res. 2013, 27, 1529–1535. [Google Scholar] [CrossRef]
- Benković, E.T.; Grohar, T.; Žigon, D.; Švajger, U.; Janeš, D.; Kreft, S.; Štrukelj, B. Chemical composition of the silver fir (Abies alba) bark extract Abigenol® and its antioxidant activity. Ind. Crops Prod. 2014, 52, 23–28. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application-A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- Hsieh, T.-C.; Wu, J.M. Unraveling and Trailblazing Cardioprotection by Resveratrol. In Resveratrol: State-of-the-Art Science and Health Applications; World Scientific: Singapore, 2019; pp. 1–28. [Google Scholar]
- Kim, H.; Seo, K.-H.; Yokoyama, W. Chemistry of Pterostilbene and Its Metabolic Effects. J. Agric. Food Chem. 2020, 68, 12836–12841. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, G.; Straniero, S.; Donati, A.; Bergamini, E. Resveratrol requires red wine polyphenols for optimum antioxidant activity. J. Nutr. Health Aging 2016, 20, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Suprun, A.R.; Dubrovina, A.S.; Aleynova, O.A.; Kiselev, K.V. The Bark of the Spruce Picea jezoensis Is a Rich Source of Stilbenes. Metabolites 2021, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Hovelstad, H.; Leirset, I.; Oyaas, K.; Fiksdahl, A. Screening Analyses of Pinosylvin Stilbenes, Resin Acids and Lignans in Norwegian Conifers. Molecules 2006, 11, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Strack, D.; Heilemann, J.; Wray, V.; Dirks, H. Structures and accumulation patterns of soluble and insoluble phenolics from norway spruce needles. Phytochemistry 1989, 28, 2071–2078. [Google Scholar] [CrossRef]
- Yeap Foo, L.; Karchesy, J.J. Procyanidin dimers and trimers from Douglas fir inner bark. Phytochemistry 1989, 28, 1743–1747. [Google Scholar] [CrossRef]
- Sut, S.; Baldan, V.; Faggian, M.; Ferrarese, I.; Maccari, E.; Teobaldo, E.; De Zordi, N.; Bertoni, P.; Peron, G.; Dall’Acqua, S. The Bark of Picea abies L., a Waste from Sawmill, as a Source of Valuable Compounds: Phytochemical Investigations and Isolation of a Novel Pimarane and a Stilbene Derivative. Plants 2021, 10, 2106. [Google Scholar] [CrossRef]
- Pietarinen, S.P.; Willför, S.M.; Ahotupa, M.O.; Hemming, J.E.; Holmbom, B.R. Knotwood and bark extracts: Strong antioxidants from waste materials. J. Wood Sci. 2006, 52, 436–444. [Google Scholar] [CrossRef]
- Willför, S.; Nisula, L.; Hemming, J.; Reunanen, M.; Holmbom, B. Bioactive phenolic substances in industrially important tree species. Part 2: Knots and stemwood of fir species. Holzforschung 2004, 58, 650–659. [Google Scholar] [CrossRef]
- Dellus, V.; Mila, I.; Scalbert, A.; Menard, C.; Michon, V.; Herve du Penhoat, C.L.M. Douglas-fir polyphenols and heartwood formation. Phytochemistry 1997, 45, 1573–1578. [Google Scholar] [CrossRef]
- Ivanova, S.Z.; Babkin, V.A. Polyphenolic compounds from Larix gmelinii phloem. Chem. Nat. Compd. 2011, 47, 124–125. [Google Scholar] [CrossRef]
- Wu, L.; Li, Y.-L.; Li, S.-M.; Yang, X.-W.; Xia, J.-H.; Zhou, L.; Zhang, W.-D. Systematic Phytochemical Investigation of Abies spectabilis. Chem. Pharm. Bull. 2010, 58, 1646–1649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yashunsky, D.V.; Men’shov, V.M.; Tsvetkov, D.E.; Tsvetkov, Y.E.; Bel’ko, A.A.; Vasiyarov, G.G.; Titova, E.V.; Pimenov, A.V.; Onuchin, A.A.; Dokichev, V.A.; et al. Analysis of content of (–)-secoisolariciresinol and related polyphenols in different morphological parts and anatomical structures of larch wood from Siberia. Russ. Chem. Bull. 2014, 63, 2571–2576. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Holmbom, B. Phenolic and Lipophilic Extractives in Scots Pine Knots and Stemwood. Wood Res. Technol. Holzforschung 2003, 57, 359–372. [Google Scholar] [CrossRef]
- Fang, J.-M.; Su, W.-C.; Cheng, Y.-S. Flavonoids and stilbenes from armand pine. Phytochemistry 1988, 27, 1395–1397. [Google Scholar] [CrossRef]
- Jaiswal, R.; Jayasinghe, L.; Kuhnert, N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS. J. Mass Spectrom. 2012, 47, 502–515. [Google Scholar] [CrossRef]
- Shen, Z.; Haslam, E.; Falshaw, C.P.; Begley, M.J. Procyanidins and polyphenols of Larix gmelini bark. Phytochemistry 1986, 25, 2629–2635. [Google Scholar] [CrossRef]
- Mbakidi-Ngouaby, H.; Pinault, E.; Gloaguen, V.; Costa, G.; Sol, V.; Millot, M.; Mambu, L. Profiling and seasonal variation of chemical constituents from Pseudotsuga menziesii wood. Industrial Crops Prod. 2018, 117, 34–49. [Google Scholar] [CrossRef]
- Razgonova, M.; Zakharenko, A.; Pikula, K.; Manakov, Y.; Ercisli, S.; Derbush, I.; Kislin, E.; Seryodkin, I.; Sabitov, A.; Kalenik, T.; et al. LC-MS/MS Screening of Phenolic Compounds in Wild and Cultivated Grapes Vitis amurensis Rupr. Molecules 2021, 26, 3650. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.; Obaid, W.A.; Alamoudi, M.O.; Mohammedsaleh, Z.M.; Annaz, H.; Abdelfattah, M.A.O.; Sobeh, M. Thymus fontanesii attenuates CCl4-induced oxidative stress and inflammation in mild liver fibrosis. Biomed. Pharmacother. 2022, 148, 112738. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Samiotaki, M.; Panayotou, G.; Oreopoulou, V. Characterization of Flavonoid Subgroups and Hydroxy Substitution by HPLC-MS/MS. Molecules 2007, 12, 593–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibi Sadeer, N.; Sinan, K.I.; Cziáky, Z.; Jekő, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; AlDhaheri, Y.; Eid, A.H.; Mahomoodally, M.F. Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.—A Traditionally Used Medicinal Halophyte. Molecules 2022, 27, 2000. [Google Scholar] [CrossRef] [PubMed]
- Koulis, G.A.; Tsagkaris, A.S.; Aalizadeh, R.; Dasenaki, M.E.; Panagopoulou, E.I.; Drivelos, S.; Halagarda, M.; Georgiou, C.A.; Proestos, C.; Thomaidis, N.S. Honey Phenolic Compound Profiling and Authenticity Assessment Using HRMS Targeted and Untargeted Metabolomics. Molecules 2021, 26, 2769. [Google Scholar] [CrossRef] [PubMed]
- Parisi, V.; Vassallo, A.; Pisano, C.; Signorino, G.; Cardile, F.; Sorrentino, M.; Colelli, F.; Fucci, A.; D’Andrea, E.L.; De Tommasi, N.; et al. A Herbal Mixture from Propolis, Pomegranate, and Grape Pomace Endowed with Anti-Inflammatory Activity in an In Vivo Rheumatoid Arthritis Model. Molecules 2020, 25, 2255. [Google Scholar] [CrossRef]
- Wu, H.; Cao, Y.; Qu, Y.; Li, T.; Wang, J.; Yang, Y.; Zhang, C.; Sun, Y. Integrating UPLC-QE-Orbitrap-MS technology and network pharmacological method to reveal the mechanism of Bailemian capsule to relieve insomnia. Nat. Prod. Res. 2022, 36, 2554–2558. [Google Scholar] [CrossRef]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and Comprehensive Evaluation of (Poly)phenolic Compounds in Pomegranate (Punica granatum L.) Juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef] [Green Version]
- Mercolini, L.; Protti, M.; Saracino, M.A.; Mandrone, M.; Antognoni, F.; Poli, F. Analytical Profiling of Bioactive Phenolic Compounds in Argan (Argania spinosa) Leaves by Combined Microextraction by Packed Sorbent (MEPS) and LC-DAD-MS/MS. Phytochem. Anal. 2016, 27, 41–49. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Cluzet, S.; Palos Pinto, A.; Dufour, M.-C.; Corio-Costet, M.-F.; Mérillon, J.-M. Pinus pinaster Knot: A Source of Polyphenols against Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 8884–8891. [Google Scholar] [CrossRef]
- Pompermaier, L.; Heiss, E.H.; Alilou, M.; Mayr, F.; Monizi, M.; Lautenschlaeger, T.; Schuster, D.; Schwaiger, S.; Stuppner, H. Dihydrochalcone Glucosides from the Subaerial Parts of Thonningia sanguinea and Their in Vitro PTP1B Inhibitory Activities. J. Nat. Prod. 2018, 81, 2091–2100. [Google Scholar] [CrossRef]
- Ahmed, A.; Li, W.; Zhang, J.S.; Sam, P.H.; Zou, Y.H.; Tang, G.H.; Yin, S. A new bisabolane sesquiterpenoid and a new abietane diterpenoid from Cephalotaxus sinensis. Nat. Prod. Res. 2018, 32, 175–181. [Google Scholar] [CrossRef]
- Sefkow, M. Enantioselective Synthesis of (−)-Wikstromol Using a New Approach via Malic Acid. J. Org. Chem. 2001, 66, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.A.; Smeds, A.I.; Sjoholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2008, 43, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Voronin, K.S.; Fenin, A.A.; Zhevlakova, A.K.; Zavadskii, S.P.; Selivanova, I.A. Polyphenolic Profile of Larch Knotwood. Pharm. Chem. J. 2021, 55, 781–786. [Google Scholar] [CrossRef]
- Stella, L.; De Rosso, M.; Panighel, A.; Vedova, A.D.; Flamini, R.; Traldi, P. Collisionally induced fragmentation of [M-H](-) species of resveratrol and piceatannol investigated by deuterium labelling and accurate mass measurements. Rapid Commun. Mass Spectrom. 2008, 22, 3867–3872. [Google Scholar] [CrossRef]
- Flamini, R.; De Rosso, M.; De Marchi, F.; Dalla Vedova, A.; Panighel, A.; Gardiman, M.; Maoz, I.; Bavaresco, L. An innovative approach to grape metabolomics: Stilbene profiling by suspect screening analysis. Metabolomics 2013, 9, 1243–1253. [Google Scholar] [CrossRef]
- Buiarelli, F.; Coccioli, F.; Jasionowska, R.; Merolle, M.; Terracciano, A. Analysis of some stilbenes in Italian wines by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 2955–2964. [Google Scholar] [CrossRef]
- Yeo, S.C.M.; Luo, W.; Wu, J.; Ho, P.C.; Lin, H.-S. Quantification of pinosylvin in rat plasma by liquid chromatography–tandem mass spectrometry: Application to a pre-clinical pharmacokinetic study. J. Chromatogr. B 2013, 931, 68–74. [Google Scholar] [CrossRef]
- Manville, J.F. Juvabione and its Analogs. Juvabione and Δ4′-Dehydrojuvabione Isolated from the Whole Wood of Abiesbalsamea, have the R,R Stereoconfigurations, not the R,S. Can. J. Chem. 1975, 53, 1579–1585. [Google Scholar] [CrossRef] [Green Version]
- Brunswick, P.; Blajkevitch, O.; Chow, L.; MacInnis, C.; van Aggelen, G.; Kim, M.; Shang, D. Trace analysis of resin acids in surface waters by direct injection liquid chromatography time of flight mass spectrometry and triple quadrupole mass spectrometry. J. Chromatogr. A 2021, 1656, 462558. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Ruttkies, C.; Krauss, M.; Brouard, C.; Kind, T.; Dührkop, K.; Allen, F.; Vaniya, A.; Verdegem, D.; Böcker, S.; et al. Critical Assessment of Small Molecule Identification 2016: Automated methods. J. Cheminformatics 2017, 9, 22. [Google Scholar] [CrossRef]
- Willför, S.; Nisula, L.; Hemming, J.; Reunanen, M.; Holmbom, B. Bioactive phenolic substances in industrially important tree species. Part 1: Knots and stemwood of different spruce species. Wood Res. Technol. Holzforschung 2004, 58, 335–344. [Google Scholar] [CrossRef]
- Willför, S.; Hemming, J.; Reunanen, M.; Eckerman, C.; Holmbom, B. Lignans and Lipophilic Extractives in Norway Spruce Knots and Stemwood. Wood Res. Technol. Holzforschung 2003, 57, 27–36. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Toledo, E.; Delgado-Rodriguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmbom, B.; Eckerman, C.; Eklund, P.; Hemming, J.; Nisula, L.; Reunanen, M.; Sjöholm, R.; Sundberg, A.; Sundberg, K.; Willför, S. Knots in trees–A new rich source of lignans. Phytochem. Rev. 2003, 2, 331–340. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Grigorchuk, V.P.; Ogneva, Z.V.; Suprun, A.R.; Dubrovina, A.S. Stilbene biosynthesis in the needles of spruce Picea jezoensis. Phytochemistry 2016, 131, 57–67. [Google Scholar] [CrossRef]
| Compounds | Retention Time [min] | UVmax [nm] | ESI 1 | MS1 [m/z] | MS2 [m/z] | Group | Presence in Pinaceae spp. |
|---|---|---|---|---|---|---|---|
| 1.gallocatechin | 3.0 | 199, 277 | [M−H]− | 305 | 219, 179, 137 | flavan-3-ol | [30] |
| 2.dimeric procyanidin B (I) | 3.3 | 230, 282 | [M−H]− | 577 | 559, 451, 407, 289 | flavan-3-ol | [31] |
| 3.dimeric procyanidin B (II) | 3.6 | 207, 238, 280 | [M−H]− | 577 | 559, 451, 407, 289 | flavan-3-ol | [31] |
| 4.trimeric procyanidin B | 4.2 | 199, 281 | [M−H]− | 865 | 695, 577, 407, 289 | flavan-3-ol | [32] |
| 5.catechin 2 | 4.5 | 232, 278 | [M−H]− | 289 | 245, 203, 179 | flavan-3-ol | [33] |
| 6.dimeric procyanidin B (III) | 5.2 | 238, 283 | [M−H]− | 577 | 559, 451, 407, 289 | flavan-3-ol | [31] |
| 7.epi-catechin 2 | 6.6 | 279 | [M−H]− | 289 | 245, 203, 179 | flavan-3-ol | [32] |
| 8.dihydromyricetin | 7.7 | 213, 225, 290 | [M−H]− | 319 | 301, 193, 125 | flavonoid | [33] |
| 9.7-hydroxylariciresinol (I) 3 | 7.8 | 199, 225, 279 | [M−H]− | 375 | 357, 345, 327, 297 | lignan | [34] |
| 10.trans-astringin | 8.1 | 192, 218, 324 | [M−H]− | 405 | 243, 225, 201, 173, 159 | stilbene | [33] |
| 11.dihydrokaempferol | 8.4 | 290 | [M−H]− | 287 | 259, 243, 181 | flavonoid | [34] |
| 12.todolactol 2 | 9.9 | 196, 225, 280 | [M−H]− | 375 | 327, 191, 176 | lignan | [33] |
| 13.taxifolin glucoside | 10.2 | 196, 287 | [M−H]− | 465 | 447, 437, 303, 285, 259 | flavonoid | [35] |
| 14.cis-astringin | 10.8 | 213, 319 | [M−H]− | 405 | 243, 225, 201, 173, 159 | stilbene | [28] |
| 15.dimeric procyanidin B (IV) | 11.6 | 243, 280 | [M−H]− | 577 | 559, 451, 407, 289 | flavan-3-ol | [31] |
| 16.7-hydroxylariciresinol (II) 3 | 11.6 | 198, 227, 279 | [M−H+HCOOH]− | 421 | 375, 357, 345, 325 | lignan | [34] |
| 17.piceid | 11.7 | 216, 319 | [M−H]− | 389 | 227, 185, 183, 157, 143 | stilbene | [33] |
| 18.taxifolin 2 | 12.4 | 280 | [M−H]− | 303 | 285, 177 | flavonoid | [34] |
| 19.isorhapontin | 12.5 | 192, 218, 325 | [M−H+HCOOH]− | 465 | 419, 257, 242, 241 | stilbene | [33] |
| 20.cyclolariciresinol 3 | 12.7 | 197, 283 | [M−H]− | 359 | 344, 313, 189 | lignan | [34] |
| 21.α-conidendric acid | 14.0 | 200, 225, 280 | [M−H+HCOOH]− | 419 | 373, 177 | lignan | [34] |
| 22.7-hydroxymatairesinol 3 | 15.0 | 197, 226, 280 | [M−H]− | 373 | 355, 340, 311, 293, 219 | lignan | [34] |
| 23.Secoisolariciresinol 3 | 15.3 | 232, 281 | [M−H]− | 361 | 346, 313, 299 | lignan | [34] |
| 24.eriodictyol | 15.4 | 194, 287 | [M−H]− | 287 | 259, 243, 151 | flavonoid | [36] |
| 25.myricetin 2 | 15.7 | 254, 373 | [M−H]− | 317 | 289, 179, 151, 137 | flavonoid | [37] |
| 26.secoisolariciresinol guaiacylglyceryl ether | 15.7 | 197, 280 | [M−H]− | 557 | 539, 525, 521, 509, 415, 361 | sesquilignan | [34] |
| 27.lariciresinol 3 | 16.1 | 197, 200, 202, 280 | [M−H+HCOOH]− | 405 | 359, 329 | lignan | [34] |
| 28.lariciresinol guaiacylglyceryl ether | 16.7 | 201, 225, 280 | [M−H]− | 555 | 525, 507, 359, 329, 315, 195, 165 | sesquilignan | [34] |
| 29.nortrachelogenin 3 | 18.3 | 199, 225, 280 | [M−H]− | 373 | 355, 327, 311, 249, 223, 191, 147 | lignan | [34] |
| 30.pinoresinol 3 | 19.1 | 201, 280 | [M−H]− | 357 | 342, 327, 311, 151, 136 | lignan | [34] |
| 31.quercetin 2 | 19.8 | 208, 368 | [M−H]− | 301 | 273, 179, 151 | flavonoid | [38] |
| 32.matairesinol 3 | 21.6 | 197, 281 | [M−H]− | 357 | 342, 313, 298, 281, 209, 191, 147 | lignan | [34] |
| 33.pinobanksin | 22.0 | 214, 284 | [M−H]− | 271 | 253 | flavonoid | [33] |
| 34.pinosylvin | 26.3 | 223, 299 | [M−H]− | 211 | 169 | stilbene | [39] |
| 35.pinocembrin | 30.3 | 214, 288 | [M−H]− | 255 | 213, 211 | flavonoid | [39] |
| 36.pinobanksin 3-O-acetate | 30.5 | 216, 292 | [M−H]− | 313 | 271, 253 | flavonoid | [40] |
| 37.pinosylvin monomethyl ether | 33.6 | 212, 223, 300 | [M−H]− | 225 | 210 | stilbene | [39] |
| 38.dehydrojuvabione | 41.0 | 224, 300 | [M+H]+ | 265 | 251, 233, 205, 187, 176 | sesquiterpene | [34] |
| 39.neoabietic acid | 52.0 | 251 | [M+H]+ | 303 | 257, 219, 179, 151, 123 | diterpene | [39] |
| 40.abietic acid 2 | 52.3 | 241 | [M+H]+ | 303 | 285, 257, 123 | diterpene | [39] |
| Compounds | Abies alba | Pinus sylvestris | Pinus mugo | Pinus cembra | Pinus strobus | Pinus ×rhaetica | Larix decidua | Larix polonica | Larix kaempferi | Pseudotsuga menziesii | Tsuga canadensis | Picea abies | Picea glauca |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.gallocatechin | + | + | + | + | + | + | + | + | + | − | − | − | − |
| 2.dimeric procyanidin B (I) | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 3.dimeric procyanidin B (II) | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 4.trimeric procyanidin B | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 5.catechin | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 6.dimeric procyanidin B (III) | − | − | − | − | − | − | + | + | + | + | − | + | − |
| 7.epi-catechin | + | − | − | + | + | − | + | + | + | + | + | − | − |
| 8.dihydromyricetin | − | − | + | − | − | + | + | + | − | − | − | − | − |
| 9.7-hydroxylariciresinol (I) | + | − | − | − | − | − | − | − | − | − | − | + | + |
| 10.trans-astringin | − | − | − | − | − | − | − | − | − | − | − | + | + |
| 11.dihydrokaempferol | − | − | − | − | − | − | + | + | − | − | − | − | − |
| 12.todolactol | + | − | − | − | − | − | + | − | + | + | + | + | + |
| 13.taxifolin hexoside | − | + | + | + | + | + | − | + | + | + | − | + | − |
| 14.cis-astringin | − | − | − | − | − | − | + | + | + | − | − | + | + |
| 15.dimeric procyanidin B (IV) | − | − | − | − | − | − | + | + | + | − | − | − | − |
| 16.7-hydroxylariciresinol (II) | + | − | + | − | + | − | + | − | + | + | + | − | − |
| 17.piceid | − | − | − | − | − | − | − | − | − | − | − | + | + |
| 18.taxifolin | − | + | + | + | + | + | + | + | + | + | − | + | + |
| 19.isorhapontin | − | − | − | − | − | − | − | − | − | − | − | + | + |
| 20.cyclolariciresinol | + | − | − | − | − | − | − | − | − | − | − | − | − |
| 21.α-conidendric acid | + | − | + | − | + | − | + | + | + | − | + | − | − |
| 22.7-hydroxymatairesinol | + | − | + | − | + | − | + | + | + | + | + | + | + |
| 23.secoisolariciresinol | + | − | + | − | + | − | + | + | + | + | + | + | + |
| 24.eriodictyol | − | − | + | + | + | − | + | + | + | + | − | − | − |
| 25.myricetin | − | − | + | − | − | − | + | + | + | + | − | − | − |
| 26.secoisolariciresinol guaiacylglyceryl ether | + | − | + | − | − | − | + | + | + | + | − | − | − |
| 27.lariciresinol | + | − | − | − | + | − | + | + | + | + | + | − | + |
| 28.lariciresinol guaiacylglyceryl ether | + | − | − | − | − | − | − | − | − | − | − | − | − |
| 29.nortrachelogenin | + | + | + | − | + | − | + | + | + | + | + | + | + |
| 30.pinoresinol | − | + | − | − | − | − | − | − | − | − | − | − | − |
| 31.quercetin | − | − | − | − | + | − | + | + | + | + | − | − | − |
| 32.matairesinol | − | + | + | + | + | + | + | + | + | − | + | + | + |
| 33.pinobanksin | − | − | + | + | + | − | + | + | + | + | − | − | + |
| 34.pinosylvin | − | + | + | − | + | − | − | − | − | − | − | − | − |
| 35.pinocembrin | − | + | + | − | + | + | − | − | − | + | − | − | − |
| 36.pinobanksin 3-O-acetate | − | − | − | − | + | − | − | − | − | − | − | − | − |
| 37.pinosylvin monomethyl ether | − | + | + | + | + | − | − | − | − | − | − | − | − |
| 38.dehydrojuvabione | + | − | − | − | − | − | − | − | − | − | − | − | − |
| 39.neoabietic acid | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 40.abietic acid | + | + | + | + | + | + | + | + | + | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patyra, A.; Dudek, M.K.; Kiss, A.K. LC-DAD–ESI-MS/MS and NMR Analysis of Conifer Wood Specialized Metabolites. Cells 2022, 11, 3332. https://doi.org/10.3390/cells11203332
Patyra A, Dudek MK, Kiss AK. LC-DAD–ESI-MS/MS and NMR Analysis of Conifer Wood Specialized Metabolites. Cells. 2022; 11(20):3332. https://doi.org/10.3390/cells11203332
Chicago/Turabian StylePatyra, Andrzej, Marta Katarzyna Dudek, and Anna Karolina Kiss. 2022. "LC-DAD–ESI-MS/MS and NMR Analysis of Conifer Wood Specialized Metabolites" Cells 11, no. 20: 3332. https://doi.org/10.3390/cells11203332
APA StylePatyra, A., Dudek, M. K., & Kiss, A. K. (2022). LC-DAD–ESI-MS/MS and NMR Analysis of Conifer Wood Specialized Metabolites. Cells, 11(20), 3332. https://doi.org/10.3390/cells11203332