LEAP-2 Counteracts Ghrelin-Induced Food Intake in a Nutrient, Growth Hormone and Age Independent Manner
Abstract
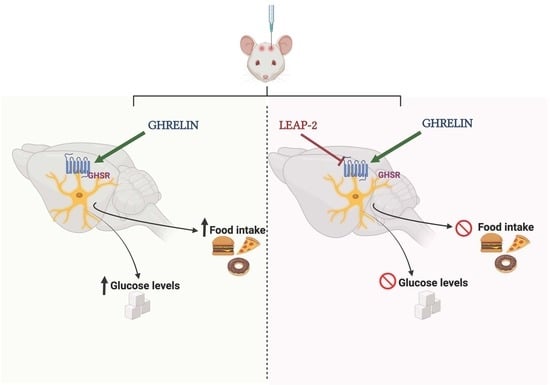
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Effects of Intracerebroventricular (ICV) LEAP-2, Ghrelin and NPY Administration on Food Intake in Rats and Mice
2.3. Conditioned Taste Aversion
2.4. Glucose Levels Measurements
2.5. Hormonal and Biochemical Assays
2.6. Statistical Analysis
3. Results
3.1. LEAP-2 Decreased Food Intake in a Dose-Dependent Manner and It Is Not Associated with Discomfort
3.2. Circulating LEAP-2 Are Modulated by Nutrient Availability and LEAP-2 Decreased Intake and Blood Glucose Levels after Fasting-Induced Refeeding
3.3. Central LEAP-2 Antagonizes Ghrelin-Induced Food Intake in a Leptin- and Other Orexigenic Signals-Independent Way
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tschop, M.H.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef]
- Wren, A.M.; Seal, L.J.; Cohen, M.A.; Brynes, A.E.; Frost, G.S.; Murphy, K.G.; Dhillo, W.S.; Ghatei, M.A.; Bloom, S.R. Ghrelin enhances appetite and increases food intake in humans. J. Clin. Endocrinol. Metabol. 2001, 86, 5992. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Guillén, L.; Barrios, V.; Campos-Barros, A.; Argente, J. Ghrelin levels in obesity and anorexia nervosa: Effect of weight reduction or recuperation. J. Pediatr. 2004, 144, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Clement, K.; Purnell, J.Q.; Vaisse, C.; Foster, K.E.; Frayo, R.S.; Schwartz, M.W.; Basdevant, A.; Weigle, D.S. Elevated plasma ghrelin levels in Prader–Willi syndrome. Nat. Med. 2002, 8, 643–644. [Google Scholar] [CrossRef] [PubMed]
- DelParigi, A.; Tschöp, M.; Heiman, M.L.; Salbe, A.D.; Vozarova, B.; Sell, S.M.; Bunt, J.C.; Tataranni, P.A. High Circulating Ghrelin: A Potential Cause for Hyperphagia and Obesity in Prader-Willi Syndrome. J. Clin. Endocrinol. Metab. 2002, 87, 5461–5464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Butte, N.F.; Garcia, J.M.; Smith, R.G. Characterization of Adult Ghrelin and Ghrelin Receptor Knockout Mice under Positive and Negative Energy Balance. Endocrinology 2007, 149, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigman, J.M.; Nakano, Y.; Coppari, R.; Balthasar, N.; Marcus, J.N.; Lee, C.E.; Jones, J.E.; Deysher, A.E.; Waxman, A.R.; White, R.D.; et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Investig. 2005, 115, 3564–3572. [Google Scholar] [CrossRef]
- Al Massadi, O.; López, M.; Tschöp, M.; Diéguez, C.; Nogueiras, R. Current Understanding of the Hypothalamic Ghrelin Pathways Inducing Appetite and Adiposity. Trends Neurosci. 2017, 40, 167–180. [Google Scholar] [CrossRef]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef]
- Mani, B.K.; Shankar, K.; Zigman, J.M. Ghrelin’s Relationship to Blood Glucose. Endocrinology 2019, 160, 1247–1261. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Asnicar, M.; Smith, R.G. Central and Peripheral Roles of Ghrelin on Glucose Homeostasis. Neuroendocrinology 2007, 86, 215–228. [Google Scholar] [CrossRef]
- Diéguez, C.; Vazquez, M.J.; Romero-Picó, A.; López, M.; Nogueiras, R. Hypothalamic Control of Lipid Metabolism: Focus on Leptin, Ghrelin and Melanocortins. Neuroendocrinology 2011, 94, 1–11. [Google Scholar] [CrossRef]
- Al-Massadi, O.; Tschöp, M.; Tong, J. Ghrelin acylation and metabolic control. Peptides 2011, 32, 2301–2308. [Google Scholar] [CrossRef]
- Albarrán-Zeckler, R.G.; Smith, R.G. The Ghrelin Receptors (GHS-R1a and GHS-R1b). Endocr. Dev. 2013, 25, 5–15. [Google Scholar] [CrossRef]
- Ge, X.; Yang, H.; Bednarek, M.A.; Galon-Tilleman, H.; Chen, P.; Chen, M.; Lichtman, J.S.; Wang, Y.; Dalmas, O.; Yin, Y.; et al. LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab. 2018, 27, 461–469. [Google Scholar] [CrossRef] [Green Version]
- M’Kadmi, C.; Cabral, A.; Barrile, F.; Giribaldi, J.; Cantel, S.; Damian, M.; Mary, S.; Denoyelle, S.; Dutertre, S.; Péraldi-Roux, S.; et al. N-Terminal Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Region Exhibits Inverse Agonist Activity toward the Ghrelin Receptor. J. Med. Chem. 2018, 62, 965–973. [Google Scholar] [CrossRef]
- Islam, M.N.; Mita, Y.; Maruyama, K.; Tanida, R.; Zhang, W.; Sakoda, H.; Nakazato, M. Liver-expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J. Endocrinol. 2020, 244, 13–23. [Google Scholar] [CrossRef]
- Shankar, K.; Metzger, N.P.; Singh, O.; Mani, B.K.; Osborne-Lawrence, S.; Varshney, S.; Gupta, D.; Ogden, S.B.; Takemi, S.; Richard, C.P.; et al. LEAP2 deletion in mice enhances ghrelin’s actions as an orexigen and growth hormone secretagogue. Mol. Metab. 2021, 53, 101327. [Google Scholar] [CrossRef] [PubMed]
- Al-Massadi, O.; Müller, T.; Tschöp, M.; Diéguez, C.; Nogueiras, R. Ghrelin and LEAP-2: Rivals in Energy Metabolism. Trends Pharmacol. Sci. 2018, 39, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Ogden, S.B.; Shankar, K.; Varshney, S.; Zigman, J.M. A LEAP 2 conclusions? Targeting the ghrelin system to treat obesity and diabetes. Mol. Metabol. 2021, 46, 101128. [Google Scholar] [CrossRef] [PubMed]
- Mani, B.K.; Puzziferri, N.; He, Z.; Rodriguez, J.A.; Osborne-Lawrence, S.; Metzger, N.P.; Chhina, N.; Gaylinn, B.; Thorner, M.O.; Thomas, E.L.; et al. LEAP2 changes with body mass and food intake in humans and mice. J. Clin. Investig. 2019, 129, 3909–3923. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, C.A.; Zhang, C.; Hansen, H.H.; Jorsal, T.; Rigbolt, K.T.G.; Madsen, M.R.; Bergmann, N.C.; Heimbürger, S.M.N.; Falkenhahn, M.; Theis, S.; et al. Identification and Metabolic Profiling of a Novel Human Gut-derived LEAP2 Fragment. J. Clin. Endocrinol. Metab. 2020, 106, e966–e981. [Google Scholar] [CrossRef]
- Sangiao-Alvarellos, S.; Varela, L.; Vazquez, M.J.; Da Boit, K.; Saha, A.K.; Cordido, F.; Diéguez, C.; López, M. Influence of Ghrelin and Growth Hormone Deficiency on AMP-Activated Protein Kinase and Hypothalamic Lipid Metabolism. J. Neuroendocr. 2010, 22, 543–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, M.; Lelliott, C.J.; Tovar, S.; Kimber, W.; Gallego, R.; Virtue, S.; Blount, M.; Vázquez, M.J.; Finer, N.; Powles, T.J.; et al. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes 2006, 55, 1327–1336. [Google Scholar] [CrossRef]
- Imbernon, M.; Beiroa, D.; Vázquez, M.J.; Morgan, D.A.; Veyrat–Durebex, C.; Porteiro, B.; Díaz–Arteaga, A.; Senra, A.; Busquets, S.; Velásquez, D.A.; et al. Central Melanin-Concentrating Hormone Influences Liver and Adipose Metabolism Via Specific Hypothalamic Nuclei and Efferent Autonomic/JNK1 Pathways. Gastroenterology 2013, 144, 636–649. [Google Scholar] [CrossRef] [Green Version]
- Barja-Fernández, S.; Lugilde, J.; Castelao, C.; Vázquez-Cobela, R.; Seoane, L.M.; Diéguez, C.; Leis, R.; Tovar, S. Circulating LEAP-2 is associated with puberty in girls. Int. J. Obes. 2020, 45, 502–514. [Google Scholar] [CrossRef]
- Zhao, T.-J.; Liang, G.; Li, R.L.; Xie, X.; Sleeman, M.W.; Murphy, A.; Valenzuela, D.M.; Yancopoulos, G.D.; Goldstein, J.L.; Brown, M.S. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. USA 2010, 107, 7467–7472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Asnicar, M.; Saha, P.K.; Chan, L.; Smith, R.G. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab. 2006, 3, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Lin, Y.; Lin, L.; Qin, G.; Pereira, F.A.; Haymond, M.W.; Butte, N.F.; Sun, Y. Ablation of ghrelin receptor in leptin-deficient ob/ob mice has paradoxical effects on glucose homeostasis when compared with ablation of ghrelin in ob/ob mice. Am. J. Physiol. Metab. 2012, 303, E422–E431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakawa, A.; Inui, A.; Kaga, T.; Katsuura, G.; Fujimiya, M.; A Fujino, M.; Kasuga, M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut 2003, 52, 947–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stark, R.; Ashley, S.E.; Andrews, Z.B. AMPK and the neuroendocrine regulation of appetite and energy expenditure. Mol. Cell. Endocrinol. 2013, 366, 215–223. [Google Scholar] [CrossRef]
- López, M.; Lage, R.; Saha, A.; Perez-Tilve, D.; Vázquez, M.J.; Varela, L.; Sangiao-Alvarellos, S.; Tovar, S.; Raghay, K.; Rodríguez-Cuenca, S.; et al. Hypothalamic Fatty Acid Metabolism Mediates the Orexigenic Action of Ghrelin. Cell Metab. 2008, 7, 389–399. [Google Scholar] [CrossRef] [Green Version]
- Romero-Pico, A.; Vázquez, M.J.; González-Touceda, D.; Folgueira, C.; Skibicka, K.P.; Alvarez-Crespo, M.; Van Gestel, M.A.; Velásquez, D.A.; Schwarzer, C.; Herzog, H.; et al. Hypothalamic kappa-opioid receptor modulates the orexigenic effect of ghrelin. Neuropsychopharmacology 2013, 38, 1296–1307. [Google Scholar] [CrossRef] [Green Version]
- Lage, R.; Vázquez, M.J.; Varela, L.; Saha, A.K.; Vidal-Puig, A.; Nogueiras, R.; Diéguez, C.; López, M. Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J. 2010, 24, 2670–2679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramírez, S.; Martins, L.; Jacas, J.; Carrasco, P.; Pozo, M.; Clotet, J.; Serra, D.; Hegardt, F.G.; Diéguez, C.; López, M.; et al. Hypothalamic Ceramide Levels Regulated by CPT1C Mediate the Orexigenic Effect of Ghrelin. Diabetes 2013, 62, 2329–2337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velásquez, D.A.; Martínez, G.; Romero, A.; Vázquez, M.J.; Boit, K.D.; Dopeso-Reyes, I.G.; López, M.; Vidal, A.; Nogueiras, R.; Diéguez, C. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes 2011, 60, 1177–1185. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.; Fernández-Mallo, D.; Novelle, M.G.; Vázquez, M.J.; Tena-Sempere, M.; Nogueiras, R.; López, M.; Diéguez, C. Hypothalamic mTOR Signaling Mediates the Orexigenic Action of Ghrelin. PLoS ONE 2012, 7, e46923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egecioglu, E.; Bjursell, M.; Ljungberg, A.; Dickson, S.L.; Kopchick, J.J.; Bergström, G.; Svensson, L.; Oscarsson, J.; Törnell, J.; Bohlooly-Y, M. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am. J. Physiol. Metab. 2006, 290, E317–E325. [Google Scholar] [CrossRef] [Green Version]
- Gilg, S.; Lutz, T. The orexigenic effect of peripheral ghrelin differs between rats of different age and with different baseline food intake, and it may in part be mediated by the area postrema. Physiol. Behav. 2006, 87, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.M.; Page, L.C.; Tong, J. Ghrelin regulation of glucose metabolism. J. Neuroendocr. 2019, 31, e12705. [Google Scholar] [CrossRef] [PubMed]
- Chuang, J.-C.; Perello, M.; Sakata, I.; Osborne-Lawrence, S.; Savitt, J.M.; Lutter, M.; Zigman, J.M. Ghrelin mediates stress-induced food-reward behavior in mice. J. Clin. Investig. 2011, 121, 2684–2692. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, C.; Uchida, A.; Chuang, J.-C.; Walker, A.; Liu, T.; Osborne-Lawrence, S.; Mason, B.L.; Mosher, C.; Berglund, E.D.; et al. Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol. Metab. 2013, 3, 64–72. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugilde, J.; Casado, S.; Beiroa, D.; Cuñarro, J.; Garcia-Lavandeira, M.; Álvarez, C.V.; Nogueiras, R.; Diéguez, C.; Tovar, S. LEAP-2 Counteracts Ghrelin-Induced Food Intake in a Nutrient, Growth Hormone and Age Independent Manner. Cells 2022, 11, 324. https://doi.org/10.3390/cells11030324
Lugilde J, Casado S, Beiroa D, Cuñarro J, Garcia-Lavandeira M, Álvarez CV, Nogueiras R, Diéguez C, Tovar S. LEAP-2 Counteracts Ghrelin-Induced Food Intake in a Nutrient, Growth Hormone and Age Independent Manner. Cells. 2022; 11(3):324. https://doi.org/10.3390/cells11030324
Chicago/Turabian StyleLugilde, Javier, Sabela Casado, Daniel Beiroa, Juan Cuñarro, Montserrat Garcia-Lavandeira, Clara V. Álvarez, Rubén Nogueiras, Carlos Diéguez, and Sulay Tovar. 2022. "LEAP-2 Counteracts Ghrelin-Induced Food Intake in a Nutrient, Growth Hormone and Age Independent Manner" Cells 11, no. 3: 324. https://doi.org/10.3390/cells11030324
APA StyleLugilde, J., Casado, S., Beiroa, D., Cuñarro, J., Garcia-Lavandeira, M., Álvarez, C. V., Nogueiras, R., Diéguez, C., & Tovar, S. (2022). LEAP-2 Counteracts Ghrelin-Induced Food Intake in a Nutrient, Growth Hormone and Age Independent Manner. Cells, 11(3), 324. https://doi.org/10.3390/cells11030324